 Found 319 hits with Last Name = 'morandi' and Initial = 'f'
Found 319 hits with Last Name = 'morandi' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-lactamase TEM
(Escherichia coli) | BDBM50225373
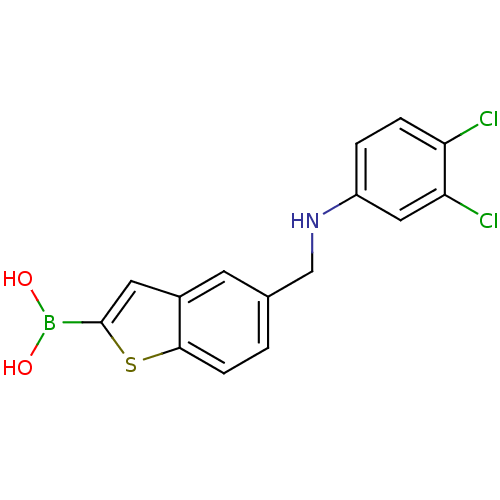
(CHEMBL396872 | pinacol 5-[(3,4-dichlorophenylamino...)Show InChI InChI=1S/C15H12BCl2NO2S/c17-12-3-2-11(7-13(12)18)19-8-9-1-4-14-10(5-9)6-15(22-14)16(20)21/h1-7,19-21H,8H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM26139
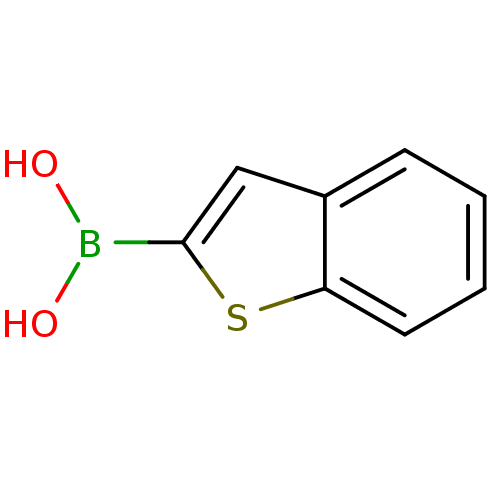
(1-benzothiophen-2-ylboranediol | 1-benzothiophen-2...)Show InChI InChI=1S/C8H7BO2S/c10-9(11)8-5-6-3-1-2-4-7(6)12-8/h1-5,10-11H | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50225393
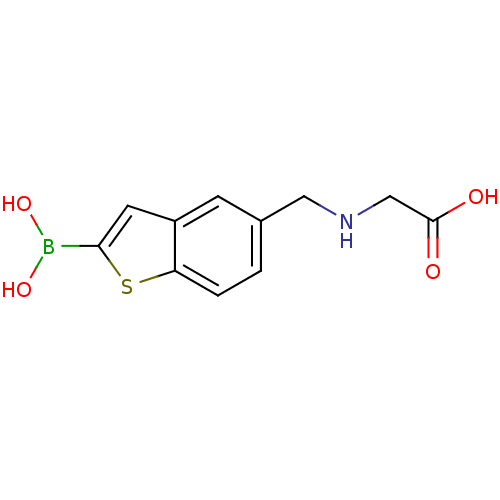
(CHEMBL235526 | pinacol 5-[(carboxymethylamino)meth...)Show InChI InChI=1S/C11H12BNO4S/c14-11(15)6-13-5-7-1-2-9-8(3-7)4-10(18-9)12(16)17/h1-4,13,16-17H,5-6H2,(H,14,15) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50225381
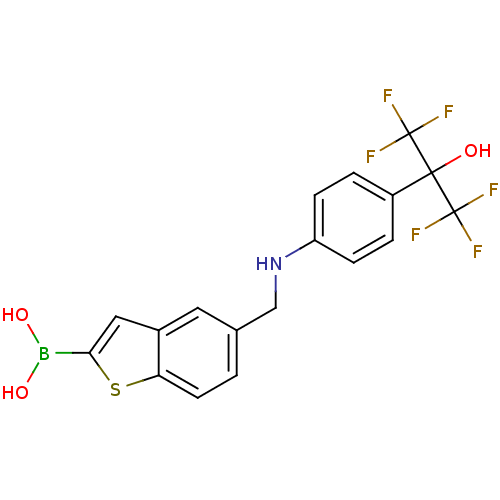
(CHEMBL235293 | pinacol5-{[4-(1-hydroxy-2,2,2-trifl...)Show SMILES OB(O)c1cc2cc(CNc3ccc(cc3)C(O)(C(F)(F)F)C(F)(F)F)ccc2s1 Show InChI InChI=1S/C18H14BF6NO3S/c20-17(21,22)16(27,18(23,24)25)12-2-4-13(5-3-12)26-9-10-1-6-14-11(7-10)8-15(30-14)19(28)29/h1-8,26-29H,9H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50225371
