

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM291573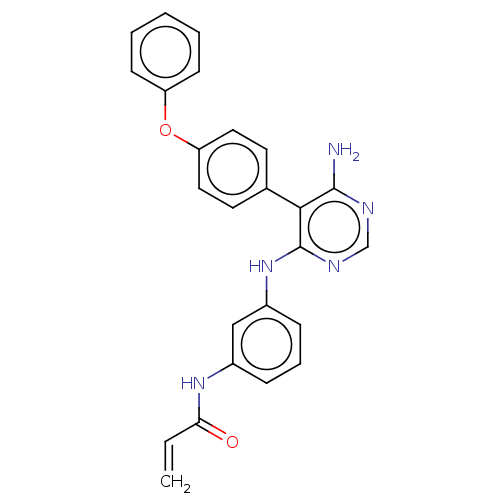 (N-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM291413 (1-(6-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM291455 (N-(4-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM291452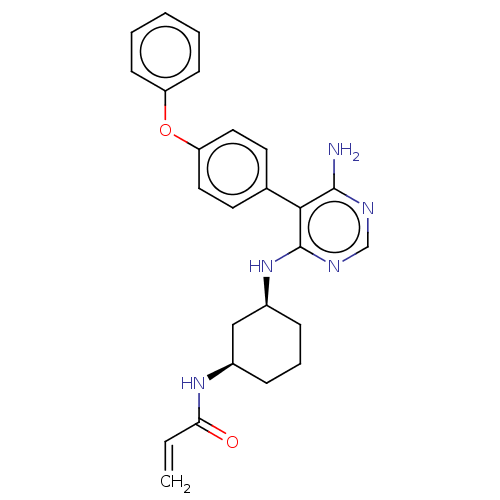 (N-((1R,3S)-3-((6-amino-5-(4-phenoxyphenyl)pyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM291522 (1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291635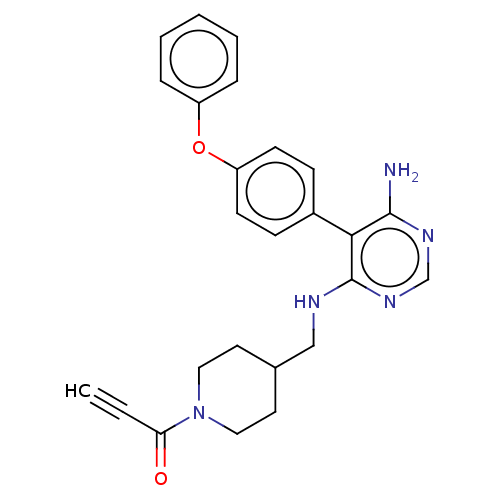 (1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50519156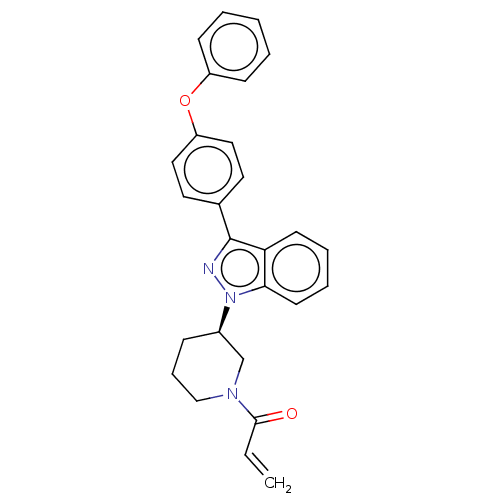 (CHEMBL4466205) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291634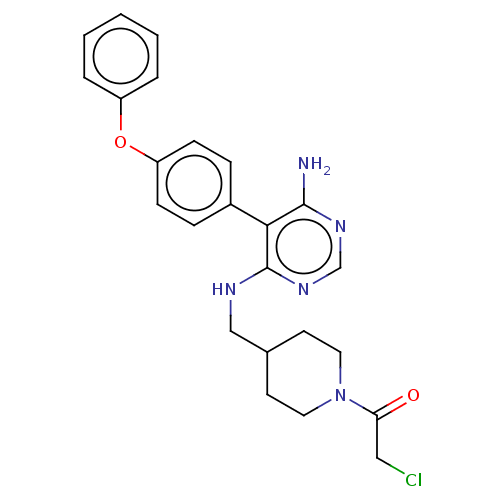 (1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291389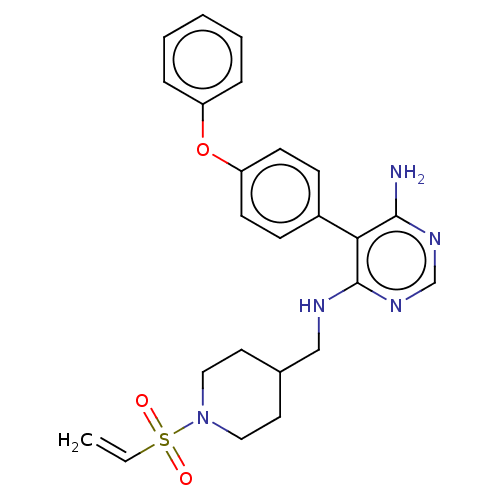 (5-(4-phenoxyphenyl)-N4-((1-(vinylsulfonyl)piperidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065485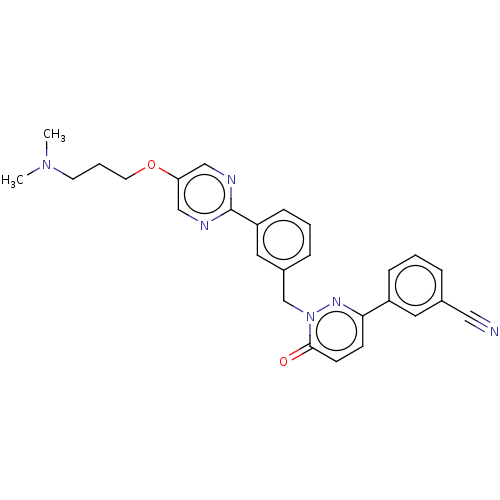 (CHEMBL3402760) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065457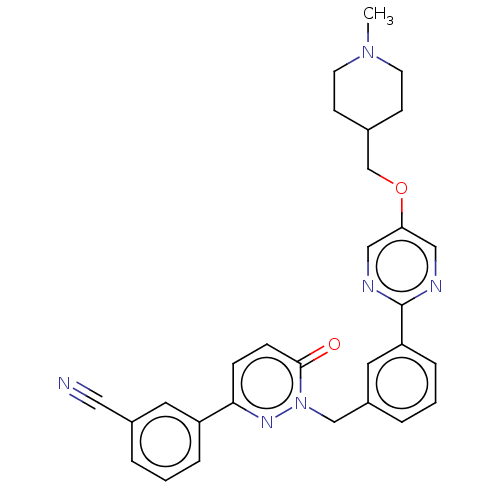 (EMD-1214063 | MSC-2156119 | MSC-2156119J | Tepotin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065490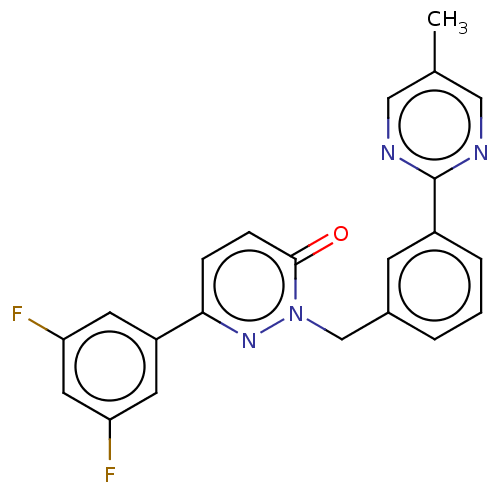 (CHEMBL3402754) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of c-Met kinase in human A549 cells assessed as inhibition of phosphorylation after 45 mins by electrochemiluminescence assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM291512 (N-(3-(4-amino-6-((4-phenoxyphenyl)amino)pyrimidin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human EGFR (696 to end aminoacids) expressed in baculovirus infected Sf21 cells | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065458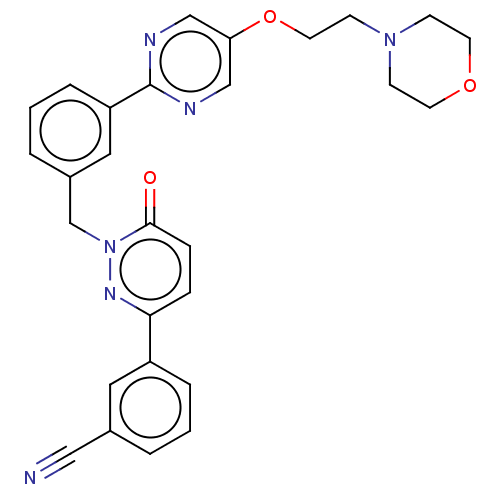 (CHEMBL3402761) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291413 (1-(6-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065489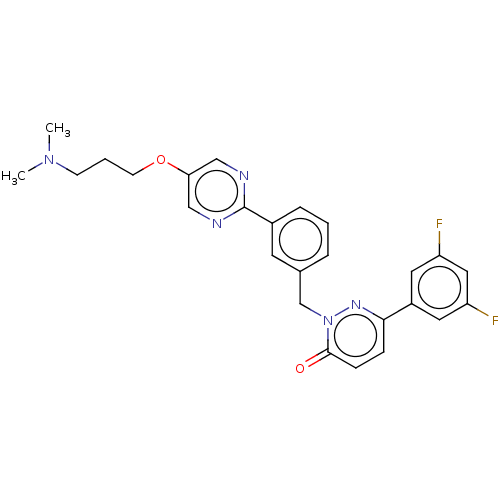 (CHEMBL3402756) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291513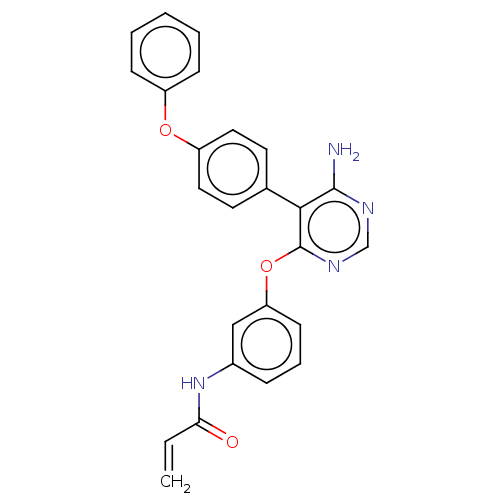 (N-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)o...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM291573 (N-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human EGFR (696 to end aminoacids) expressed in baculovirus infected Sf21 cells | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291573 (N-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291515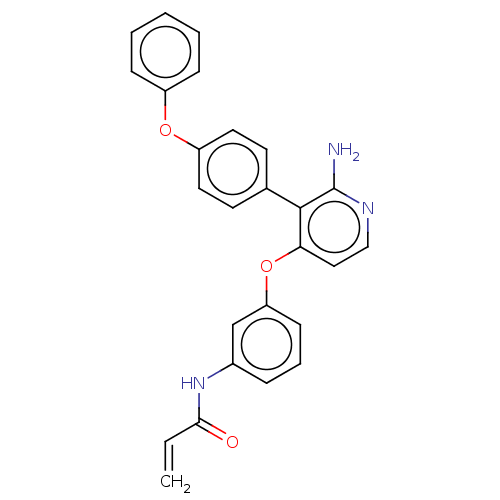 (N-(3-((2-amino-3-(4-phenoxyphenyl)pyridin-4-yl)oxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291425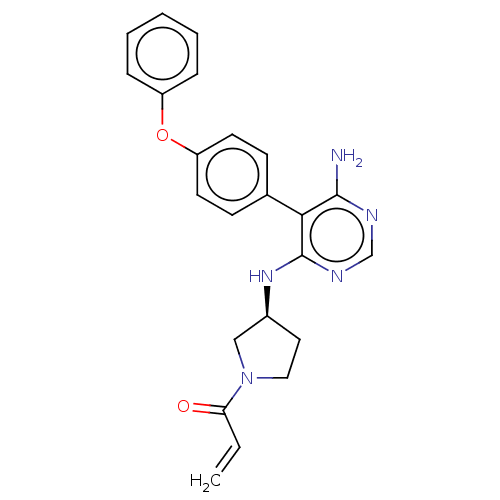 ((S)-1-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065486 (CHEMBL3402759) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291625 (1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065488 (CHEMBL3402757) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291549 ((S)-1-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291512 (N-(3-(4-amino-6-((4-phenoxyphenyl)amino)pyrimidin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291522 (1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291610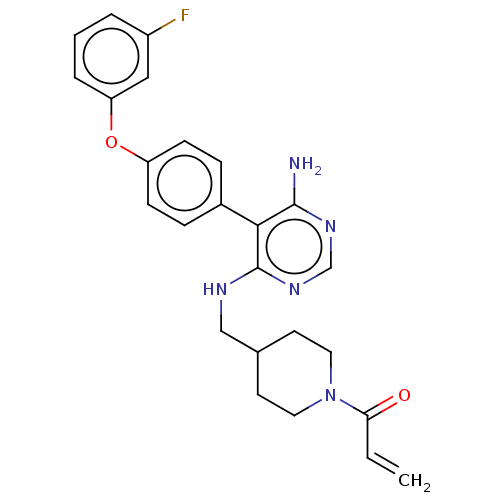 (1-(4-(((6-amino-5-(4-(3-fluorophenoxy)phenyl)pyrim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291455 (N-(4-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291513 (N-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)o...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of BTK in human PBMC cells assessed as reduction in anti-IgM-stimulated CD69 expression on B cells preincubated for 60 mins followed by go... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065487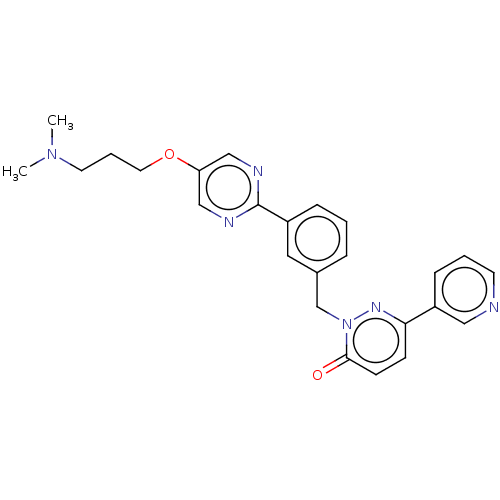 (CHEMBL3402758) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065493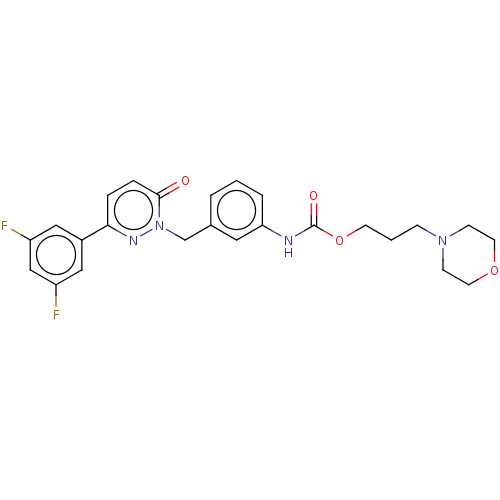 (CHEMBL3402765) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065457 (EMD-1214063 | MSC-2156119 | MSC-2156119J | Tepotin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of c-Met kinase in human A549 cells assessed as inhibition of phosphorylation after 45 mins by electrochemiluminescence assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291452 (N-((1R,3S)-3-((6-amino-5-(4-phenoxyphenyl)pyrimidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065489 (CHEMBL3402756) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of c-Met kinase in human A549 cells assessed as inhibition of phosphorylation after 45 mins by electrochemiluminescence assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065485 (CHEMBL3402760) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of c-Met kinase in human A549 cells