

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Homoisocitrate dehydrogenase, mitochondrial (Saccharomyces cerevisiae) | BDBM23220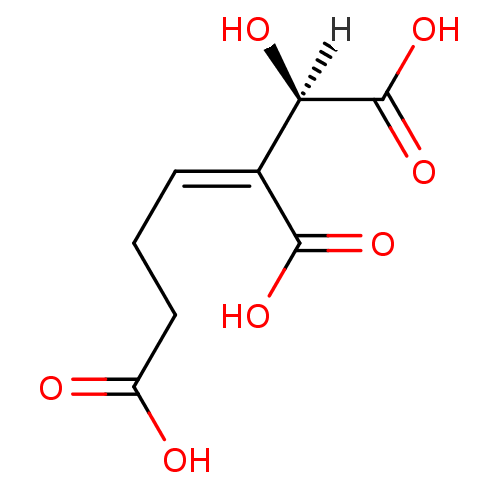 ((1R,2Z)-1-hydroxypent-2-ene-1,2,5-tricarboxylic ac...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 7.20E+4 | -24.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 36 |
Tokyo Institute of Technology | Assay Description Enzyme reaction was monitored by measuring the NADH absorption at 340 nm on a UV-Vis spectrometer. The formation of NADH was measured for 30 s. Data ... | Bioorg Med Chem 15: 1346-55 (2007) Article DOI: 10.1016/j.bmc.2006.11.008 BindingDB Entry DOI: 10.7270/Q2N87824 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Homoisocitrate dehydrogenase (Deinococcus radiodurans) | BDBM23221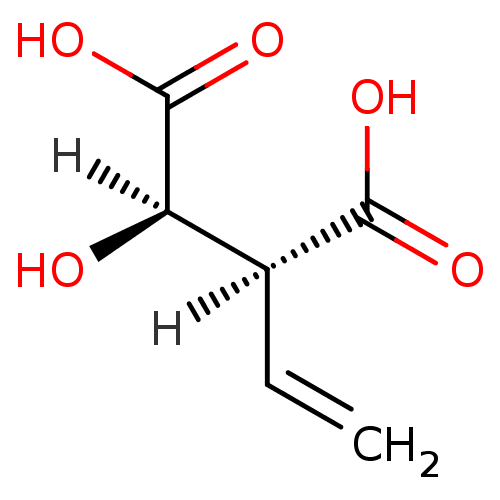 ((2S,3R)-2-ethenyl-3-hydroxybutanedioic acid | Viny...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 8.80E+4 | -23.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 28 |
Tokyo Institute of Technology | Assay Description Enzyme reaction was monitored by measuring the NADH absorption at 340 nm on a UV-Vis spectrometer. The formation of NADH was measured for 30 s. Data ... | Bioorg Med Chem 15: 1346-55 (2007) Article DOI: 10.1016/j.bmc.2006.11.008 BindingDB Entry DOI: 10.7270/Q2N87824 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Homoisocitrate dehydrogenase (Deinococcus radiodurans) | BDBM23220 ((1R,2Z)-1-hydroxypent-2-ene-1,2,5-tricarboxylic ac...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.60E+5 | -20.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 28 |
Tokyo Institute of Technology | Assay Description Enzyme reaction was monitored by measuring the NADH absorption at 340 nm on a UV-Vis spectrometer. The formation of NADH was measured for 30 s. Data ... | Bioorg Med Chem 15: 1346-55 (2007) Article DOI: 10.1016/j.bmc.2006.11.008 BindingDB Entry DOI: 10.7270/Q2N87824 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Homoisocitrate dehydrogenase, mitochondrial (Saccharomyces cerevisiae) | BDBM23218 ((2R,3Z)-2-hydroxy-3-(3-hydroxypropylidene)butanedi...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 5.10E+5 | -19.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 36 |