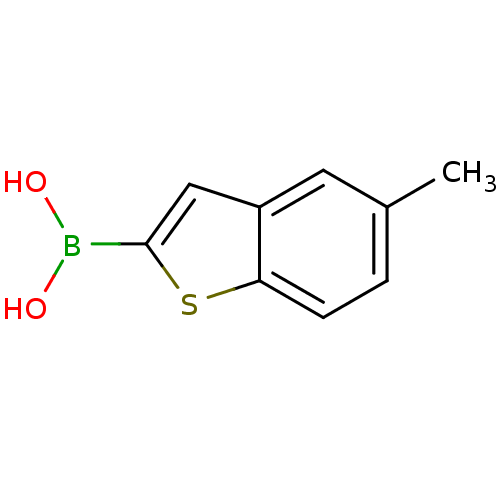
(5-methyl-benzo[b]thiophen-2-ylboronic acid | CHEMB...)Show InChI InChI=1S/C9H9BO2S/c1-6-2-3-8-7(4-6)5-9(13-8)10(11)12/h2-5,11-12H,1H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50225376
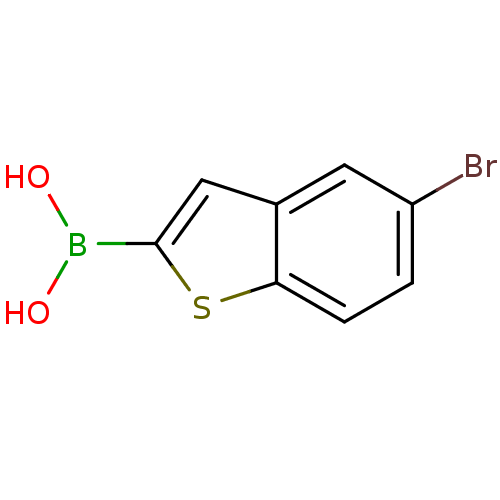
(CHEMBL397522 | pinacol 5-bromomethylbenzo[b]thioph...)Show InChI InChI=1S/C8H6BBrO2S/c10-6-1-2-7-5(3-6)4-8(13-7)9(11)12/h1-4,11-12H | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50225379
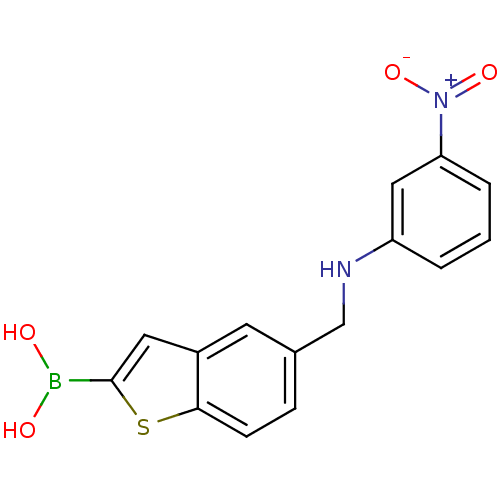
(CHEMBL235292 | pinacol 5-[(3-nitrophenylamino)meth...)Show SMILES OB(O)c1cc2cc(CNc3cccc(c3)[N+]([O-])=O)ccc2s1 Show InChI InChI=1S/C15H13BN2O4S/c19-16(20)15-7-11-6-10(4-5-14(11)23-15)9-17-12-2-1-3-13(8-12)18(21)22/h1-8,17,19-20H,9H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50225378
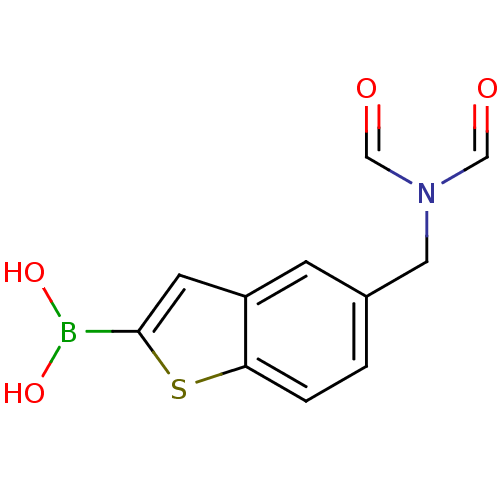
(CHEMBL235073 | pinacol 5-diformylaminomethylbenzo[...)Show InChI InChI=1S/C11H10BNO4S/c14-6-13(7-15)5-8-1-2-10-9(3-8)4-11(18-10)12(16)17/h1-4,6-7,16-17H,5H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50225392
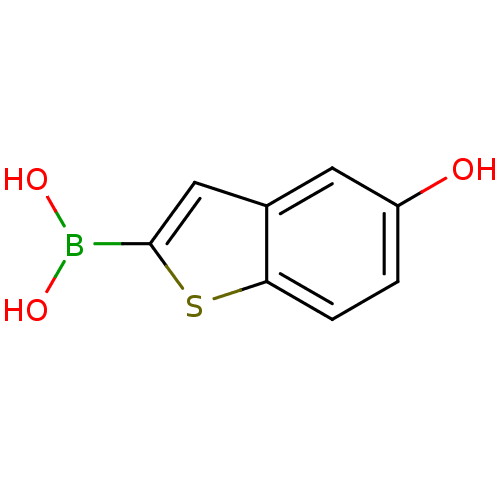
(5-hydroxymethylbenzo[b]thiophen-2-ylboronic acid |...)Show InChI InChI=1S/C8H7BO3S/c10-6-1-2-7-5(3-6)4-8(13-7)9(11)12/h1-4,10-12H | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50225380
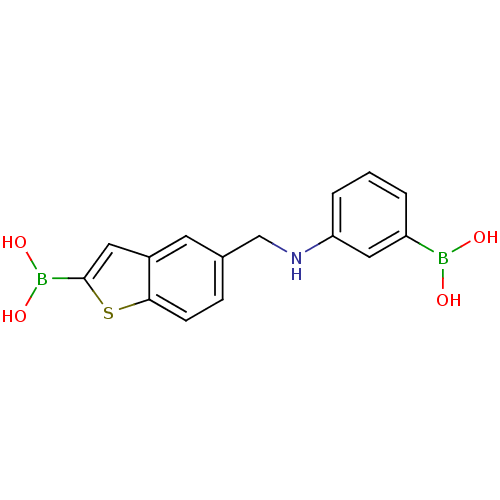
