

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28681 (5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Tumori"Giovanni Paolo II" Curated by ChEMBL | Assay Description Displacement of [3H]rosiglitazone from N-terminal His-tagged human PPARgamma ligand binding domain expressed in Escherichia coli BL21 DE3 cells by sc... | J Med Chem 55: 37-54 (2012) Article DOI: 10.1021/jm201306q BindingDB Entry DOI: 10.7270/Q23N24JP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28762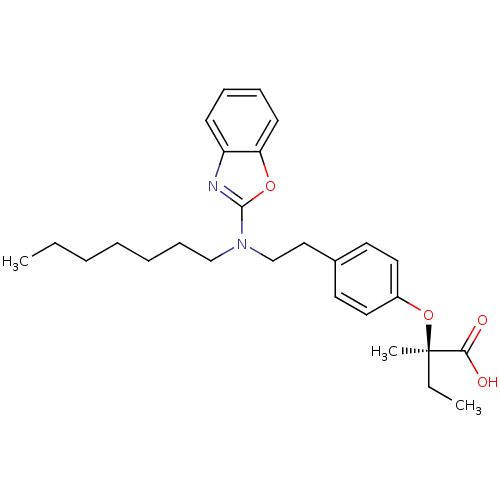 ((2R)-2-(4-{2-[1,3-benzoxazol-2-yl(heptyl)amino]eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Tumori"Giovanni Paolo II" Curated by ChEMBL | Assay Description Displacement of [3H]rosiglitazone from N-terminal His-tagged human PPARgamma ligand binding domain expressed in Escherichia coli BL21 DE3 cells by sc... | J Med Chem 55: 37-54 (2012) Article DOI: 10.1021/jm201306q BindingDB Entry DOI: 10.7270/Q23N24JP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28763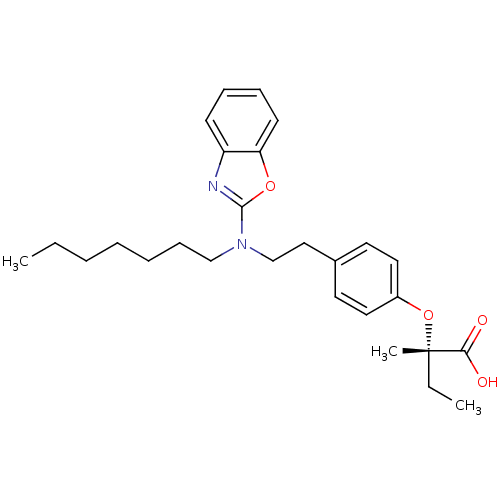 ((2S)-2-(4-{2-[1,3-benzoxazol-2-yl(heptyl)amino]eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 971 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Tumori"Giovanni Paolo II" Curated by ChEMBL | Assay Description Displacement of [3H]rosiglitazone from N-terminal His-tagged human PPARgamma ligand binding domain expressed in Escherichia coli BL21 DE3 cells by sc... | J Med Chem 55: 37-54 (2012) Article DOI: 10.1021/jm201306q BindingDB Entry DOI: 10.7270/Q23N24JP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM27878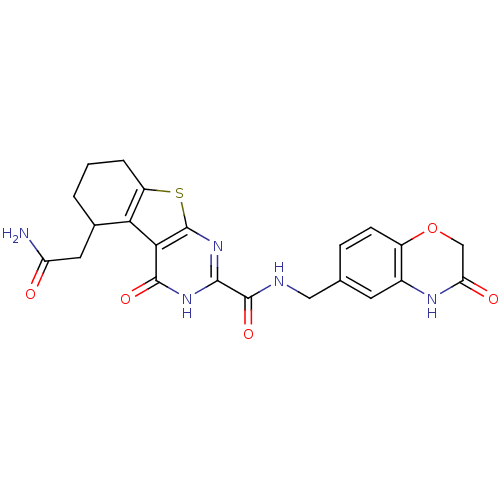 (13-(carbamoylmethyl)-3-oxo-N-[(3-oxo-3,4-dihydro-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Universita degli Studi | Assay Description Inhibition of the matrix metalloproteinases MMPs has been determined by continuously monitoring the hydrolysis of the fluorescent substrate. The hydr... | J Med Chem 52: 1040-9 (2009) Article DOI: 10.1021/jm801166j BindingDB Entry DOI: 10.7270/Q28S4N80 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50030474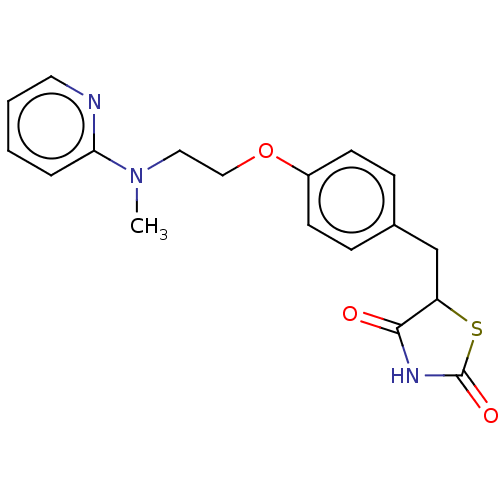 (Avandamet | Avandaryl | Avandia | BRL-49653 | CHEB...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at PPARg-LBD (unknown origin) expressed in HEK293 cells assessed as displacement of SMRT incubated for 16 hrs by luciferase reporter... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01555 BindingDB Entry DOI: 10.7270/Q20V8HGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM27877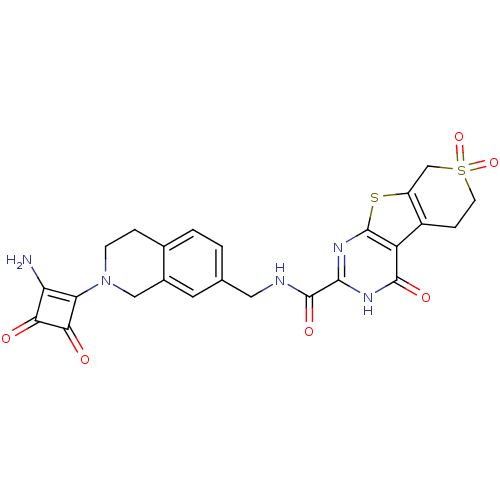 (N-{[2-(2-amino-3,4-dioxocyclobut-1-en-1-yl)-1,2,3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | <25 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Universita degli Studi | Assay Description Inhibition of the matrix metalloproteinases MMPs has been determined by continuously monitoring the hydrolysis of the fluorescent substrate. The hydr... | J Med Chem 52: 1040-9 (2009) Article DOI: 10.1021/jm801166j