 Found 1287 hits with Last Name = 'migliore' and Initial = 'm'
Found 1287 hits with Last Name = 'migliore' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50153378
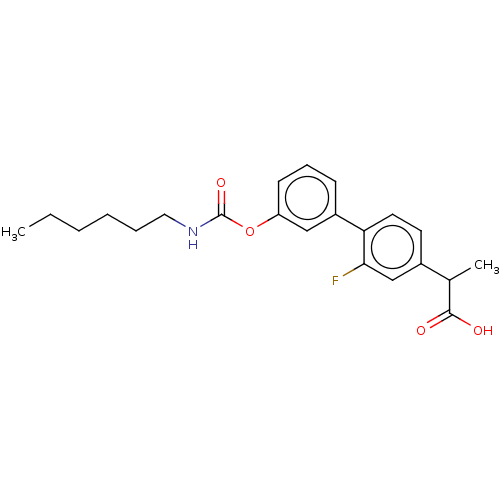
(CHEMBL3775041 | US9630914, Compound 12)Show SMILES CCCCCCNC(=O)Oc1cccc(c1)-c1ccc(cc1F)C(C)C(O)=O Show InChI InChI=1S/C22H26FNO4/c1-3-4-5-6-12-24-22(27)28-18-9-7-8-17(13-18)19-11-10-16(14-20(19)23)15(2)21(25)26/h7-11,13-15H,3-6,12H2,1-2H3,(H,24,27)(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano Di Tecnologia; The Regents of the University of California; Alma Mater Studiorum—Universita' Di Bologna
US Patent
| Assay Description
COX activity was measured using a commercial kit (COX Inhibitor Screening Assay Kit Cayman Chemical N. 560131) which includes both ovine COX-1 and hu... |
US Patent US9630914 (2017)
BindingDB Entry DOI: 10.7270/Q2JW8H0S |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612206

(CHEMBL5277668)Show SMILES CN1c2nnnn2CC(C(=O)Nc2ccc3[nH]nc(-c4ccnc(c4)N4CCOCC4)c3c2)=C1C |c:37| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612230