(CHEMBL235308 | pinacol 5-[(phenylamino3-boronicaci...)Show InChI InChI=1S/C15H15B2NO4S/c19-16(20)12-2-1-3-13(8-12)18-9-10-4-5-14-11(6-10)7-15(23-14)17(21)22/h1-8,18-22H,9H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50225383
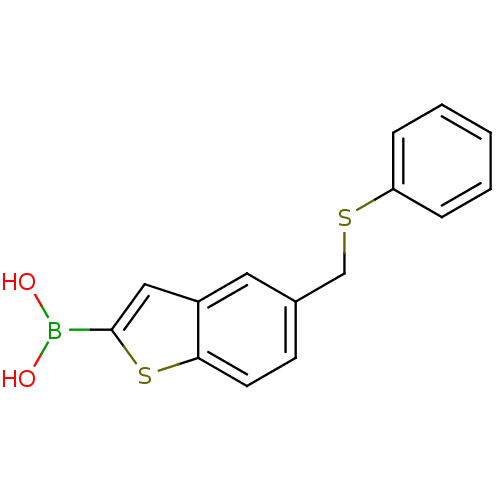
(CHEMBL236203 | pinacol 5-phenylsulphanylmethylbenz...)Show InChI InChI=1S/C15H13BO2S2/c17-16(18)15-9-12-8-11(6-7-14(12)20-15)10-19-13-4-2-1-3-5-13/h1-9,17-18H,10H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50225389
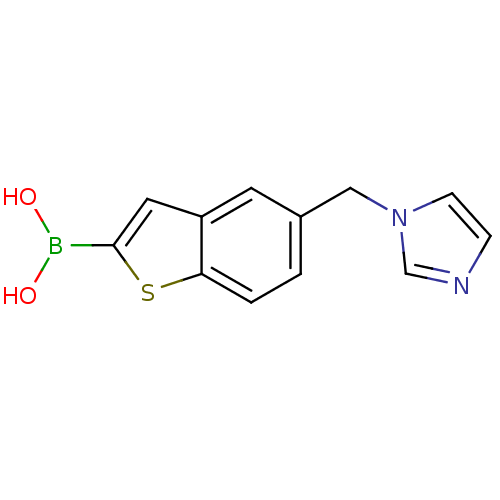
(5-imidazol-1-yl-methylbenzo[b]thiophen-2-ylboronic...)Show InChI InChI=1S/C12H11BN2O2S/c16-13(17)12-6-10-5-9(1-2-11(10)18-12)7-15-4-3-14-8-15/h1-6,8,16-17H,7H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50225377
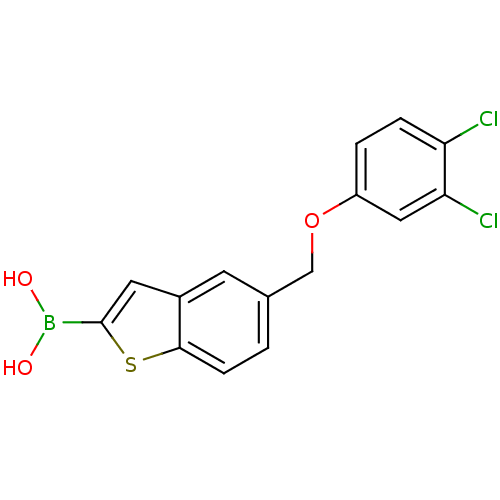
(CHEMBL237390 | pinacol 5-(3,4-dichlorophenoxymethy...)Show InChI InChI=1S/C15H11BCl2O3S/c17-12-3-2-11(7-13(12)18)21-8-9-1-4-14-10(5-9)6-15(22-14)16(19)20/h1-7,19-20H,8H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50225374
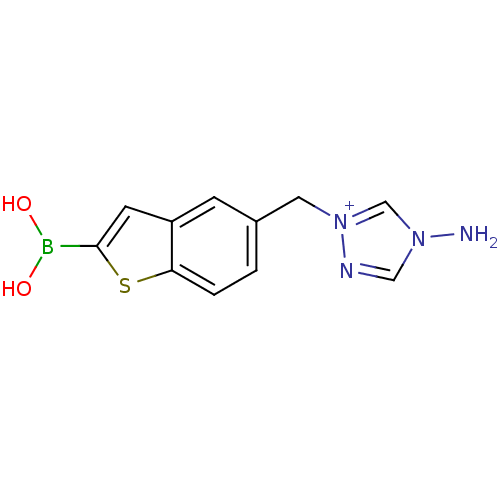
(5 [(1,2,4-triazol-1-ylmethyl]benzo[b]thiophen-2-yl...)Show InChI InChI=1S/C11H12BN4O2S/c13-15-6-14-16(7-15)5-8-1-2-10-9(3-8)4-11(19-10)12(17)18/h1-4,6-7,17-18H,5,13H2/q+1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50225390
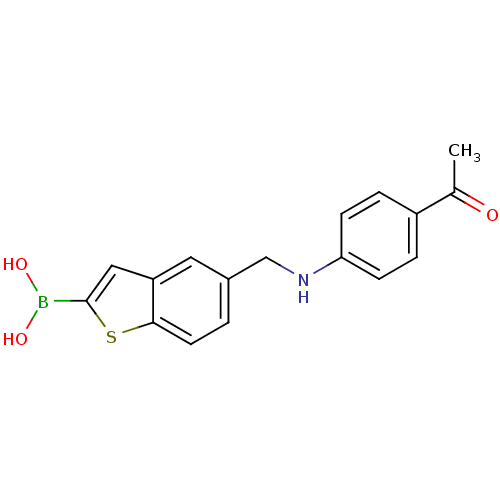