BindingDB Entry DOI: 10.7270/Q28S4N80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM27878 (13-(carbamoylmethyl)-3-oxo-N-[(3-oxo-3,4-dihydro-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | <25 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Universita degli Studi | Assay Description Inhibition of the matrix metalloproteinases MMPs has been determined by continuously monitoring the hydrolysis of the fluorescent substrate. The hydr... | J Med Chem 52: 1040-9 (2009) Article DOI: 10.1021/jm801166j BindingDB Entry DOI: 10.7270/Q28S4N80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM27877 (N-{[2-(2-amino-3,4-dioxocyclobut-1-en-1-yl)-1,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Universita degli Studi | Assay Description Inhibition of the matrix metalloproteinases MMPs has been determined by continuously monitoring the hydrolysis of the fluorescent substrate. The hydr... | J Med Chem 52: 1040-9 (2009) Article DOI: 10.1021/jm801166j BindingDB Entry DOI: 10.7270/Q28S4N80 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50030474 (Avandamet | Avandaryl | Avandia | BRL-49653 | CHEB...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at PPARg-LBD (unknown origin) expressed in HEK293 cells assessed as displacement of NCoR incubated for 16 hrs by luciferase reporter... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01555 BindingDB Entry DOI: 10.7270/Q20V8HGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM27877 (N-{[2-(2-amino-3,4-dioxocyclobut-1-en-1-yl)-1,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.0 | 22 |
Universita degli Studi | Assay Description Inhibition of the matrix metalloproteinases MMPs has been determined by continuously monitoring the hydrolysis of the fluorescent substrate. The hydr... | J Med Chem 52: 1040-9 (2009) Article DOI: 10.1021/jm801166j BindingDB Entry DOI: 10.7270/Q28S4N80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50554669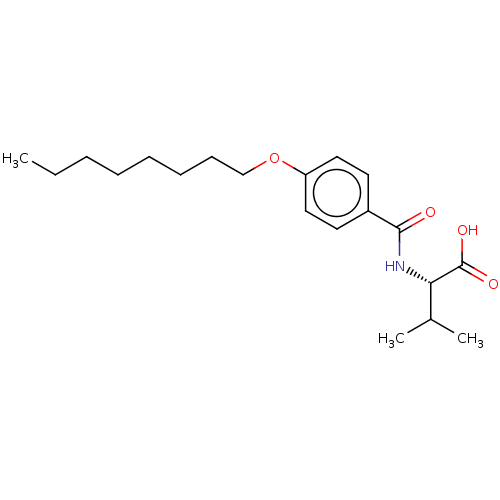 (CHEMBL4743677) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 956 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at PPARg-LBD (unknown origin) expressed in HEK293 cells assessed as displacement of NCoR incubated for 16 hrs by luciferase reporter... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01555 BindingDB Entry DOI: 10.7270/Q20V8HGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50554669 (CHEMBL4743677) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at PPARg-LBD (unknown origin) expressed in HEK293 cells assessed as displacement of SMRT incubated for 16 hrs by luciferase reporter... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01555 BindingDB Entry DOI: 10.7270/Q20V8HGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM27878 (13-(carbamoylmethyl)-3-oxo-N-[(3-oxo-3,4-dihydro-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | 6.0 | 22 |