(CHEMBL5270706)Show SMILES CN1c2nnnn2C(C)(C)C(C(=O)Nc2ccc3[nH]nc(-c4ccnc(c4)N4CCOCC4)c3c2)=C1C |c:39| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612227
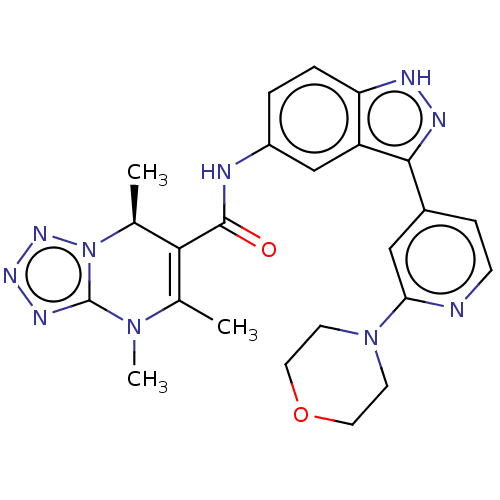
(CHEMBL5289675)Show SMILES C[C@H]1C(C(=O)Nc2ccc3[nH]nc(-c4ccnc(c4)N4CCOCC4)c3c2)=C(C)N(C)c2nnnn12 |r,t:30| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612226
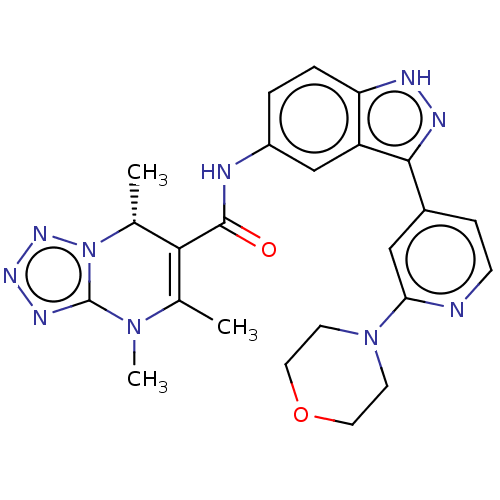
(CHEMBL5281154)Show SMILES C[C@@H]1C(C(=O)Nc2ccc3[nH]nc(-c4ccnc(c4)N4CCOCC4)c3c2)=C(C)N(C)c2nnnn12 |r,t:30| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612225
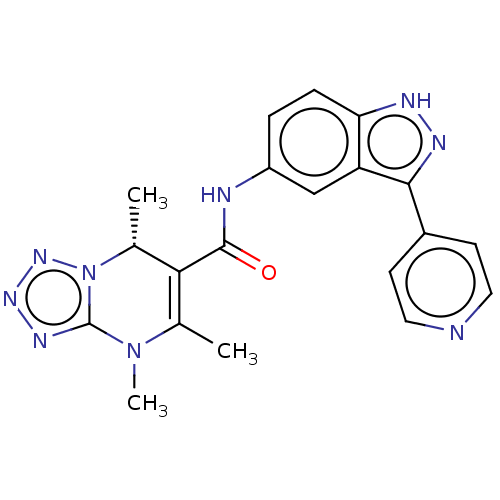
(CHEMBL5286893)Show SMILES C[C@@H]1C(C(=O)Nc2ccc3[nH]nc(-c4ccncc4)c3c2)=C(C)N(C)c2nnnn12 |r,t:23| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612209
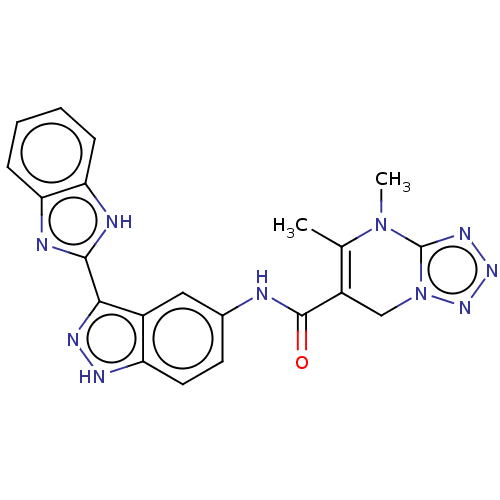
(CHEMBL5288427)Show SMILES CN1c2nnnn2CC(C(=O)Nc2ccc3[nH]nc(-c4nc5ccccc5[nH]4)c3c2)=C1C |c:34| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612208

(CHEMBL5284027)Show SMILES CN1c2nnnn2CC(C(=O)Nc2ccc3[nH]nc(-c4ccc(cc4)S(N)(=O)=O)c3c2)=C1C |c:34| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612231
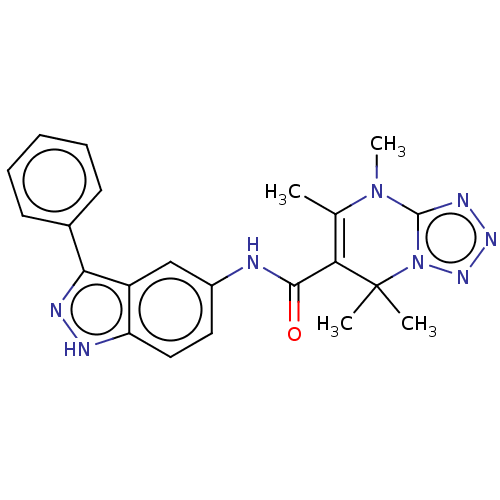
(CHEMBL5280373)Show SMILES CN1c2nnnn2C(C)(C)C(C(=O)Nc2ccc3[nH]nc(-c4ccccc4)c3c2)=C1C |c:32| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612226

(CHEMBL5281154)Show SMILES C[C@@H]1C(C(=O)Nc2ccc3[nH]nc(-c4ccnc(c4)N4CCOCC4)c3c2)=C(C)N(C)c2nnnn12 |r,t:30| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612206

(CHEMBL5277668)Show SMILES CN1c2nnnn2CC(C(=O)Nc2ccc3[nH]nc(-c4ccnc(c4)N4CCOCC4)c3c2)=C1C |c:37| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612225