(CHEMBL235523 | pinacol 5-[(4-acetylphenylamino)met...)Show InChI InChI=1S/C17H16BNO3S/c1-11(20)13-3-5-15(6-4-13)19-10-12-2-7-16-14(8-12)9-17(23-16)18(21)22/h2-9,19,21-22H,10H2,1H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50225386
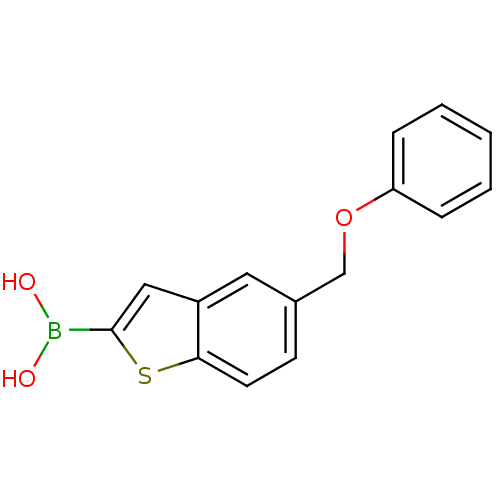
(CHEMBL396871 | pinacol 5-phenoxymethylbenzo[b]thio...)Show InChI InChI=1S/C15H13BO3S/c17-16(18)15-9-12-8-11(6-7-14(12)20-15)10-19-13-4-2-1-3-5-13/h1-9,17-18H,10H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50225387
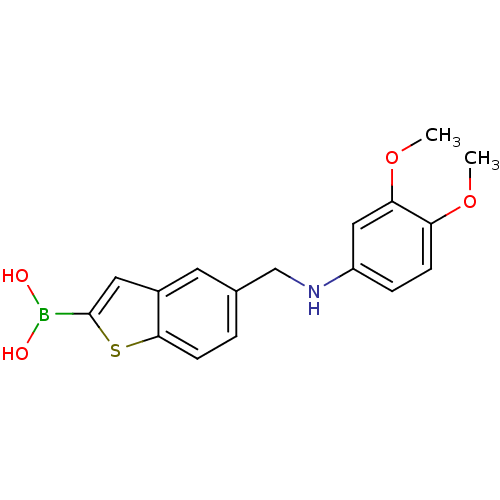
(CHEMBL396889 | pinacol 5-[(3,4-dimethoxyphenylamin...)Show InChI InChI=1S/C17H18BNO4S/c1-22-14-5-4-13(9-15(14)23-2)19-10-11-3-6-16-12(7-11)8-17(24-16)18(20)21/h3-9,19-21H,10H2,1-2H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50225375
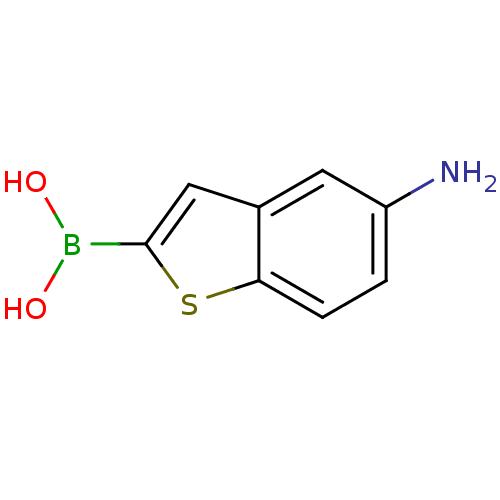
(CHEMBL396390 | pinacol 5-aminomethylbenzo[b]thioph...)Show InChI InChI=1S/C8H8BNO2S/c10-6-1-2-7-5(3-6)4-8(13-7)9(11)12/h1-4,11-12H,10H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50225384
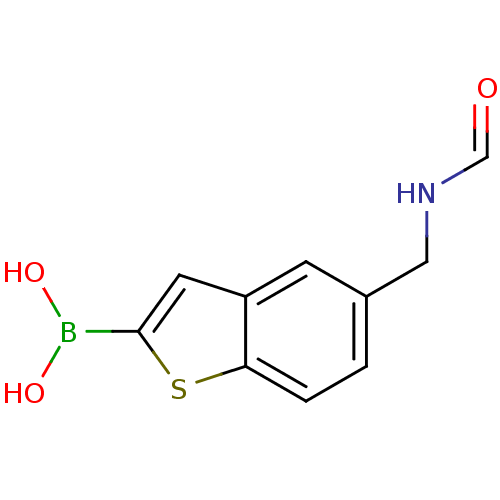
(5-formylaminomethylbenzo[b]thiophen-2-ylboronic ac...)Show InChI InChI=1S/C10H10BNO3S/c13-6-12-5-7-1-2-9-8(3-7)4-10(16-9)11(14)15/h1-4,6,14-15H,5H2,(H,12,13) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM291573
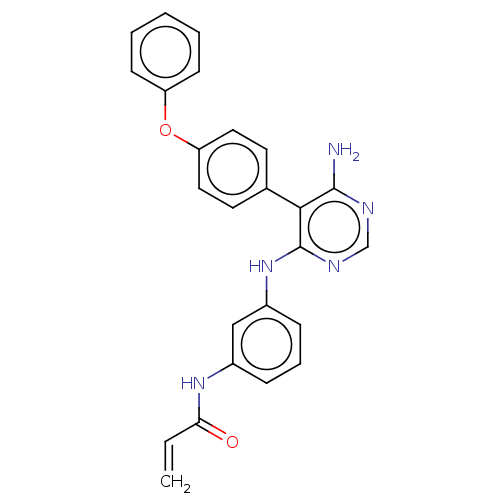