Tokyo Institute of Technology | Assay Description Enzyme reaction was monitored by measuring the NADH absorption at 340 nm on a UV-Vis spectrometer. The formation of NADH was measured for 30 s. Data ... | Bioorg Med Chem 15: 1346-55 (2007) Article DOI: 10.1016/j.bmc.2006.11.008 BindingDB Entry DOI: 10.7270/Q2N87824 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Homoisocitrate dehydrogenase, mitochondrial (Saccharomyces cerevisiae) | BDBM23219 ((1R,2E)-1-hydroxypent-2-ene-1,2,5-tricarboxylic ac...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 7.90E+5 | -18.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 36 |
Tokyo Institute of Technology | Assay Description Enzyme reaction was monitored by measuring the NADH absorption at 340 nm on a UV-Vis spectrometer. The formation of NADH was measured for 30 s. Data ... | Bioorg Med Chem 15: 1346-55 (2007) Article DOI: 10.1016/j.bmc.2006.11.008 BindingDB Entry DOI: 10.7270/Q2N87824 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Homoisocitrate dehydrogenase, mitochondrial (Saccharomyces cerevisiae) | BDBM23217 ((2R,3E)-2-hydroxy-3-(3-hydroxypropylidene)butanedi...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.40E+6 | -16.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 36 |
Tokyo Institute of Technology | Assay Description Enzyme reaction was monitored by measuring the NADH absorption at 340 nm on a UV-Vis spectrometer. The formation of NADH was measured for 30 s. Data ... | Bioorg Med Chem 15: 1346-55 (2007) Article DOI: 10.1016/j.bmc.2006.11.008 BindingDB Entry DOI: 10.7270/Q2N87824 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Homoisocitrate dehydrogenase (Deinococcus radiodurans) | BDBM23219 ((1R,2E)-1-hydroxypent-2-ene-1,2,5-tricarboxylic ac...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.10E+6 | -14.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 28 |