(CHEMBL5286893)Show SMILES C[C@@H]1C(C(=O)Nc2ccc3[nH]nc(-c4ccncc4)c3c2)=C(C)N(C)c2nnnn12 |r,t:23| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50153360
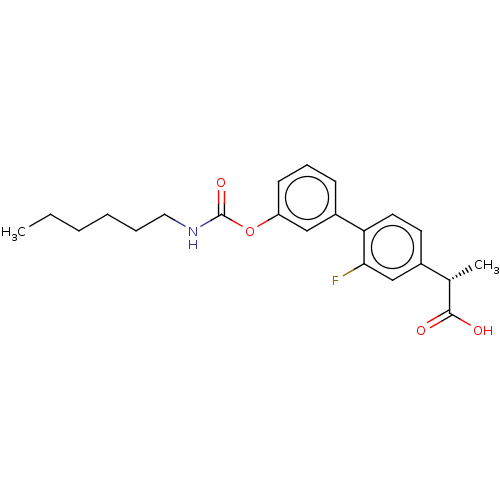
(CHEMBL3774784)Show SMILES CCCCCCNC(=O)Oc1cccc(c1)-c1ccc(cc1F)[C@H](C)C(O)=O |r| Show InChI InChI=1S/C22H26FNO4/c1-3-4-5-6-12-24-22(27)28-18-9-7-8-17(13-18)19-11-10-16(14-20(19)23)15(2)21(25)26/h7-11,13-15H,3-6,12H2,1-2H3,(H,24,27)(H,25,26)/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano di Tecnologia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 assessed as production of PGF2-alpha preincubated with compound followed by the addition of 5 uM arachidonic acid as substra... |
Eur J Med Chem 109: 216-37 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.036
BindingDB Entry DOI: 10.7270/Q21C1ZRJ |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612230

(CHEMBL5270706)Show SMILES CN1c2nnnn2C(C)(C)C(C(=O)Nc2ccc3[nH]nc(-c4ccnc(c4)N4CCOCC4)c3c2)=C1C |c:39| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612207

(CHEMBL5274608)Show SMILES CN1c2nnnn2CC(C(=O)Nc2ccc3[nH]nc(-c4cc(ncn4)N4CCOCC4)c3c2)=C1C |c:37| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612205
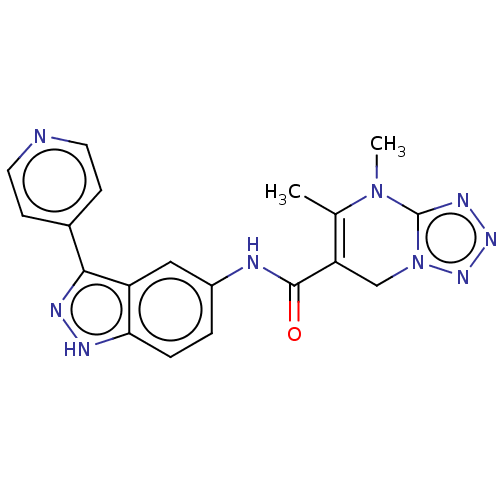
(CHEMBL5279036)Show SMILES CN1c2nnnn2CC(C(=O)Nc2ccc3[nH]nc(-c4ccncc4)c3c2)=C1C |c:30| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612209

(CHEMBL5288427)Show SMILES CN1c2nnnn2CC(C(=O)Nc2ccc3[nH]nc(-c4nc5ccccc5[nH]4)c3c2)=C1C |c:34| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612208

(CHEMBL5284027)Show SMILES CN1c2nnnn2CC(C(=O)Nc2ccc3[nH]nc(-c4ccc(cc4)S(N)(=O)=O)c3c2)=C1C |c:34| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612229