(N-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...)Show SMILES Nc1ncnc(Nc2cccc(NC(=O)C=C)c2)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H21N5O2/c1-2-22(31)29-18-7-6-8-19(15-18)30-25-23(24(26)27-16-28-25)17-11-13-21(14-12-17)32-20-9-4-3-5-10-20/h2-16H,1H2,(H,29,31)(H3,26,27,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50225391
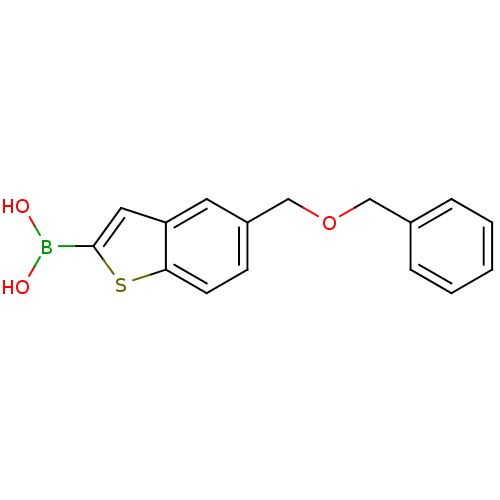
(CHEMBL237181 | pinacol 5-benzyloxymethylbenzo[b]th...)Show InChI InChI=1S/C16H15BO3S/c18-17(19)16-9-14-8-13(6-7-15(14)21-16)11-20-10-12-4-2-1-3-5-12/h1-9,18-19H,10-11H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM291413

(1-(6-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...)Show SMILES Nc1ncnc(NC2CC3(C2)CN(C3)C(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H25N5O2/c1-2-21(31)30-14-25(15-30)12-18(13-25)29-24-22(23(26)27-16-28-24)17-8-10-20(11-9-17)32-19-6-4-3-5-7-19/h2-11,16,18H,1,12-15H2,(H3,26,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50225385
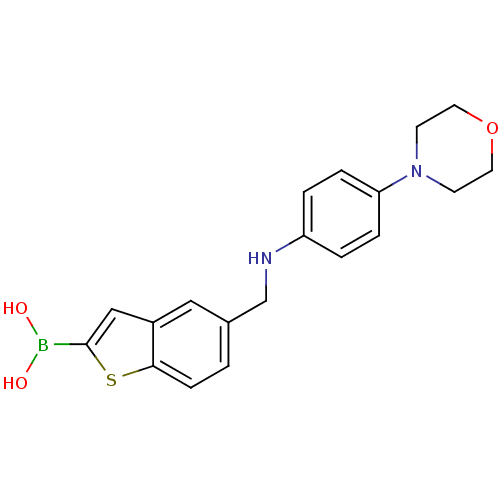
(CHEMBL391537 | pinacol 5-[(4-morpholin-4-yl-phenyl...)Show InChI InChI=1S/C19H21BN2O3S/c23-20(24)19-12-15-11-14(1-6-18(15)26-19)13-21-16-2-4-17(5-3-16)22-7-9-25-10-8-22/h1-6,11-12,21,23-24H,7-10,13H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50225372
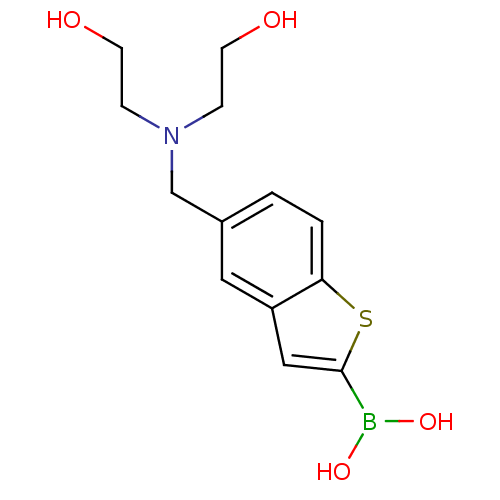