Universita degli Studi | Assay Description Inhibition of the matrix metalloproteinases MMPs has been determined by continuously monitoring the hydrolysis of the fluorescent substrate. The hydr... | J Med Chem 52: 1040-9 (2009) Article DOI: 10.1021/jm801166j BindingDB Entry DOI: 10.7270/Q28S4N80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM27877 (N-{[2-(2-amino-3,4-dioxocyclobut-1-en-1-yl)-1,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Universita degli Studi | Assay Description Inhibition of the matrix metalloproteinases MMPs has been determined by continuously monitoring the hydrolysis of the fluorescent substrate. The hydr... | J Med Chem 52: 1040-9 (2009) Article DOI: 10.1021/jm801166j BindingDB Entry DOI: 10.7270/Q28S4N80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM27877 (N-{[2-(2-amino-3,4-dioxocyclobut-1-en-1-yl)-1,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Universita degli Studi | Assay Description Inhibition of the matrix metalloproteinases MMPs has been determined by continuously monitoring the hydrolysis of the fluorescent substrate. The hydr... | J Med Chem 52: 1040-9 (2009) Article DOI: 10.1021/jm801166j BindingDB Entry DOI: 10.7270/Q28S4N80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM27878 (13-(carbamoylmethyl)-3-oxo-N-[(3-oxo-3,4-dihydro-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Universita degli Studi | Assay Description Inhibition of the matrix metalloproteinases MMPs has been determined by continuously monitoring the hydrolysis of the fluorescent substrate. The hydr... | J Med Chem 52: 1040-9 (2009) Article DOI: 10.1021/jm801166j BindingDB Entry DOI: 10.7270/Q28S4N80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM27877 (N-{[2-(2-amino-3,4-dioxocyclobut-1-en-1-yl)-1,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Universita degli Studi | Assay Description Inhibition of the matrix metalloproteinases MMPs has been determined by continuously monitoring the hydrolysis of the fluorescent substrate. The hydr... | J Med Chem 52: 1040-9 (2009) Article DOI: 10.1021/jm801166j BindingDB Entry DOI: 10.7270/Q28S4N80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM27878 (13-(carbamoylmethyl)-3-oxo-N-[(3-oxo-3,4-dihydro-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Universita degli Studi | Assay Description Inhibition of the matrix metalloproteinases MMPs has been determined by continuously monitoring the hydrolysis of the fluorescent substrate. The hydr... | J Med Chem 52: 1040-9 (2009) Article DOI: 10.1021/jm801166j BindingDB Entry DOI: 10.7270/Q28S4N80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM27878 (13-(carbamoylmethyl)-3-oxo-N-[(3-oxo-3,4-dihydro-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Universita degli Studi | Assay Description Inhibition of the matrix metalloproteinases MMPs has been determined by continuously monitoring the hydrolysis of the fluorescent substrate. The hydr... | J Med Chem 52: 1040-9 (2009) Article DOI: 10.1021/jm801166j BindingDB Entry DOI: 10.7270/Q28S4N80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM27878 (13-(carbamoylmethyl)-3-oxo-N-[(3-oxo-3,4-dihydro-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Universita degli Studi | Assay Description Inhibition of the matrix metalloproteinases MMPs has been determined by continuously monitoring the hydrolysis of the fluorescent substrate. The hydr... | J Med Chem 52: 1040-9 (2009) Article DOI: 10.1021/jm801166j BindingDB Entry DOI: 10.7270/Q28S4N80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM27877 (N-{[2-(2-amino-3,4-dioxocyclobut-1-en-1-yl)-1,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Universita degli Studi | Assay Description Inhibition of the matrix metalloproteinases MMPs has been determined by continuously monitoring the hydrolysis of the fluorescent substrate. The hydr... | J Med Chem 52: 1040-9 (2009) Article DOI: 10.1021/jm801166j BindingDB Entry DOI: 10.7270/Q28S4N80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM27878 (13-(carbamoylmethyl)-3-oxo-N-[(3-oxo-3,4-dihydro-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Universita degli Studi | Assay Description Inhibition of the matrix metalloproteinases MMPs has been determined by continuously monitoring the hydrolysis of the fluorescent substrate. The hydr... | J Med Chem 52: 1040-9 (2009) Article DOI: 10.1021/jm801166j BindingDB Entry DOI: 10.7270/Q28S4N80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM27878 (13-(carbamoylmethyl)-3-oxo-N-[(3-oxo-3,4-dihydro-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Universita degli Studi | Assay Description Inhibition of the matrix metalloproteinases MMPs has been determined by continuously monitoring the hydrolysis of the fluorescent substrate. The hydr... | J Med Chem 52: 1040-9 (2009) Article DOI: 10.1021/jm801166j BindingDB Entry DOI: 10.7270/Q28S4N80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM27877 (N-{[2-(2-amino-3,4-dioxocyclobut-1-en-1-yl)-1,2,3,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Universita degli Studi | Assay Description Inhibition of the matrix metalloproteinases MMPs has been determined by continuously monitoring the hydrolysis of the fluorescent substrate. The hydr... | J Med Chem 52: 1040-9 (2009) Article DOI: 10.1021/jm801166j BindingDB Entry DOI: 10.7270/Q28S4N80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM27877 (N-{[2-(2-amino-3,4-dioxocyclobut-1-en-1-yl)-1,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Universita degli Studi | Assay Description Inhibition of the matrix metalloproteinases MMPs has been determined by continuously monitoring the hydrolysis of the fluorescent substrate. The hydr... | J Med Chem 52: 1040-9 (2009) Article DOI: 10.1021/jm801166j BindingDB Entry DOI: 10.7270/Q28S4N80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28759 ((2S)-3-phenyl-2-(4-phenylphenoxy)propanoic acid | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | 480 | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche | Assay Description The HepG2 cells were