(CHEMBL5280577)Show SMILES CN1c2nnnn2C(C)(C)C(C(=O)Nc2ccc3[nH]nc(C)c3c2)=C1C |c:26| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612223
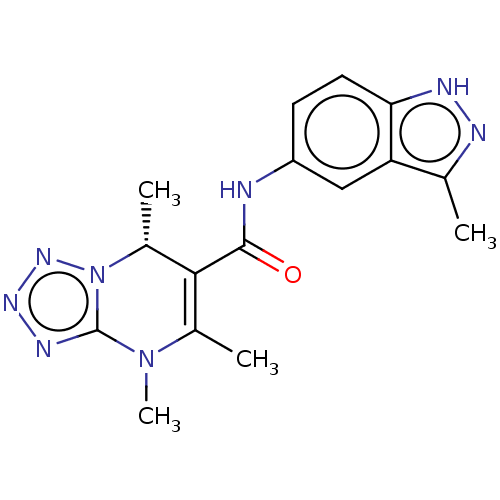
(CHEMBL5279816)Show SMILES C[C@@H]1C(C(=O)Nc2ccc3[nH]nc(C)c3c2)=C(C)N(C)c2nnnn12 |r,t:17| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612207

(CHEMBL5274608)Show SMILES CN1c2nnnn2CC(C(=O)Nc2ccc3[nH]nc(-c4cc(ncn4)N4CCOCC4)c3c2)=C1C |c:37| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612230

(CHEMBL5270706)Show SMILES CN1c2nnnn2C(C)(C)C(C(=O)Nc2ccc3[nH]nc(-c4ccnc(c4)N4CCOCC4)c3c2)=C1C |c:39| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612227

(CHEMBL5289675)Show SMILES C[C@H]1C(C(=O)Nc2ccc3[nH]nc(-c4ccnc(c4)N4CCOCC4)c3c2)=C(C)N(C)c2nnnn12 |r,t:30| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612230

(CHEMBL5270706)Show SMILES CN1c2nnnn2C(C)(C)C(C(=O)Nc2ccc3[nH]nc(-c4ccnc(c4)N4CCOCC4)c3c2)=C1C |c:39| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612226

(CHEMBL5281154)Show SMILES C[C@@H]1C(C(=O)Nc2ccc3[nH]nc(-c4ccnc(c4)N4CCOCC4)c3c2)=C(C)N(C)c2nnnn12 |r,t:30| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612230

(CHEMBL5270706)Show SMILES CN1c2nnnn2C(C)(C)C(C(=O)Nc2ccc3[nH]nc(-c4ccnc(c4)N4CCOCC4)c3c2)=C1C |c:39| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612221

(CHEMBL5289513)Show SMILES C[C@@H]1C(C(=O)Nc2ccc3[nH]ncc3c2)=C(C)N(C)c2nnnn12 |r,t:16| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano di Tecnologia
Curated by ChEMBL
| Assay Description
Inhibition of FAAH in Sprague-Dawley rat brain homogenates preincubated for 10 mins followed by addition of substrate measured after 30 mins by liqui... |
Eur J Med Chem 109: 216-37 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.036
BindingDB Entry DOI: 10.7270/Q21C1ZRJ |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612231

(CHEMBL5280373)Show SMILES CN1c2nnnn2C(C)(C)C(C(=O)Nc2ccc3[nH]nc(-c4ccccc4)c3c2)=C1C |c:32| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612226

(CHEMBL5281154)Show SMILES C[C@@H]1C(C(=O)Nc2ccc3[nH]nc(-c4ccnc(c4)N4CCOCC4)c3c2)=C(C)N(C)c2nnnn12 |r,t:30| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612210
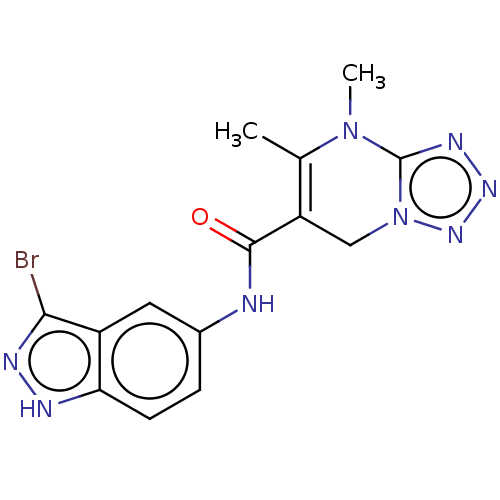
(CHEMBL5271931)Show SMILES CN1c2nnnn2CC(C(=O)Nc2ccc3[nH]nc(Br)c3c2)=C1C |c:24| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612231