Tokyo Institute of Technology | Assay Description Enzyme reaction was monitored by measuring the NADH absorption at 340 nm on a UV-Vis spectrometer. The formation of NADH was measured for 30 s. Data ... | Bioorg Med Chem 15: 1346-55 (2007) Article DOI: 10.1016/j.bmc.2006.11.008 BindingDB Entry DOI: 10.7270/Q2N87824 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Homoisocitrate dehydrogenase (Deinococcus radiodurans) | BDBM23217 ((2R,3E)-2-hydroxy-3-(3-hydroxypropylidene)butanedi...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 5.20E+6 | -13.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 28 |
Tokyo Institute of Technology | Assay Description Enzyme reaction was monitored by measuring the NADH absorption at 340 nm on a UV-Vis spectrometer. The formation of NADH was measured for 30 s. Data ... | Bioorg Med Chem 15: 1346-55 (2007) Article DOI: 10.1016/j.bmc.2006.11.008 BindingDB Entry DOI: 10.7270/Q2N87824 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Homoisocitrate dehydrogenase (Deinococcus radiodurans) | BDBM23218 ((2R,3Z)-2-hydroxy-3-(3-hydroxypropylidene)butanedi...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.53E+7 | -10.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 28 |
Tokyo Institute of Technology | Assay Description Enzyme reaction was monitored by measuring the NADH absorption at 340 nm on a UV-Vis spectrometer. The formation of NADH was measured for 30 s. Data ... | Bioorg Med Chem 15: 1346-55 (2007) Article DOI: 10.1016/j.bmc.2006.11.008 BindingDB Entry DOI: 10.7270/Q2N87824 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50089825 (1-[4-(3-Chloro-4-methoxy-benzylamino)-6-nitro-phth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50089837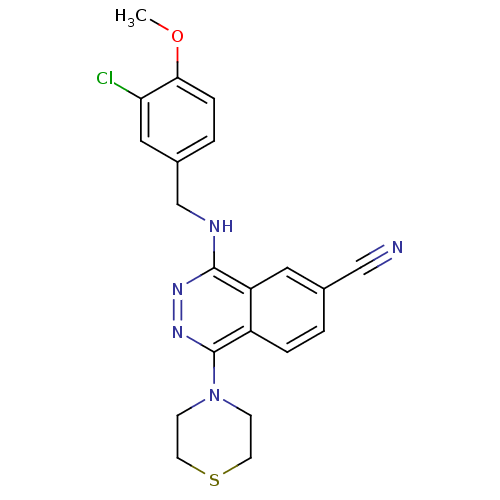 (4-(3-Chloro-4-methoxy-benzylamino)-1-thiomorpholin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50058752 (CHEMBL3335630) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd Curated by ChEMBL | Assay Description Inhibition of HIF1 signaling in human U251 cells expressing VEGF by VEGF promoter-driven PLAP reporter gene assay | Bioorg Med Chem 22: 5513-29 (2014) Article DOI: 10.1016/j.bmc.2014.07.020 BindingDB Entry DOI: 10.7270/Q24J0GSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50089826 (1-[4-(3-Chloro-4-methoxy-benzylamino)-6-cyano-phth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50089807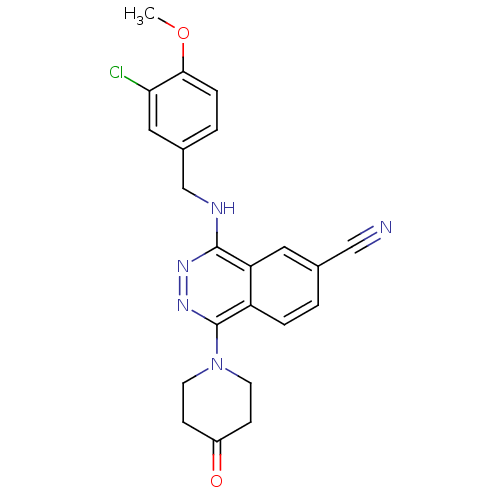 (4-(3-Chloro-4-methoxy-benzylamino)-1-(4-oxo-piperi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50089833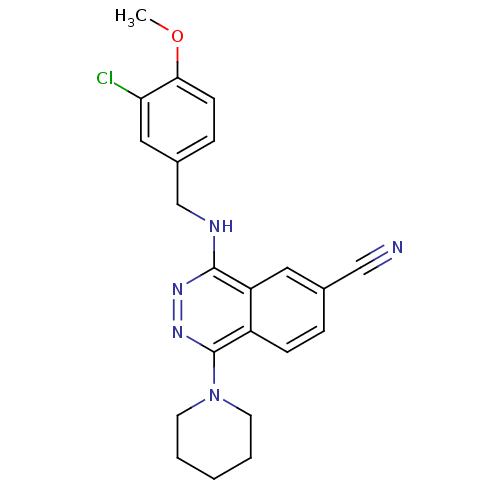 (4-(3-Chloro-4-methoxy-benzylamino)-1-piperidin-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50089814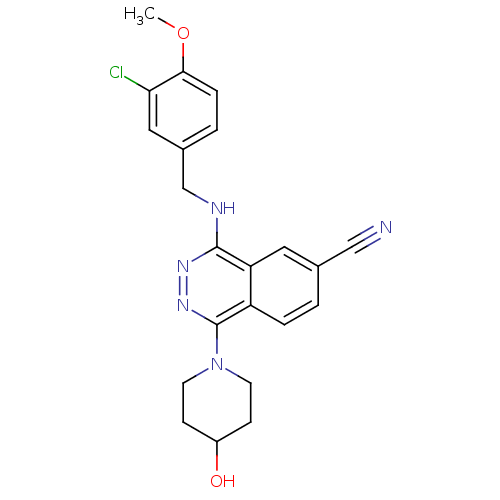 (4-(3-Chloro-4-methoxy-benzylamino)-1-(4-hydroxy-pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 (PDE5) from porcine platelets, range 0.442-0.710 | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50089827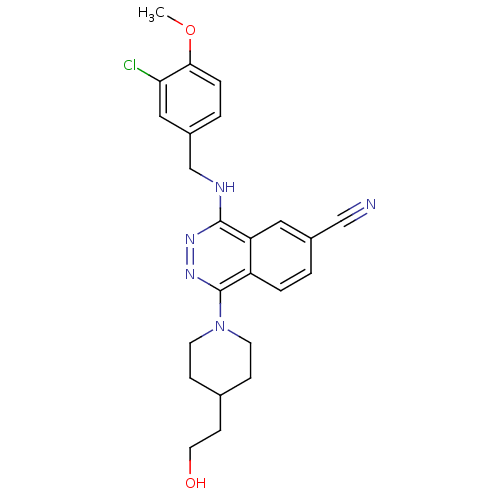 (4-(3-Chloro-4-methoxy-benzylamino)-1-[4-(2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50089821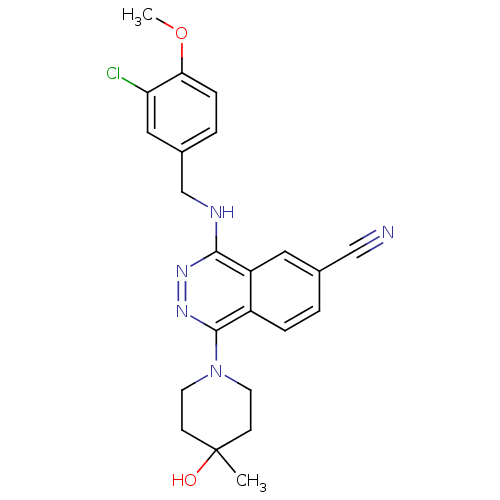 (4-(3-Chloro-4-methoxy-benzylamino)-1-(4-hydroxy-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50369544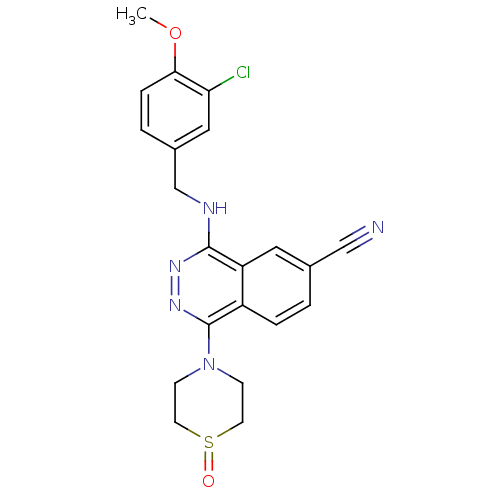 (CHEMBL545012) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50089815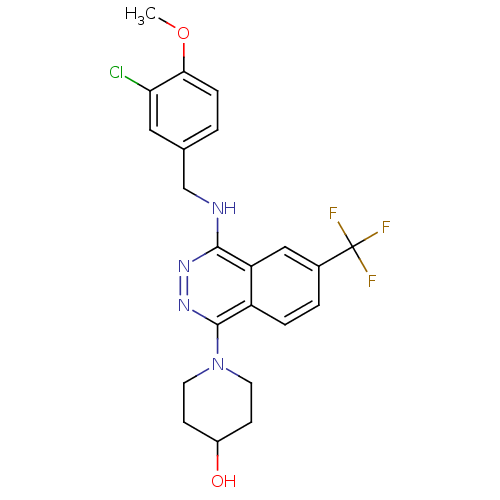 (1-[4-(3-Chloro-4-methoxy-benzylamino)-6-trifluorom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50089841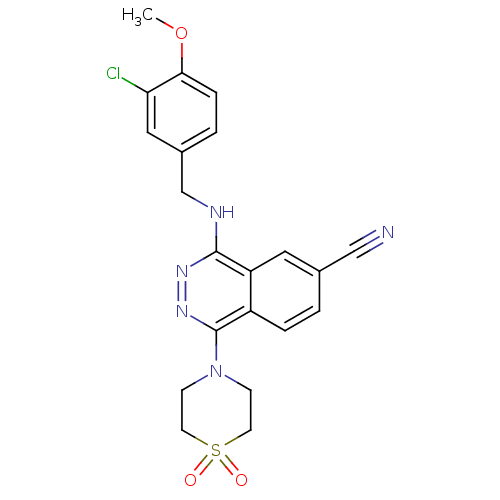 (4-(3-Chloro-4-methoxy-benzylamino)-1-(1,1-dioxo-1l...