assessed as inhibition of phosphorylation after 45 mins by electrochemiluminescence assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291684 (1-(4-(((6-amino-5-(4-(p-tolyloxy)phenyl)pyrimidin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291573 (N-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of BTK in human PBMC cells assessed as reduction in anti-IgM-stimulated CD69 expression on B cells preincubated for 60 mins followed by go... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291460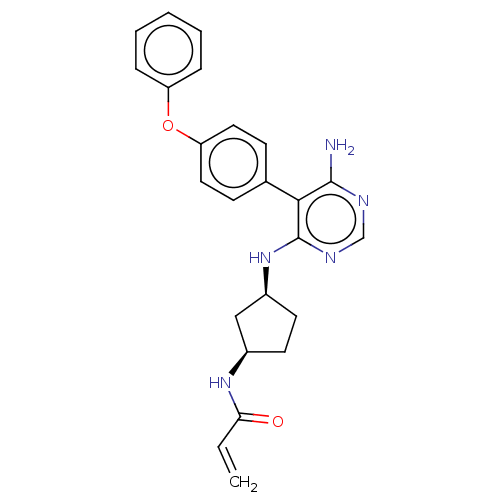 (N-(cis-3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291527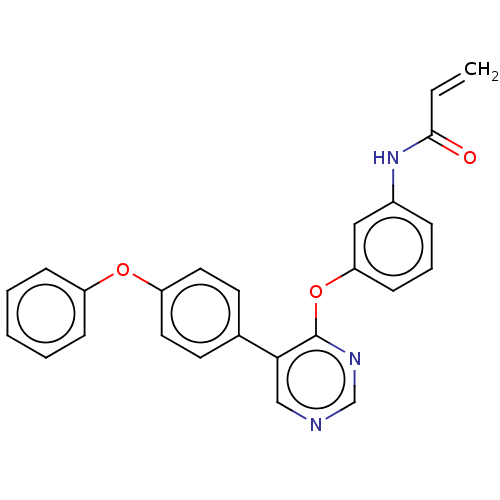 (N-(3-((5-(4-phenoxyphenyl)pyrimidin-4-yl)oxy)pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065492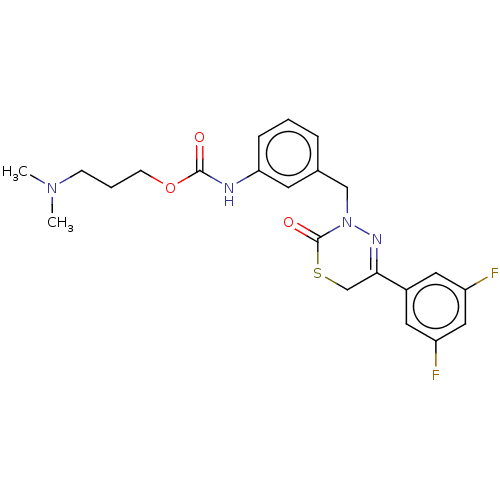 (CHEMBL3402742) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291625 (1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of BTK in human PBMC cells assessed as reduction in anti-IgM-stimulated CD69 expression on B cells preincubated for 60 mins followed by go... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065487 (CHEMBL3402758) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of c-Met kinase in human A549 cells assessed as inhibition of phosphorylation after 45 mins by electrochemiluminescence assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291539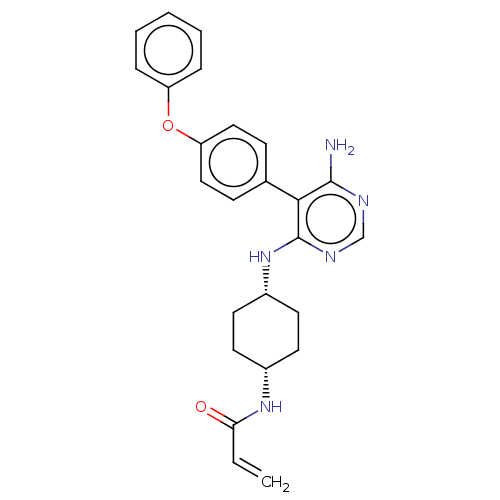 (N-((1s,4s)-4-((6-amino-5-(4-phenoxyphenyl)pyrimidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065458 (CHEMBL3402761) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of c-Met kinase in human A549 cells assessed as inhibition of phosphorylation after 45 mins by electrochemiluminescence assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065490 (CHEMBL3402754) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50065491 (CHEMBL3402743) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research& Development Curated by ChEMBL | Assay Description Inhibition of GST-His6-tagged recombinant human c-Met kinase domain (956 to 1390) after 60 mins by anoff-chip mobility shift assay | Bioorg Med Chem Lett 25: 1597-602 (2015) Article DOI: 10.1016/j.bmcl.2015.02.002 BindingDB Entry DOI: 10.7270/Q2HX1FBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291544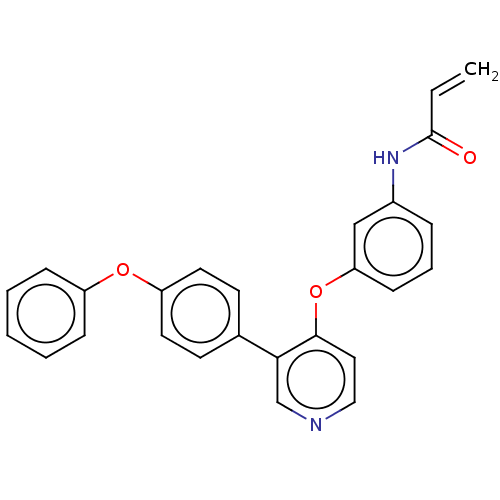 (N-(3-((3-(4-phenoxyphenyl)pyridin-4-yl)oxy)phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291389 (5-(4-phenoxyphenyl)-N4-((1-(vinylsulfonyl)piperidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of BTK in human PBMC cells assessed as reduction in anti-IgM-stimulated CD69 expression on B cells preincubated for 60 mins followed by go... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM291522 (1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of BTK in human PBMC cells assessed as reduction in anti-IgM-stimulated CD69 expression on B cells preincubated for 60 mins followed by go... | J Med Chem 62: 7643-7655 (2019) Article DOI: 10.1021/acs.jmedchem.9b00794 BindingDB Entry DOI: 10.7270/Q21R6TXT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 94 total ) | Next | Last >> |