(CHEMBL5280373)Show SMILES CN1c2nnnn2C(C)(C)C(C(=O)Nc2ccc3[nH]nc(-c4ccccc4)c3c2)=C1C |c:32| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50153438
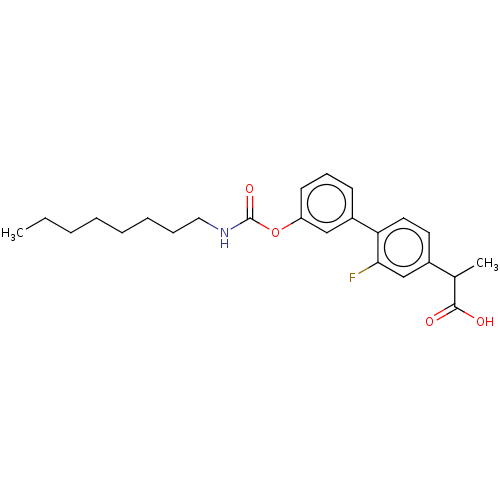
(CHEMBL3774873 | US9630914, Example 14)Show SMILES CCCCCCCCNC(=O)Oc1cccc(c1)-c1ccc(cc1F)C(C)C(O)=O Show InChI InChI=1S/C9H12N2O2S/c1-14(12,13)6-7-2-4-8(5-3-7)9(10)11/h2-5H,6H2,1H3,(H3,10,11) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano Di Tecnologia; The Regents of the University of California; Alma Mater Studiorum—Universita' Di Bologna
US Patent
| Assay Description
Rat FAAH was prepared from male Sprague Dawley rat brains, homogenized in a potter in 20 mM of Tris HCl pH 7.4, 0.32 M sucrose.The radiometric assay ... |
US Patent US9630914 (2017)
BindingDB Entry DOI: 10.7270/Q2JW8H0S |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612219

(CHEMBL5286615)Show SMILES CC1=C(Cn2nnnc2N1c1ccccc1)C(=O)Nc1ccc2[nH]ncc2c1 |t:1| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50153438