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50089832 (1-[6,7-Dichloro-4-(3-chloro-4-methoxy-benzylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50089820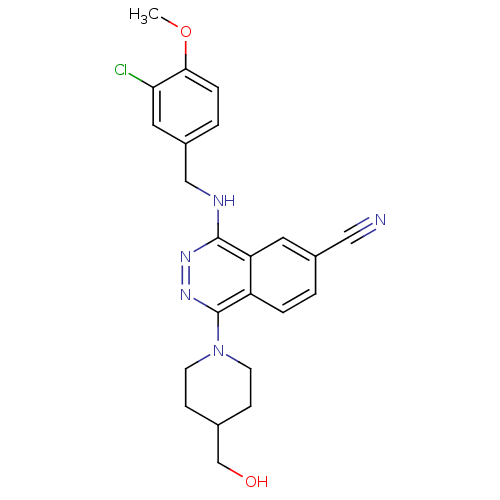 (4-(3-Chloro-4-methoxy-benzylamino)-1-(4-hydroxymet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50089830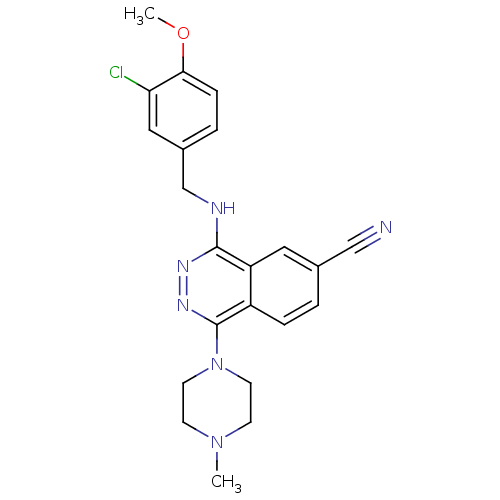 (4-(3-Chloro-4-methoxy-benzylamino)-1-(4-methyl-pip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50058757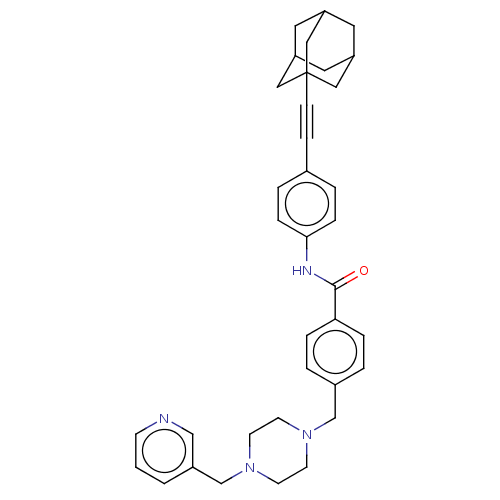 (CHEMBL3335423) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd Curated by ChEMBL | Assay Description Inhibition of HIF1 signaling in human U251 cells expressing VEGF by VEGF promoter-driven PLAP reporter gene assay | Bioorg Med Chem 22: 5513-29 (2014) Article DOI: 10.1016/j.bmc.2014.07.020 BindingDB Entry DOI: 10.7270/Q24J0GSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50058753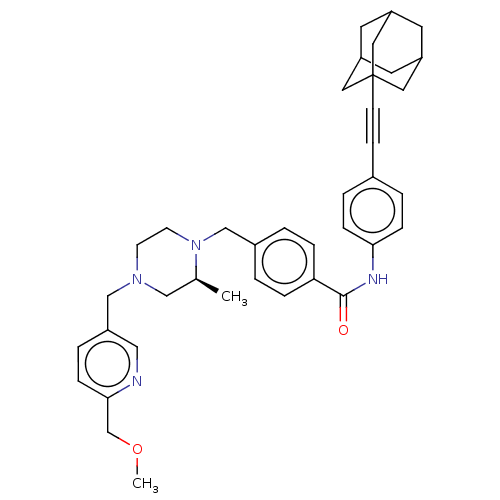 (CHEMBL3335427) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd Curated by ChEMBL | Assay Description Inhibition of HIF1 signaling in human U251 cells expressing VEGF by VEGF promoter-driven PLAP reporter gene assay | Bioorg Med Chem 22: 5513-29 (2014) Article DOI: 10.1016/j.bmc.2014.07.020 BindingDB Entry DOI: 10.7270/Q24J0GSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50089816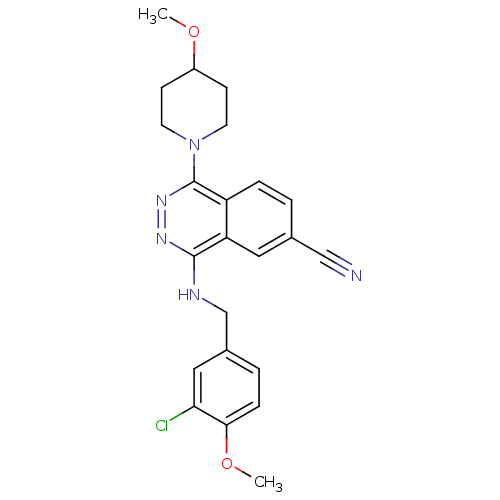 (4-(3-Chloro-4-methoxy-benzylamino)-1-(4-methoxy-pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50089809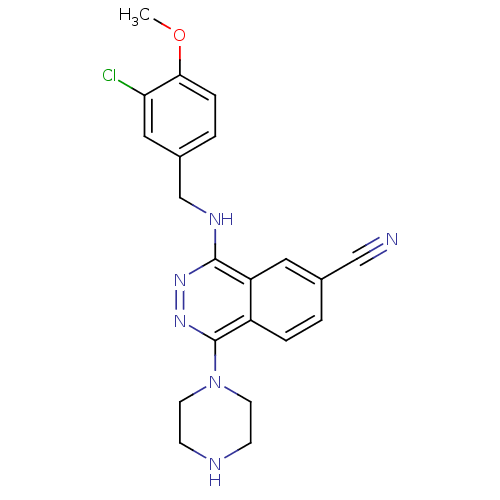 (4-(3-Chloro-4-methoxy-benzylamino)-1-piperazin-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50089838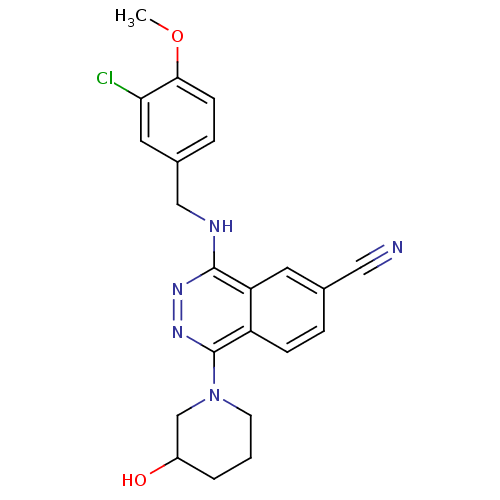 (4-(3-Chloro-4-methoxy-benzylamino)-1-(3-hydroxy-pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50089842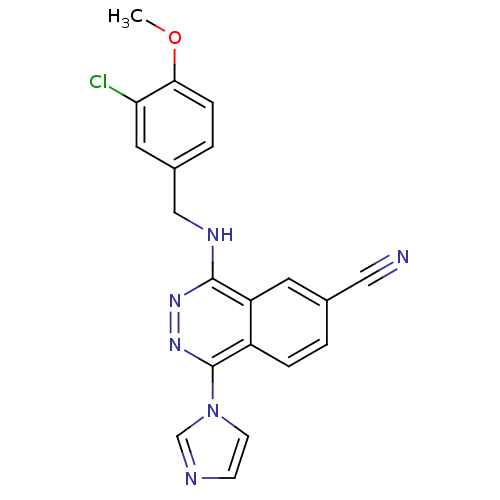 (4-(3-Chloro-4-methoxy-benzylamino)-1-imidazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50089818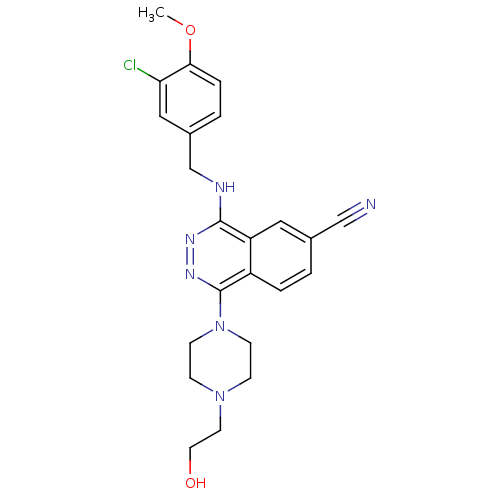 (4-(3-Chloro-4-methoxy-benzylamino)-1-[4-(2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50089812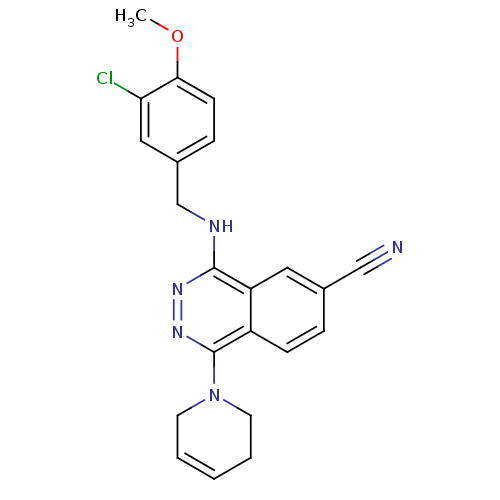 (4-(3-Chloro-4-methoxy-benzylamino)-1-(3,6-dihydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM307410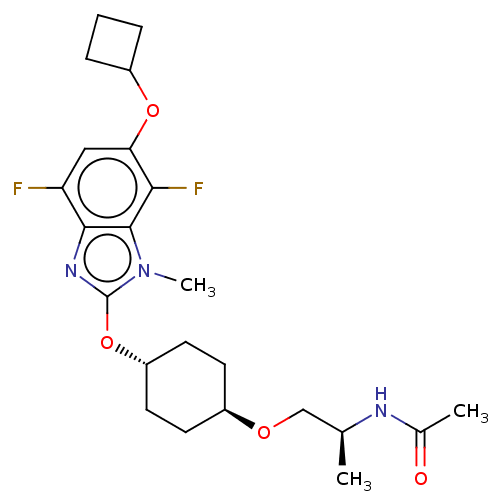 (US10150728, Example I-639) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Recombinant human ACC1 and recombinant human ACC2, which were prepared by the method mentioned above, were preincubated with assay buffer solution (5... | US Patent US10150728 (2018) BindingDB Entry DOI: 10.7270/Q2WD42M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50089836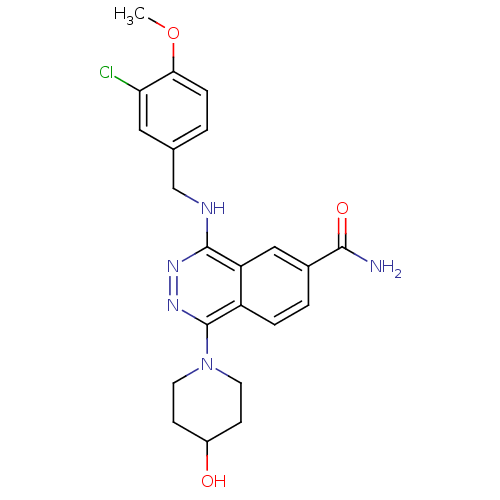 (4-(3-Chloro-4-methoxy-benzylamino)-1-(4-hydroxy-pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM307424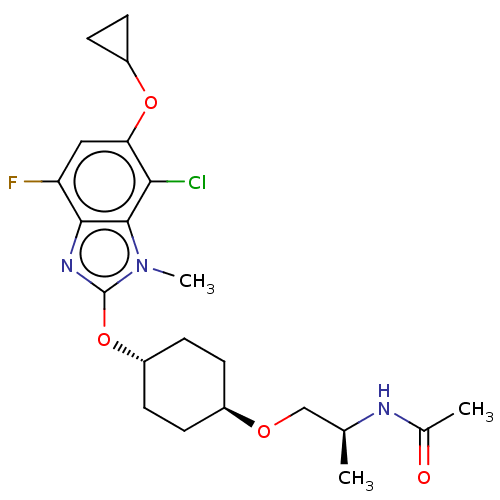 (US10150728, Example I-655) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Recombinant human ACC1 and recombinant human ACC2, which were prepared by the method mentioned above, were preincubated with assay buffer solution (5... | US Patent US10150728 (2018) BindingDB Entry DOI: 10.7270/Q2WD42M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14390 (5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50089817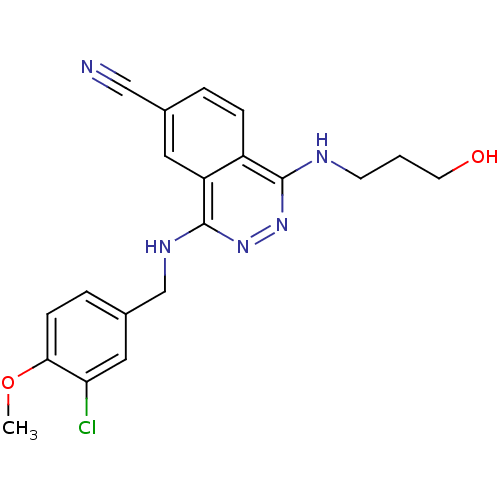 (4-(3-Chloro-4-methoxy-benzylamino)-1-(3-hydroxy-pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50058754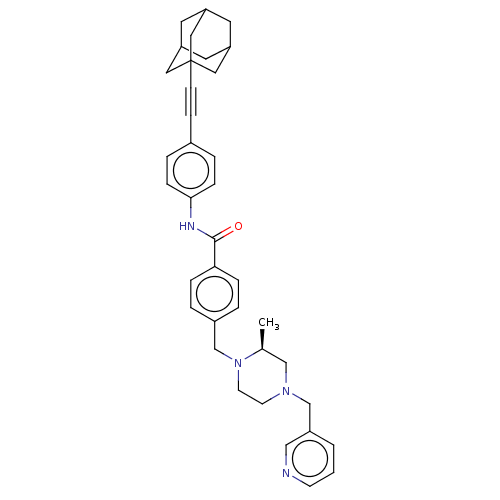 (CHEMBL3335426) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd Curated by ChEMBL | Assay Description Inhibition of HIF1 signaling in human U251 cells expressing VEGF by VEGF promoter-driven PLAP reporter gene assay | Bioorg Med Chem 22: 5513-29 (2014) Article DOI: 10.1016/j.bmc.2014.07.020 BindingDB Entry DOI: 10.7270/Q24J0GSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50089823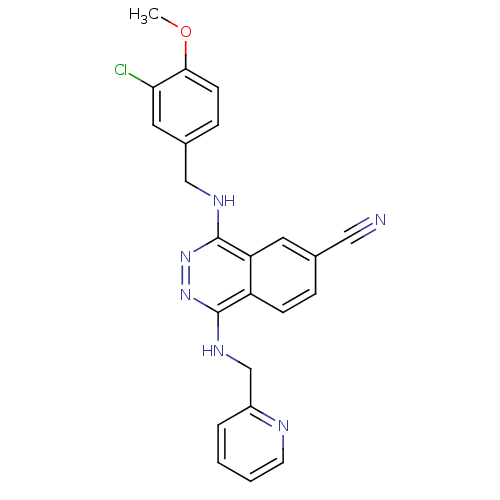 (4-(3-Chloro-4-methoxy-benzylamino)-1-[(pyridin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM307449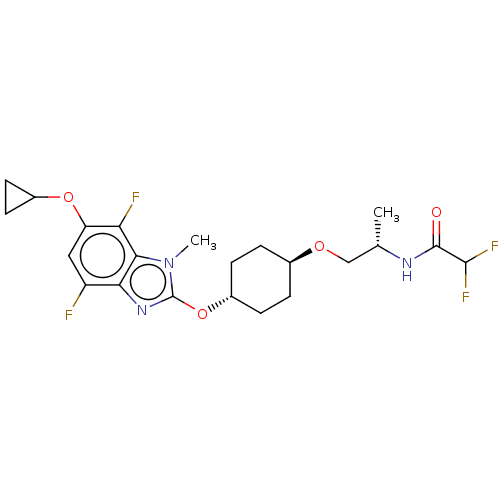 (US10150728, Example I-683) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Recombinant human ACC1 and recombinant human ACC2, which were prepared by the method mentioned above, were