

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuraminidase (Influenza A virus (A/Tokyo/3/67(H2N2))) | BDBM5202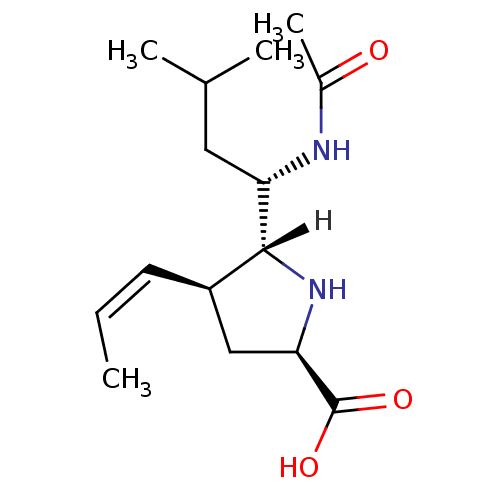 ((+/-)-(2R,4S,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Memphis/3/93)) | BDBM5202 ((+/-)-(2R,4S,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Tokyo/3/67(H2N2))) | BDBM5200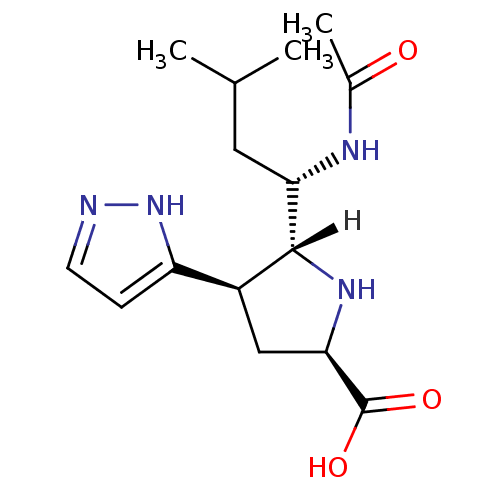 ((+/-)-(2R,4R,5R,1 S)-5-(1 -Acetylamino-3 -methylbu...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 45 | -41.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Memphis/3/93)) | BDBM5200 ((+/-)-(2R,4R,5R,1 S)-5-(1 -Acetylamino-3 -methylbu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 57 | -40.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Tokyo/3/67(H2N2))) | BDBM5207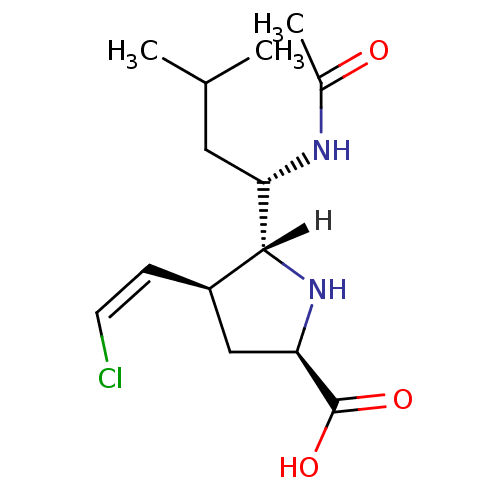 ((+/-)-(2R,4S,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Memphis/3/93)) | BDBM5207 ((+/-)-(2R,4S,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Tokyo/3/67(H2N2))) | BDBM5202 ((+/-)-(2R,4S,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Memphis/3/93)) | BDBM5195 ((+/-)-(2R,4R,5S,1 S)-5-(1 -Acetylamino-3 -methylbu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 150 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Memphis/3/93)) | BDBM5202 ((+/-)-(2R,4S,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Memphis/3/93)) | BDBM5201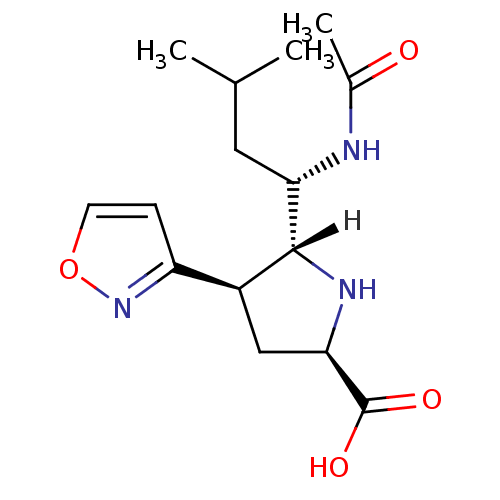 ((2R,4R,5R)-5-[(1S)-1-acetamido-3-methylbutyl]-4-(1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Tokyo/3/67(H2N2))) | BDBM5195 ((+/-)-(2R,4R,5S,1 S)-5-(1 -Acetylamino-3 -methylbu...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 260 | -37.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Tokyo/3/67(H2N2))) | BDBM5201 ((2R,4R,5R)-5-[(1S)-1-acetamido-3-methylbutyl]-4-(1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Tokyo/3/67(H2N2))) | BDBM5203 ((+/-)-(2R,4S,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Tokyo/3/67(H2N2))) | BDBM5198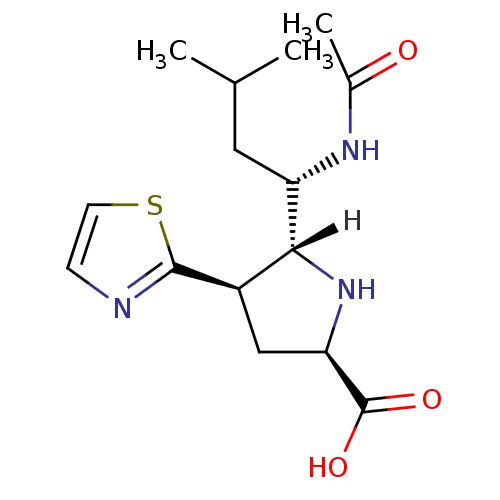 ((+/-)-(2R,4R,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 310 | -36.