(CHEMBL235097 | pinacol 5-{[bis(2-hydroxyethyl)amin...)Show InChI InChI=1S/C13H18BNO4S/c16-5-3-15(4-6-17)9-10-1-2-12-11(7-10)8-13(20-12)14(18)19/h1-2,7-8,16-19H,3-6,9H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 28: 2939-2944 (2018)
Article DOI: 10.1016/j.bmcl.2018.07.008
BindingDB Entry DOI: 10.7270/Q2QR50TC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM291455

(N-(4-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)a...)Show SMILES Nc1ncnc(NC23CC(C2)(CC3)NC(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 |(-.82,-6.28,;-2.15,-5.51,;-3.48,-6.28,;-4.82,-5.51,;-4.82,-3.97,;-3.48,-3.2,;-3.48,-1.66,;-4.82,-.89,;-4.82,.65,;-6.28,1.13,;-5.88,-.36,;-7.19,-.12,;-6.28,-1.37,;-6.68,2.61,;-5.59,3.7,;-4.1,3.3,;-5.99,5.19,;-4.9,6.28,;-2.15,-3.97,;-.82,-3.2,;.52,-3.97,;1.85,-3.2,;1.85,-1.66,;3.19,-.89,;4.52,-1.66,;4.52,-3.2,;5.85,-3.97,;7.19,-3.2,;7.19,-1.66,;5.85,-.89,;.52,-.89,;-.82,-1.66,)| Show InChI InChI=1S/C25H25N5O2/c1-2-20(31)29-24-12-13-25(14-24,15-24)30-23-21(22(26)27-16-28-23)17-8-10-19(11-9-17)32-18-6-4-3-5-7-18/h2-11,16H,1,12-15H2,(H,29,31)(H3,26,27,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM291452
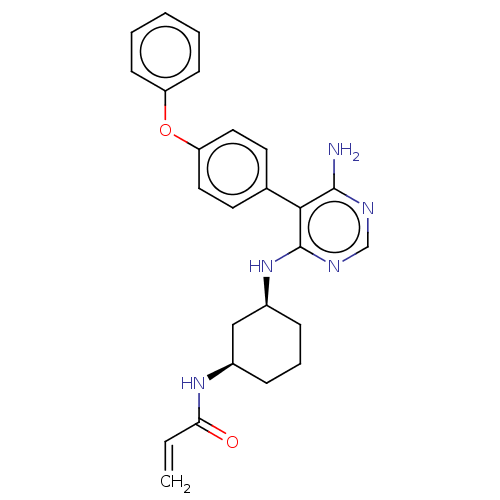
(N-((1R,3S)-3-((6-amino-5-(4-phenoxyphenyl)pyrimidi...)Show SMILES Nc1ncnc(N[C@H]2CCC[C@H](C2)NC(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C25H27N5O2/c1-2-22(31)29-18-7-6-8-19(15-18)30-25-23(24(26)27-16-28-25)17-11-13-21(14-12-17)32-20-9-4-3-5-10-20/h2-5,9-14,16,18-19H,1,6-8,15H2,(H,29,31)(H3,26,27,28,30)/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50225382