co-transfected with DNA construct containing PPAR-Gal4 chimeric receptor and Gal4-luciferase reporter. The luciferase activity w... | J Med Chem 51: 7768-76 (2008) Article DOI: 10.1021/jm800733h BindingDB Entry DOI: 10.7270/Q2CC0Z0D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28760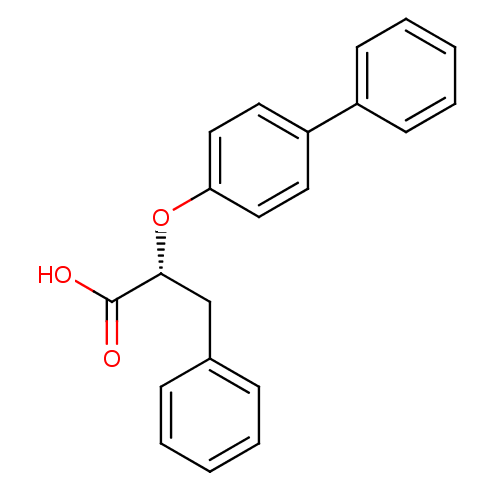 ((2R)-3-phenyl-2-(4-phenylphenoxy)propanoic acid | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | n/a | n/a | n/a | n/a | 5.93E+3 | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche | Assay Description The HepG2 cells were co-transfected with DNA construct containing PPAR-Gal4 chimeric receptor and Gal4-luciferase reporter. The luciferase activity w... | J Med Chem 51: 7768-76 (2008) Article DOI: 10.1021/jm800733h BindingDB Entry DOI: 10.7270/Q2CC0Z0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28681 (5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche | Assay Description The HepG2 cells were co-transfected with DNA construct containing PPAR-Gal4 chimeric receptor and Gal4-luciferase reporter. The luciferase activity w... | J Med Chem 51: 7768-76 (2008) Article DOI: 10.1021/jm800733h BindingDB Entry DOI: 10.7270/Q2CC0Z0D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM28759 ((2S)-3-phenyl-2-(4-phenylphenoxy)propanoic acid | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche | Assay Description The HepG2 cells were co-transfected with DNA construct containing PPAR-Gal4 chimeric receptor and Gal4-luciferase reporter. The luciferase activity w... | J Med Chem 51: 7768-76 (2008) Article DOI: 10.1021/jm800733h BindingDB Entry DOI: 10.7270/Q2CC0Z0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM24566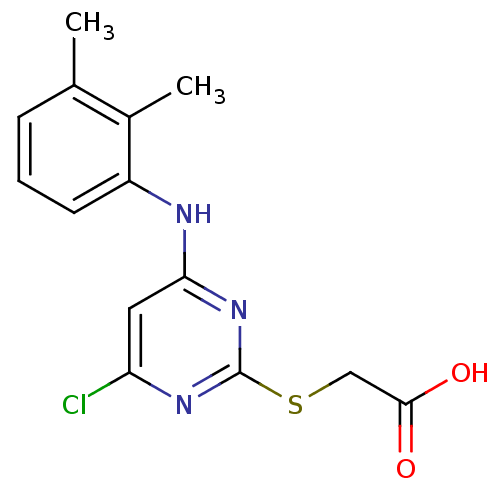 (2-({4-chloro-6-[(2,3-dimethylphenyl)amino]pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche | Assay Description The HepG2 cells were co-transfected with DNA construct containing PPAR-Gal4 chimeric receptor and Gal4-luciferase reporter. The luciferase activity w... | J Med Chem 51: 7768-76 (2008) Article DOI: 10.1021/jm800733h BindingDB Entry DOI: 10.7270/Q2CC0Z0D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Mus musculus) | BDBM28759 ((2S)-3-phenyl-2-(4-phenylphenoxy)propanoic acid | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche | Assay Description The HepG2 cells were co-transfected with DNA construct containing PPAR-Gal4 chimeric receptor and Gal4-luciferase reporter. The luciferase activity w... | J Med Chem 51: 7768-76 (2008) Article DOI: 10.1021/jm800733h BindingDB Entry DOI: 10.7270/Q2CC0Z0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Mus musculus) | BDBM24566 (2-({4-chloro-6-[(2,3-dimethylphenyl)amino]pyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche | Assay Description The HepG2 cells were co-transfected with DNA construct containing PPAR-Gal4 chimeric receptor and Gal4-luciferase reporter. The luciferase activity w... | J Med Chem 51: 7768-76 (2008) Article DOI: 10.1021/jm800733h