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Memphis/3/93)) | BDBM5205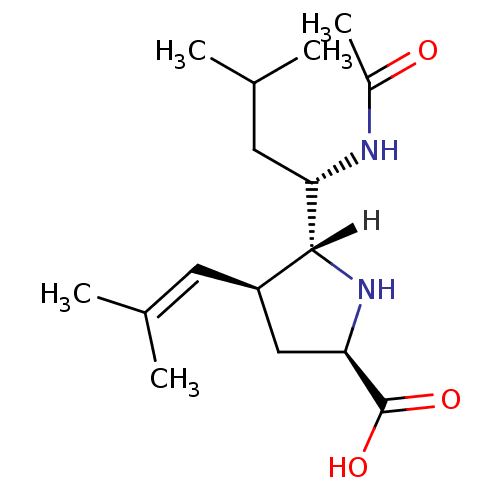 ((+/-)-(2R,4S,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Tokyo/3/67(H2N2))) | BDBM5197 ((+/-)-(2R,4R,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 350 | -36.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Tokyo/3/67(H2N2))) | BDBM5199 ((+/-)-(2R,4R,5R,1 S)-5-(1 -Acetylamino-3 -methylbu...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 350 | -36.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Memphis/3/93)) | BDBM5198 ((+/-)-(2R,4R,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 370 | -36.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Tokyo/3/67(H2N2))) | BDBM5205 ((+/-)-(2R,4S,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Tokyo/3/67(H2N2))) | BDBM5206 ((+/-)-(2R,4S,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Memphis/3/93)) | BDBM5203 ((+/-)-(2R,4S,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Memphis/3/93)) | BDBM5197 ((+/-)-(2R,4R,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 490 | -35.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Memphis/3/93)) | BDBM5196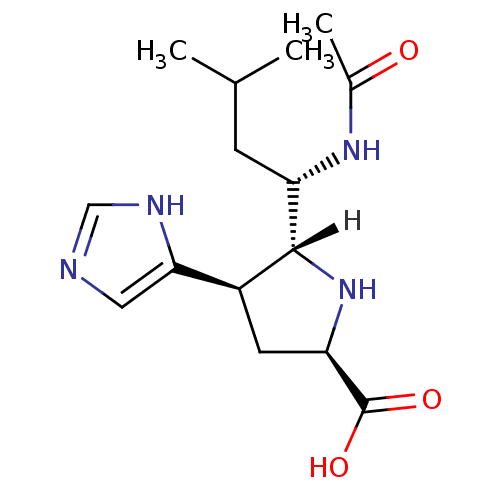 ((+/-)-(2R,4R,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 610 | -35.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Tokyo/3/67(H2N2))) | BDBM5196 ((+/-)-(2R,4R,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 610 | -35.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Memphis/3/93)) | BDBM5206 ((+/-)-(2R,4S,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Memphis/3/93)) | BDBM5199 ((+/-)-(2R,4R,5R,1 S)-5-(1 -Acetylamino-3 -methylbu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 830 | -34.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Memphis/3/93)) | BDBM5194 ((+/-)-(2R,4R,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.56E+3 | -32.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Tokyo/3/67(H2N2))) | BDBM5207 ((+/-)-(2R,4S,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Memphis/3/93)) | BDBM5207 ((+/-)-(2R,4S,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Memphis/3/93)) | BDBM5192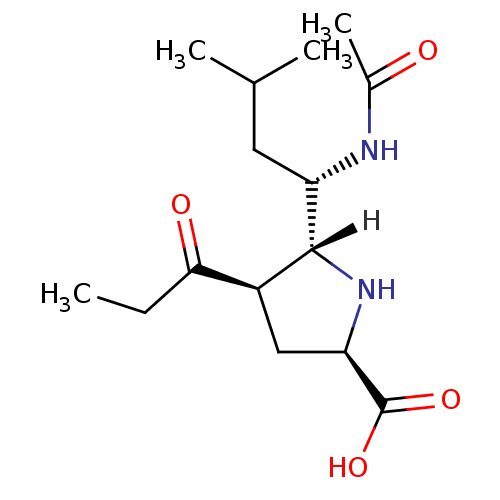 ((+/-)-(2R,4R,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.67E+3 | -29.