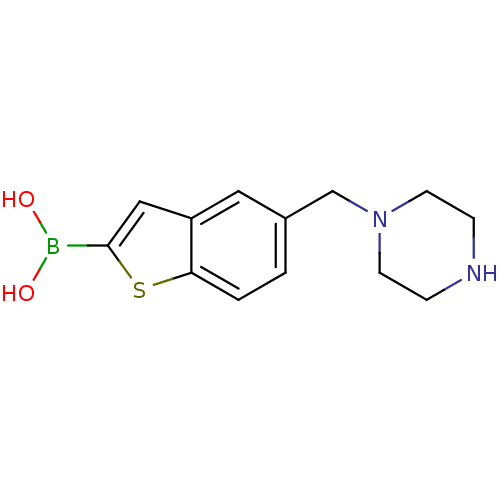
(CHEMBL398112 | pinacol 5-piperazin-1-ylmethylbenzo...)Show InChI InChI=1S/C13H17BN2O2S/c17-14(18)13-8-11-7-10(1-2-12(11)19-13)9-16-5-3-15-4-6-16/h1-2,7-8,15,17-18H,3-6,9H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50463725

(CHEMBL4242598)Show SMILES [H][C@]12CN(c3ccc(C(N)=O)c(Oc4ccc(Oc5ccccc5)cc4)n3)[C@]([H])(CN1C(=O)C=C)C2 |r| Show InChI InChI=1S/C26H24N4O4/c1-2-24(31)30-16-17-14-18(30)15-29(17)23-13-12-22(25(27)32)26(28-23)34-21-10-8-20(9-11-21)33-19-6-4-3-5-7-19/h2-13,17-18H,1,14-16H2,(H2,27,32)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 28: 2939-2944 (2018)
Article DOI: 10.1016/j.bmcl.2018.07.008
BindingDB Entry DOI: 10.7270/Q2QR50TC |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50225388
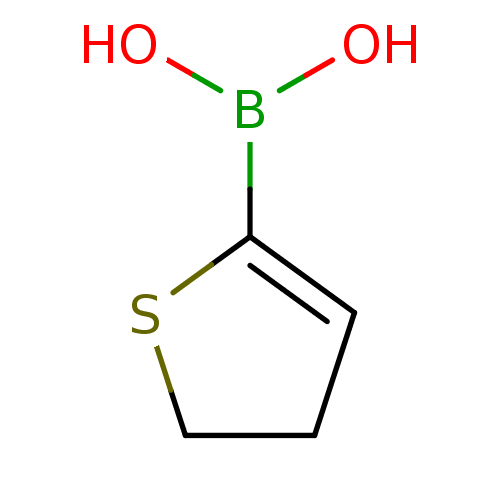
(CHEMBL236759 | thiophene-2-ylboronic acid)Show InChI InChI=1S/C4H7BO2S/c6-5(7)4-2-1-3-8-4/h2,6-7H,1,3H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli beta-lactamase |
J Med Chem 50: 5644-54 (2007)
Article DOI: 10.1021/jm070643q
BindingDB Entry DOI: 10.7270/Q21N80W5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM291522