(CHEMBL3774873 | US9630914, Example 14)Show SMILES CCCCCCCCNC(=O)Oc1cccc(c1)-c1ccc(cc1F)C(C)C(O)=O Show InChI InChI=1S/C9H12N2O2S/c1-14(12,13)6-7-2-4-8(5-3-7)9(10)11/h2-5H,6H2,1H3,(H3,10,11) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano di Tecnologia
Curated by ChEMBL
| Assay Description
Inhibition of FAAH in Sprague-Dawley rat brain homogenates preincubated for 10 mins followed by addition of substrate measured after 30 mins by liqui... |
Eur J Med Chem 109: 216-37 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.036
BindingDB Entry DOI: 10.7270/Q21C1ZRJ |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50153356
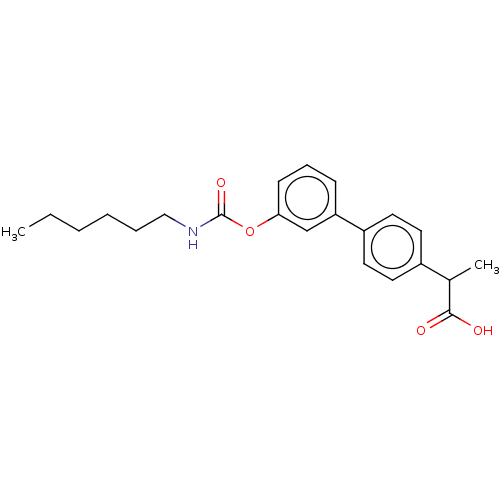
(CHEMBL3774686)Show SMILES CCCCCCNC(=O)Oc1cccc(c1)-c1ccc(cc1)C(C)C(O)=O Show InChI InChI=1S/C22H27NO4/c1-3-4-5-6-14-23-22(26)27-20-9-7-8-19(15-20)18-12-10-17(11-13-18)16(2)21(24)25/h7-13,15-16H,3-6,14H2,1-2H3,(H,23,26)(H,24,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano di Tecnologia
Curated by ChEMBL
| Assay Description
Inhibition of FAAH in Sprague-Dawley rat brain homogenates preincubated for 10 mins followed by addition of substrate measured after 30 mins by liqui... |
Eur J Med Chem 109: 216-37 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.036
BindingDB Entry DOI: 10.7270/Q21C1ZRJ |
More data for this
Ligand-Target Pair | |
Acid ceramidase
(Homo sapiens (Human)) | BDBM50511300
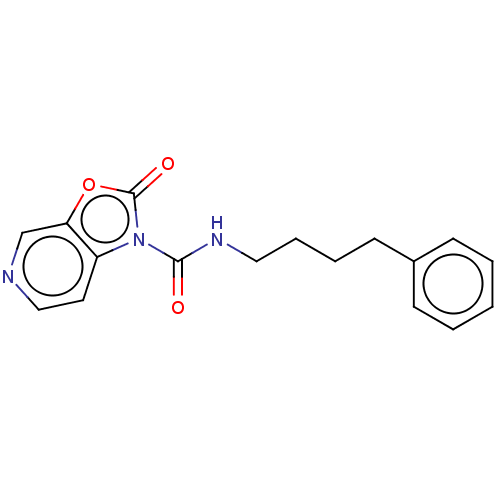
(CHEMBL4580541)Show InChI InChI=1S/C17H17N3O3/c21-16(19-10-5-4-8-13-6-2-1-3-7-13)20-14-9-11-18-12-15(14)23-17(20)22/h1-3,6-7,9,11-12H,4-5,8,10H2,(H,19,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human acid ceramidase expressed in HEK293 cells using Rbm14-12 as substrate preincubated for 10 mins followed by substrate addition and... |
J Med Chem 63: 3634-3664 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02004
BindingDB Entry DOI: 10.7270/Q20C503N |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612212
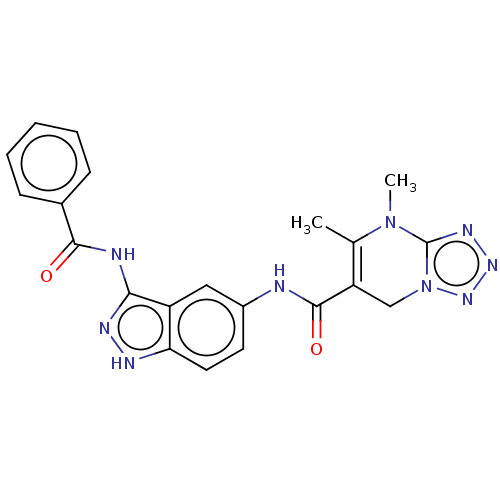
(CHEMBL5288038)Show SMILES CN1c2nnnn2CC(C(=O)Nc2ccc3[nH]nc(NC(=O)c4ccccc4)c3c2)=C1C |c:33| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50153445
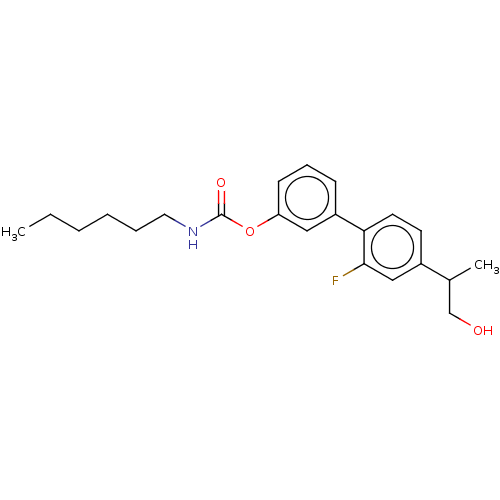
(CHEMBL3775280 | US9630914, Example 15)Show InChI InChI=1S/C9H12N2O/c10-9(11)8-3-1-7(2-4-8)5-6-12/h1-4,12H,5-6H2,(H3,10,11) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano di Tecnologia
Curated by ChEMBL
| Assay Description
Inhibition of FAAH in Sprague-Dawley rat brain homogenates preincubated for 10 mins followed by addition of substrate measured after 30 mins by liqui... |
Eur J Med Chem 109: 216-37 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.036
BindingDB Entry DOI: 10.7270/Q21C1ZRJ |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612231