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Memphis/3/93)) | BDBM5190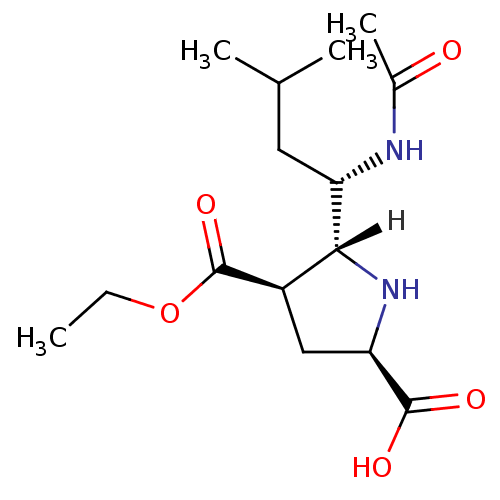 ((+/-)-(2R,4R,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.24E+4 | -27.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Memphis/3/93)) | BDBM5191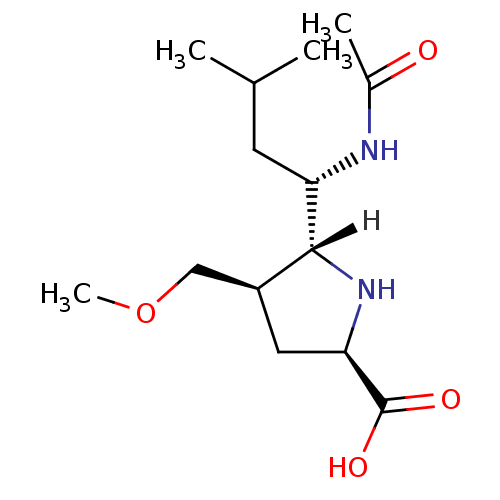 ((+/-)-(2R,4R,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.51E+4 | -27.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Memphis/3/93)) | BDBM5193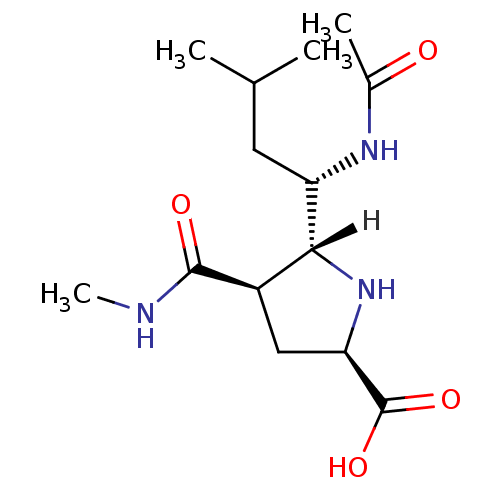 ((+/-)-(2R,4R,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >4.00E+4 | >-24.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50389975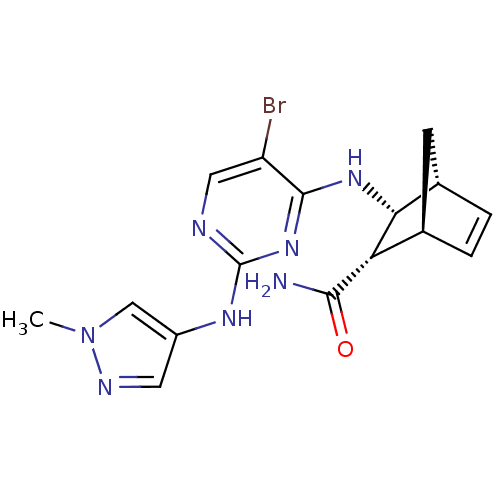 (CHEMBL2071201) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha by TR-FRET analysis | Bioorg Med Chem Lett 22: 4750-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.067 BindingDB Entry DOI: 10.7270/Q20C4WTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50389975 (CHEMBL2071201) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of Flt3 by TR-FRET analysis | Bioorg Med Chem Lett 22: 4750-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.067 BindingDB Entry DOI: 10.7270/Q20C4WTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50389991 (CHEMBL2071271) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of KDR by HTRF analysis in presence of 1 mM ATP | Bioorg Med Chem Lett 22: 4750-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.067 BindingDB Entry DOI: 10.7270/Q20C4WTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50389995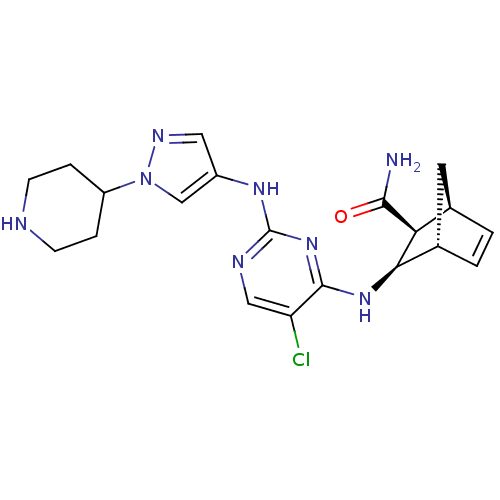 (CHEMBL2071275) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of KDR by HTRF analysis in presence of 1 mM ATP | Bioorg Med Chem Lett 22: 4750-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.067 BindingDB Entry DOI: 10.7270/Q20C4WTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM50389975 (CHEMBL2071201) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of FGFR2 by TR-FRET analysis | Bioorg Med Chem Lett 22: 4750-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.067 BindingDB Entry DOI: 10.7270/Q20C4WTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50381716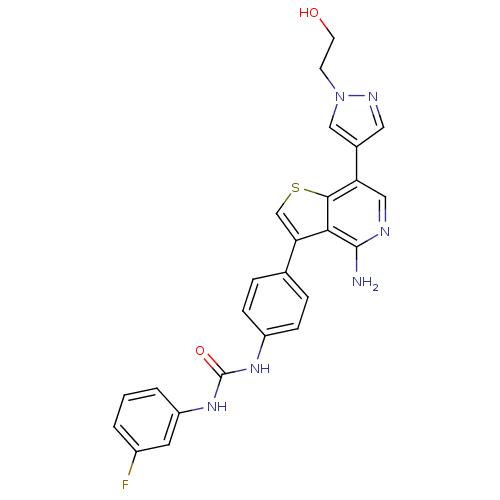 (ABT-348 | ILORASERTIB | US8722890, 1 | US8722890, ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of KDR by HTRF analysis in presence of 1 mM ATP | Bioorg Med Chem Lett 22: 4750-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.067 BindingDB Entry DOI: 10.7270/Q20C4WTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50389998 (CHEMBL2071276) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of KDR by HTRF analysis in presence of 1 mM ATP | Bioorg Med Chem Lett 22: 4750-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.067 BindingDB Entry DOI: 10.7270/Q20C4WTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50389988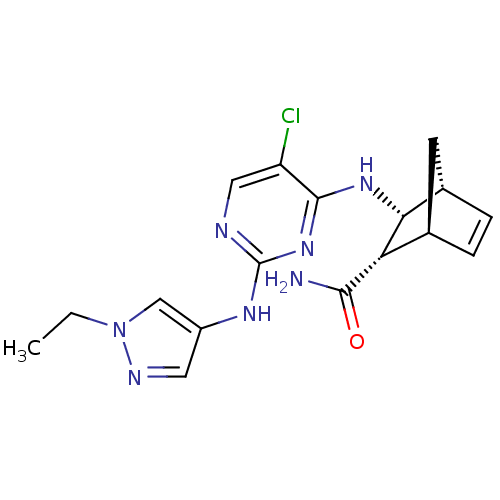 (CHEMBL2071216) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of KDR by HTRF analysis in presence of 1 mM ATP | Bioorg Med Chem Lett 22: 4750-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.067 BindingDB Entry DOI: 10.7270/Q20C4WTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50381716 (ABT-348 | ILORASERTIB | US8722890, 1 | US8722890, ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human KDR autophosphorylation expressed in mouse NIH/3T3 cells | Bioorg Med Chem Lett 22: 4750-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.067 BindingDB Entry DOI: 10.7270/Q20C4WTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50389975 (CHEMBL2071201) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of Aurora A by TR-FRET analysis | Bioorg Med Chem Lett 22: 4750-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.067 BindingDB Entry DOI: 10.7270/Q20C4WTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50389993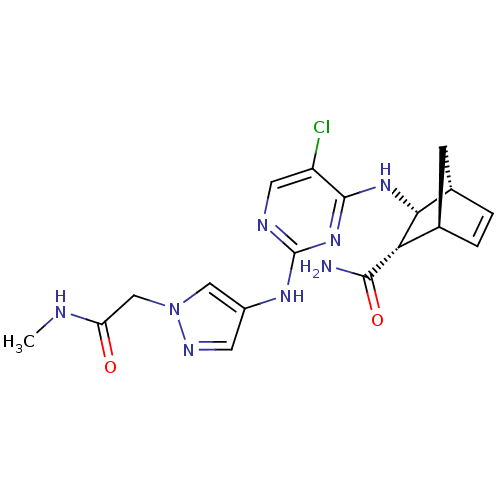 (CHEMBL2071273) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of KDR by HTRF analysis in presence of 1 mM ATP | Bioorg Med Chem