BindingDB Entry DOI: 10.7270/Q2CC0Z0D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM28760 ((2R)-3-phenyl-2-(4-phenylphenoxy)propanoic acid | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche | Assay Description The HepG2 cells were co-transfected with DNA construct containing PPAR-Gal4 chimeric receptor and Gal4-luciferase reporter. The luciferase activity w... | J Med Chem 51: 7768-76 (2008) Article DOI: 10.1021/jm800733h BindingDB Entry DOI: 10.7270/Q2CC0Z0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Mus musculus) | BDBM28760 ((2R)-3-phenyl-2-(4-phenylphenoxy)propanoic acid | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche | Assay Description The HepG2 cells were co-transfected with DNA construct containing PPAR-Gal4 chimeric receptor and Gal4-luciferase reporter. The luciferase activity w... | J Med Chem 51: 7768-76 (2008) Article DOI: 10.1021/jm800733h BindingDB Entry DOI: 10.7270/Q2CC0Z0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50299112 ((2S)-2-(4-phenethylphenoxy)-3-phenyl-propanoic aci...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | 860 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Curated by ChEMBL | Assay Description Agonist activity at GAL4-tagged human PPARgamma ligand binding domain expressed in human HepG2 cells assessed as receptor transactivation by lucifera... | J Med Chem 52: 6382-93 (2009) Article DOI: 10.1021/jm900941b BindingDB Entry DOI: 10.7270/Q2862GHF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50299109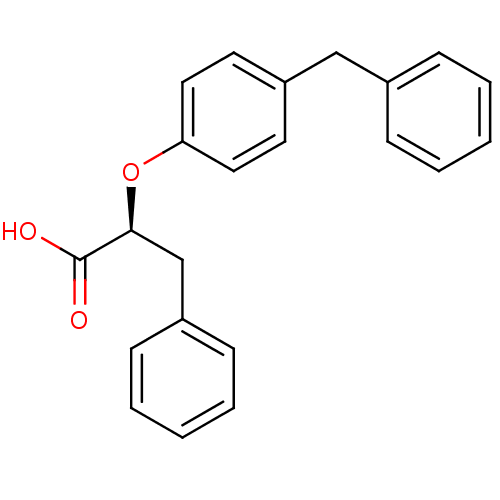 ((2S)-2-(4-benzylphenoxy)-3-phenylpropanoic acid | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | 580 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Curated by ChEMBL | Assay Description Agonist activity at GAL4-tagged human PPARgamma ligand binding domain expressed in human HepG2 cells assessed as receptor transactivation by lucifera... | J Med Chem 52: 6382-93 (2009) Article DOI: 10.1021/jm900941b BindingDB Entry DOI: 10.7270/Q2862GHF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50299109 ((2S)-2-(4-benzylphenoxy)-3-phenylpropanoic acid | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Curated by ChEMBL | Assay Description Agonist activity at GAL4-tagged human PPARalpha ligand binding domain expressed in human HepG2 cells assessed as receptor transactivation by lucifera... | J Med Chem 52: 6382-93 (2009) Article DOI: 10.1021/jm900941b BindingDB Entry DOI: 10.7270/Q2862GHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50299112 ((2S)-2-(4-phenethylphenoxy)-3-phenyl-propanoic aci...