(CHEMBL5280373)Show SMILES CN1c2nnnn2C(C)(C)C(C(=O)Nc2ccc3[nH]nc(-c4ccccc4)c3c2)=C1C |c:32| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50153445

(CHEMBL3775280 | US9630914, Example 15)Show InChI InChI=1S/C9H12N2O/c10-9(11)8-3-1-7(2-4-8)5-6-12/h1-4,12H,5-6H2,(H3,10,11) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano Di Tecnologia; The Regents of the University of California; Alma Mater Studiorum—Universita' Di Bologna
US Patent
| Assay Description
Rat FAAH was prepared from male Sprague Dawley rat brains, homogenized in a potter in 20 mM of Tris HCl pH 7.4, 0.32 M sucrose.The radiometric assay ... |
US Patent US9630914 (2017)
BindingDB Entry DOI: 10.7270/Q2JW8H0S |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50153438

(CHEMBL3774873 | US9630914, Example 14)Show SMILES CCCCCCCCNC(=O)Oc1cccc(c1)-c1ccc(cc1F)C(C)C(O)=O Show InChI InChI=1S/C9H12N2O2S/c1-14(12,13)6-7-2-4-8(5-3-7)9(10)11/h2-5H,6H2,1H3,(H3,10,11) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano Di Tecnologia; The Regents of the University of California; Alma Mater Studiorum—Universita' Di Bologna
US Patent
| Assay Description
Human recombinant FAAH was obtained from a HEK-293 FAAH-1 overexpressing stable cell line. Cells were grown in DMEM medium containing 10% FBS, 1% pen... |
US Patent US9630914 (2017)
BindingDB Entry DOI: 10.7270/Q2JW8H0S |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612230

(CHEMBL5270706)Show SMILES CN1c2nnnn2C(C)(C)C(C(=O)Nc2ccc3[nH]nc(-c4ccnc(c4)N4CCOCC4)c3c2)=C1C |c:39| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acid ceramidase
(Homo sapiens (Human)) | BDBM50556798
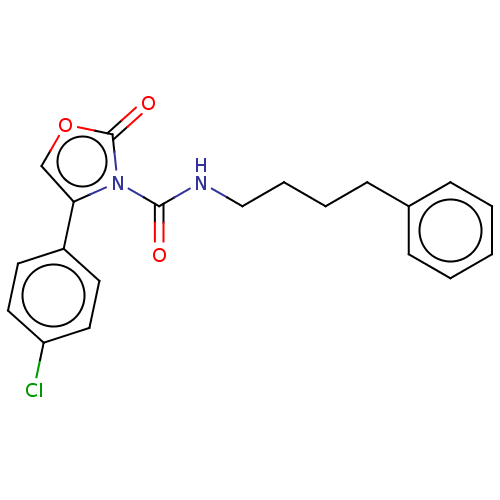
(CHEMBL4742096) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human acid ceramidase using N-lauroyl ceramide incubated for 1 hr by LC/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01561
BindingDB Entry DOI: 10.7270/Q2G73JC2 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50153354
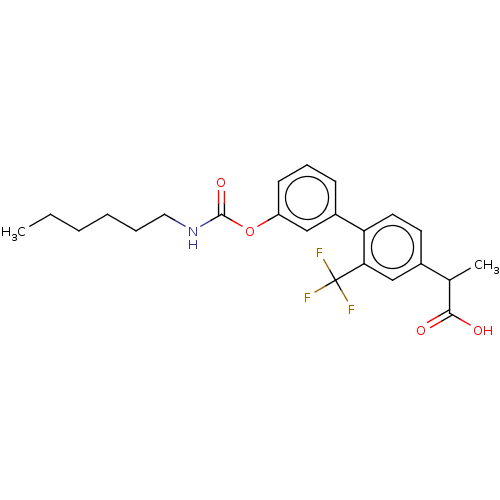
(CHEMBL3775510)Show SMILES CCCCCCNC(=O)Oc1cccc(c1)-c1ccc(cc1C(F)(F)F)C(C)C(O)=O Show InChI InChI=1S/C23H26F3NO4/c1-3-4-5-6-12-27-22(30)31-18-9-7-8-17(13-18)19-11-10-16(15(2)21(28)29)14-20(19)23(24,25)26/h7-11,13-15H,3-6,12H2,1-2H3,(H,27,30)(H,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano di Tecnologia
Curated by ChEMBL
| Assay Description
Inhibition of FAAH in Sprague-Dawley rat brain homogenates preincubated for 10 mins followed by addition of substrate measured after 30 mins by liqui... |
Eur J Med Chem 109: 216-37 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.036
BindingDB Entry DOI: 10.7270/Q21C1ZRJ |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612219