Lett 22: 4750-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.067 BindingDB Entry DOI: 10.7270/Q20C4WTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50389990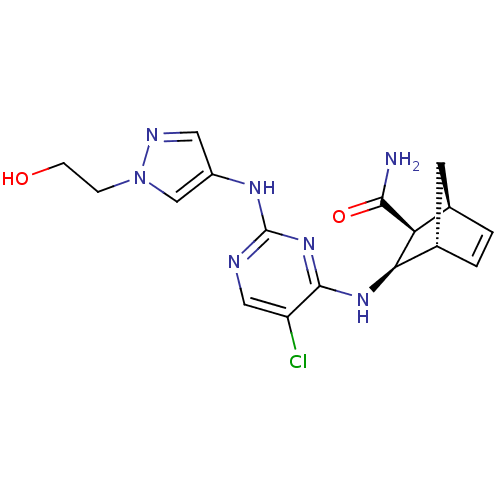 (CHEMBL2071270) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of KDR by HTRF analysis in presence of 1 mM ATP | Bioorg Med Chem Lett 22: 4750-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.067 BindingDB Entry DOI: 10.7270/Q20C4WTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50389975 (CHEMBL2071201) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of KDR by TR-FRET analysis | Bioorg Med Chem Lett 22: 4750-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.067 BindingDB Entry DOI: 10.7270/Q20C4WTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50381716 (ABT-348 | ILORASERTIB | US8722890, 1 | US8722890, ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of Aurora B kinase by HTRF analysis in presence of 1 mM ATP | Bioorg Med Chem Lett 22: 4750-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.067 BindingDB Entry DOI: 10.7270/Q20C4WTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50389975 (CHEMBL2071201) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck by TR-FRET analysis | Bioorg Med Chem Lett 22: 4750-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.067 BindingDB Entry DOI: 10.7270/Q20C4WTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50389989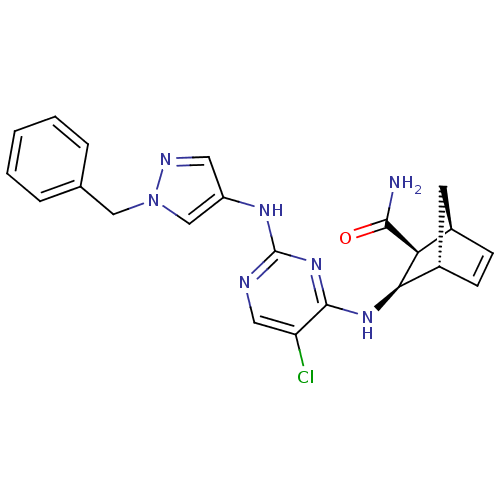 (CHEMBL2071217) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of KDR by HTRF analysis in presence of 1 mM ATP | Bioorg Med Chem Lett 22: 4750-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.067 BindingDB Entry DOI: 10.7270/Q20C4WTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50389988 (CHEMBL2071216) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human KDR autophosphorylation expressed in mouse NIH/3T3 cells | Bioorg Med Chem Lett 22: 4750-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.067 BindingDB Entry DOI: 10.7270/Q20C4WTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 133 total ) | Next | Last >> |