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Curated by ChEMBL | Assay Description Agonist activity at GAL4-tagged human PPARalpha ligand binding domain expressed in human HepG2 cells assessed as receptor transactivation by lucifera... | J Med Chem 52: 6382-93 (2009) Article DOI: 10.1021/jm900941b BindingDB Entry DOI: 10.7270/Q2862GHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50299109 ((2S)-2-(4-benzylphenoxy)-3-phenylpropanoic acid | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Curated by ChEMBL | Assay Description Agonist activity at GAL4-tagged human PPARalpha ligand binding domain expressed in human HepG2 cells assessed as receptor transactivation by lucifera... | J Med Chem 52: 6382-93 (2009) Article DOI: 10.1021/jm900941b BindingDB Entry DOI: 10.7270/Q2862GHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28681 (5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 39 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Curated by ChEMBL | Assay Description Agonist activity at GAL4-tagged human PPARgamma ligand binding domain expressed in human HepG2 cells assessed as receptor transactivation by lucifera... | J Med Chem 52: 6382-93 (2009) Article DOI: 10.1021/jm900941b BindingDB Entry DOI: 10.7270/Q2862GHF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50299110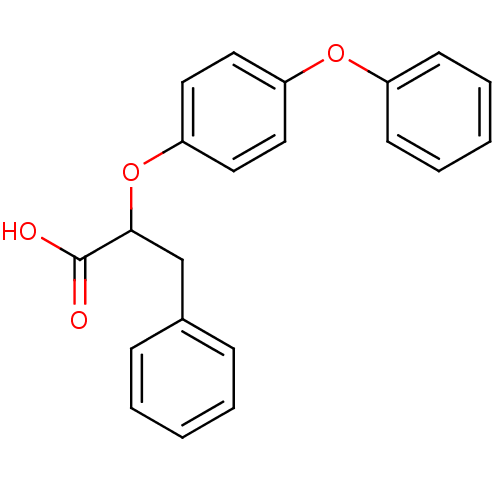 (CHEMBL578860 | rac-2-(4-Phenoxy-phenoxy)-3-phenyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Curated by ChEMBL | Assay Description Agonist activity at GAL4-tagged human PPARalpha ligand binding domain expressed in human HepG2 cells assessed as receptor transactivation by lucifera... | J Med Chem 52: 6382-93 (2009) Article DOI: 10.1021/jm900941b BindingDB Entry DOI: 10.7270/Q2862GHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50299111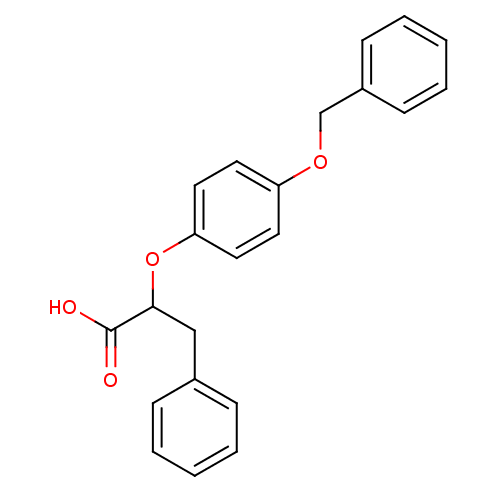 (CHEMBL572565 | rac-2-(4-Benzyloxy-phenoxy)-3-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 107 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Curated by ChEMBL | Assay Description Agonist activity at GAL4-tagged human PPARalpha ligand binding domain expressed in human HepG2 cells assessed as receptor transactivation by lucifera... | J Med Chem 52: 6382-93 (2009) Article DOI: 10.1021/jm900941b BindingDB Entry DOI: 10.7270/Q2862GHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50299113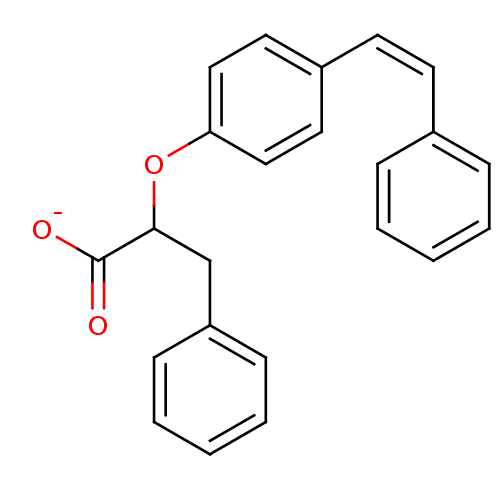 (CHEMBL572795 | rac-2-(4-cis-Styryl-phenoxy)-3-phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Curated by ChEMBL | Assay Description Agonist activity at GAL4-tagged human PPARalpha ligand binding domain expressed in human HepG2 cells assessed as receptor transactivation by lucifera... | J Med Chem 52: 6382-93 (2009) Article DOI: 10.1021/jm900941b BindingDB Entry DOI: 10.7270/Q2862GHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM28759 ((2S)-3-phenyl-2-(4-phenylphenoxy)propanoic acid | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 190 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Curated by ChEMBL | Assay Description Agonist activity at GAL4-tagged human PPARalpha ligand binding domain expressed in human HepG2 cells assessed as receptor transactivation by lucifera... | J Med Chem 52: 6382-93 (2009) Article DOI: 10.1021/jm900941b BindingDB Entry DOI: 10.7270/Q2862GHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50299114 (CHEMBL573015 | rac-2-(4-Phenylthiomethyl-phenoxy)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 310 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Curated by ChEMBL | Assay Description Agonist activity at GAL4-tagged human PPARalpha ligand binding domain expressed in human HepG2 cells assessed as receptor transactivation by lucifera... | J Med Chem 52: 6382-93 (2009) Article DOI: 10.1021/jm900941b BindingDB Entry DOI: 10.7270/Q2862GHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50299112 ((2S)-2-(4-phenethylphenoxy)-3-phenyl-propanoic aci...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Curated by ChEMBL | Assay Description Agonist activity at GAL4-tagged human PPARalpha ligand binding domain expressed in human HepG2 cells assessed as receptor transactivation by lucifera... | J Med Chem 52: 6382-93 (2009) Article DOI: 10.1021/jm900941b BindingDB Entry DOI: 10.7270/Q2862GHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50299112 ((2S)-2-(4-phenethylphenoxy)-3-phenyl-propanoic aci...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Curated by ChEMBL | Assay Description Agonist activity at GAL4-tagged human PPARgamma ligand binding domain expressed in human HepG2 cells assessed as receptor transactivation by lucifera... | J Med Chem 52: 6382-93 (2009) Article DOI: 10.1021/jm900941b BindingDB Entry DOI: 10.7270/Q2862GHF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50299114 (CHEMBL573015 | rac-2-(4-Phenylthiomethyl-phenoxy)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Curated by ChEMBL | Assay Description Agonist activity at GAL4-tagged human PPARgamma ligand binding domain expressed in human HepG2 cells assessed as receptor transactivation by lucifera... | J Med Chem 52: 6382-93 (2009) Article DOI: 10.1021/jm900941b BindingDB Entry DOI: 10.7270/Q2862GHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50299115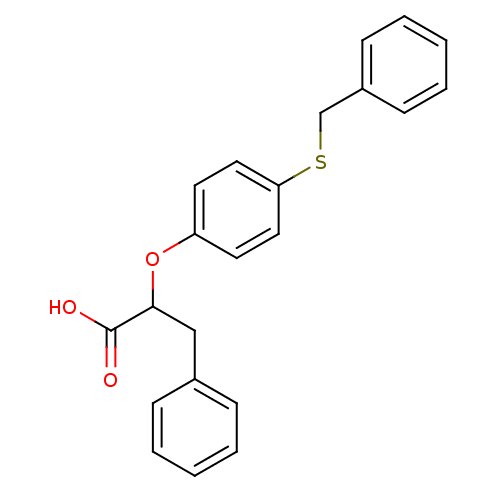 (CHEMBL573252 | rac-2-(4-Benzylthio-phenoxy)-3-phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 488 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Curated by ChEMBL | Assay Description Agonist activity at GAL4-tagged human PPARalpha ligand binding domain expressed in human HepG2 cells assessed as receptor transactivation by lucifera... | J Med Chem 52: 6382-93 (2009) Article DOI: 10.1021/jm900941b BindingDB Entry DOI: 10.7270/Q2862GHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50299116 (CHEMBL574606 | rac-2-(4-Phenylethynyl-phenoxy)-3-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 540 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Curated by ChEMBL | Assay Description Agonist activity at GAL4-tagged human PPARgamma ligand binding domain expressed in human HepG2 cells assessed as receptor transactivation by lucifera... | J Med Chem 52: 6382-93 (2009) Article DOI: 10.1021/jm900941b BindingDB Entry DOI: 10.7270/Q2862GHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 215 total ) | Next | Last >> |