preincubated with assay buffer solution (5... | US Patent US10150728 (2018) BindingDB Entry DOI: 10.7270/Q2WD42M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM307497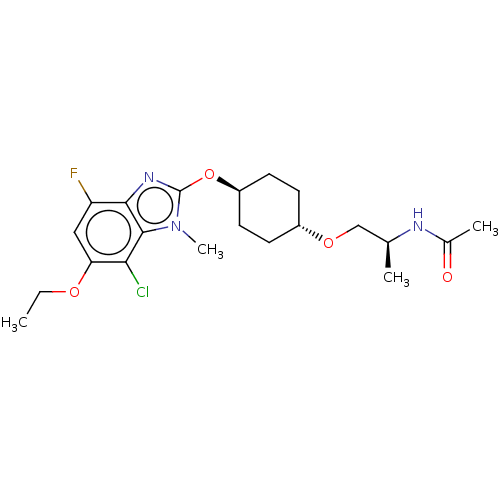 (US10150728, Example I-740) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Recombinant human ACC1 and recombinant human ACC2, which were prepared by the method mentioned above, were preincubated with assay buffer solution (5... | US Patent US10150728 (2018) BindingDB Entry DOI: 10.7270/Q2WD42M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM306944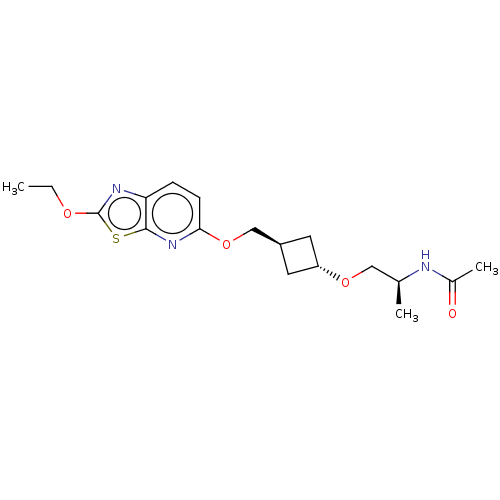 (US10150728, Example I-157) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Recombinant human ACC1 and recombinant human ACC2, which were prepared by the method mentioned above, were preincubated with assay buffer solution (5... | US Patent US10150728 (2018) BindingDB Entry DOI: 10.7270/Q2WD42M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM307475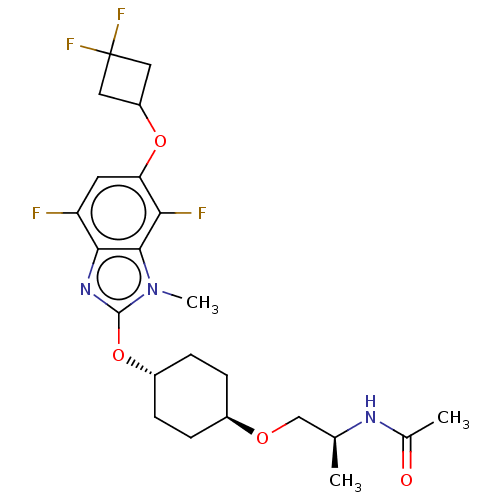 (US10150728, Example I-713) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Recombinant human ACC1 and recombinant human ACC2, which were prepared by the method mentioned above, were preincubated with assay buffer solution (5... | US Patent US10150728 (2018) BindingDB Entry DOI: 10.7270/Q2WD42M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM307463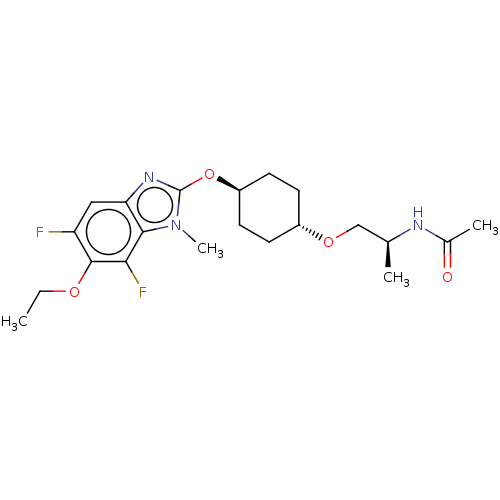 (US10150728, Example I-698) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Recombinant human ACC1 and recombinant human ACC2, which were prepared by the method mentioned above, were preincubated with assay buffer solution (5... | US Patent US10150728 (2018) BindingDB Entry DOI: 10.7270/Q2WD42M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50066450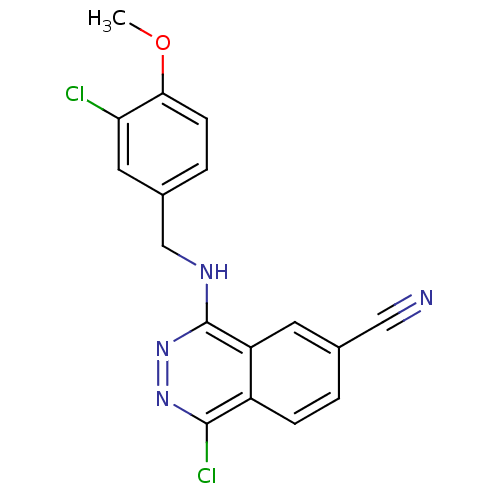 (1-Chloro-4-(3-chloro-4-methoxy-benzylamino)-phthal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM307385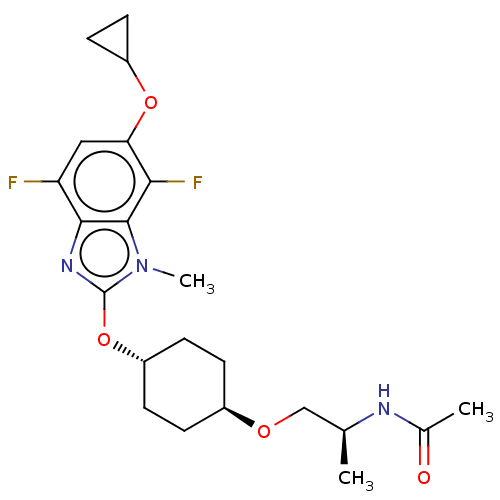 (US10150728, Example