(CHEMBL5286615)Show SMILES CC1=C(Cn2nnnc2N1c1ccccc1)C(=O)Nc1ccc2[nH]ncc2c1 |t:1| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acid ceramidase
(Homo sapiens (Human)) | BDBM50511282
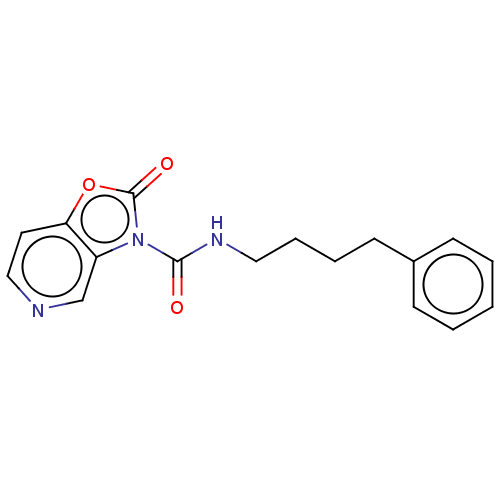
(CHEMBL4592427)Show InChI InChI=1S/C17H17N3O3/c21-16(19-10-5-4-8-13-6-2-1-3-7-13)20-14-12-18-11-9-15(14)23-17(20)22/h1-3,6-7,9,11-12H,4-5,8,10H2,(H,19,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human acid ceramidase expressed in HEK293 cells using Rbm14-12 as substrate preincubated for 10 mins followed by substrate addition and... |
J Med Chem 63: 3634-3664 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02004
BindingDB Entry DOI: 10.7270/Q20C503N |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50153379

(CHEMBL3775979 | US9630914, Example 13)Show SMILES CCCCCCCNC(=O)Oc1cccc(c1)-c1ccc(cc1F)C(C)C(O)=O Show InChI InChI=1S/C23H28FNO4/c1-3-4-5-6-7-13-25-23(28)29-19-10-8-9-18(14-19)20-12-11-17(15-21(20)24)16(2)22(26)27/h8-12,14-16H,3-7,13H2,1-2H3,(H,25,28)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano Di Tecnologia; The Regents of the University of California; Alma Mater Studiorum—Universita' Di Bologna
US Patent
| Assay Description
Human recombinant FAAH was obtained from a HEK-293 FAAH-1 overexpressing stable cell line. Cells were grown in DMEM medium containing 10% FBS, 1% pen... |
US Patent US9630914 (2017)
BindingDB Entry DOI: 10.7270/Q2JW8H0S |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612211
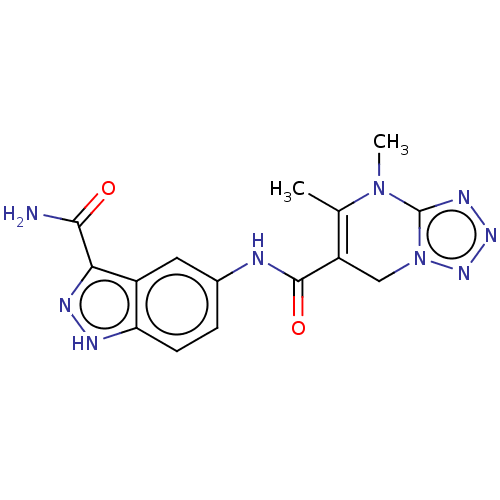
(CHEMBL5266676)Show SMILES CN1c2nnnn2CC(C(=O)Nc2ccc3[nH]nc(C(N)=O)c3c2)=C1C |c:26| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50612231

(CHEMBL5280373)Show SMILES CN1c2nnnn2C(C)(C)C(C(=O)Nc2ccc3[nH]nc(-c4ccccc4)c3c2)=C1C |c:32| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data