I-611) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Recombinant human ACC1 and recombinant human ACC2, which were prepared by the method mentioned above, were preincubated with assay buffer solution (5... | US Patent US10150728 (2018) BindingDB Entry DOI: 10.7270/Q2WD42M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM306965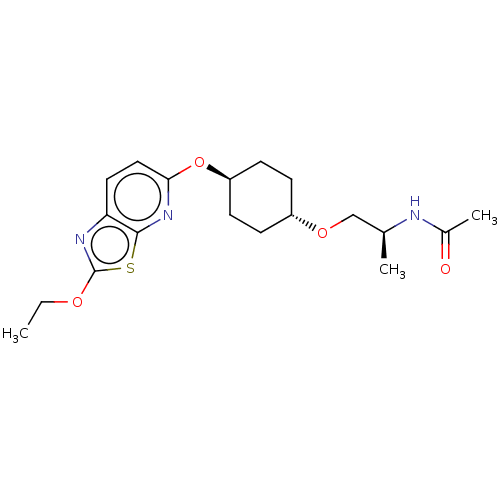 (US10150728, Example I-178) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Recombinant human ACC1 and recombinant human ACC2, which were prepared by the method mentioned above, were preincubated with assay buffer solution (5... | US Patent US10150728 (2018) BindingDB Entry DOI: 10.7270/Q2WD42M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50058760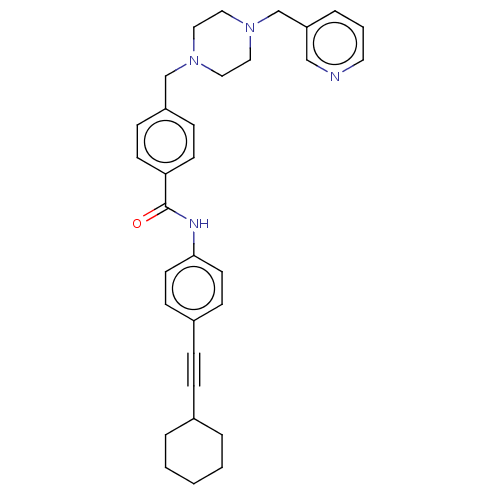 (CHEMBL3335421) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd Curated by ChEMBL | Assay Description Inhibition of HIF1 signaling in human U251 cells expressing VEGF by VEGF promoter-driven PLAP reporter gene assay | Bioorg Med Chem 22: 5513-29 (2014) Article DOI: 10.1016/j.bmc.2014.07.020 BindingDB Entry DOI: 10.7270/Q24J0GSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50089839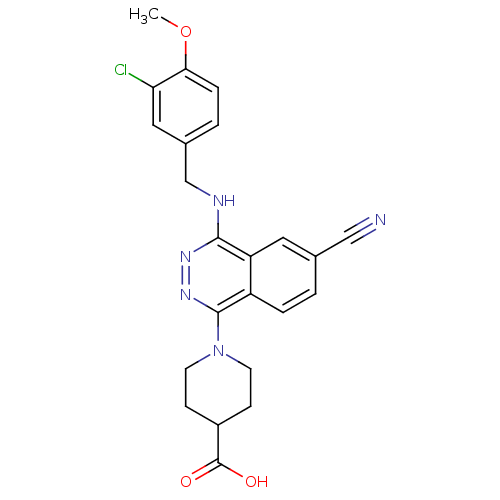 (1-[4-(3-Chloro-4-methoxy-benzylamino)-6-cyano-phth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description 50% inhibitory concentration against phosphodiesterase 5 (PDE5) from porcine platelets | J Med Chem 43: 2523-9 (2000) BindingDB Entry DOI: 10.7270/Q2T72J4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50058766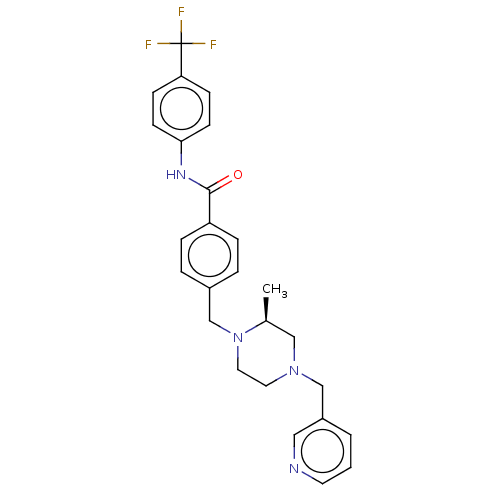 (CHEMBL3335415) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd Curated by ChEMBL | Assay Description Inhibition of HIF1 signaling in human U251 cells expressing VEGF by VEGF promoter-driven PLAP reporter gene assay | Bioorg Med Chem 22: 5513-29 (2014) Article DOI: 10.1016/j.bmc.2014.07.020 BindingDB Entry DOI: 10.7270/Q24J0GSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 930 total ) | Next | Last >> |