(1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...)Show SMILES Nc1ncnc(NCC2CCN(CC2)C(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H27N5O2/c1-2-22(31)30-14-12-18(13-15-30)16-27-25-23(24(26)28-17-29-25)19-8-10-21(11-9-19)32-20-6-4-3-5-7-20/h2-11,17-18H,1,12-16H2,(H3,26,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysis |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50466323
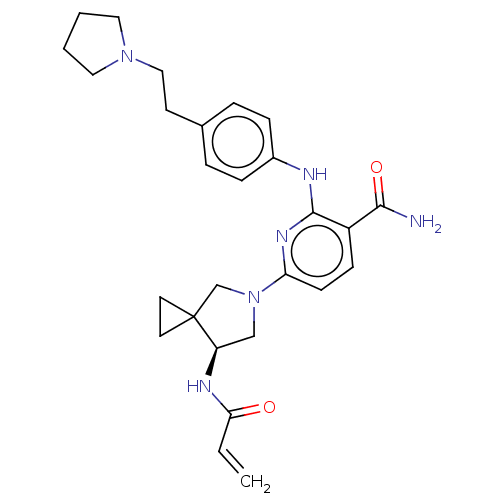
(CHEMBL4279353)Show SMILES NC(=O)c1ccc(nc1Nc1ccc(CCN2CCCC2)cc1)N1C[C@@H](NC(=O)C=C)C2(CC2)C1 |r| Show InChI InChI=1S/C27H34N6O2/c1-2-24(34)30-22-17-33(18-27(22)12-13-27)23-10-9-21(25(28)35)26(31-23)29-20-7-5-19(6-8-20)11-16-32-14-3-4-15-32/h2,5-10,22H,1,3-4,11-18H2,(H2,28,35)(H,29,31)(H,30,34)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length N-terminal GST-tagged BTK (2 to 659 residues) expressed in baculovirus expression system using FITC-AHA-E... |
Bioorg Med Chem Lett 28: 3419-3424 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.033
BindingDB Entry DOI: 10.7270/Q218395S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50466316
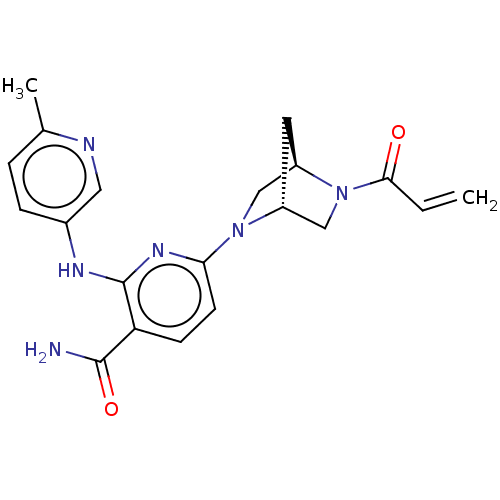
(CHEMBL4276669)Show SMILES [H][C@@]12CN(c3ccc(C(N)=O)c(Nc4ccc(C)nc4)n3)[C@@]([H])(CN1C(=O)C=C)C2 |r| Show InChI InChI=1S/C20H22N6O2/c1-3-18(27)26-11-14-8-15(26)10-25(14)17-7-6-16(19(21)28)20(24-17)23-13-5-4-12(2)22-9-13/h3-7,9,14-15H,1,8,10-11H2,2H3,(H2,21,28)(H,23,24)/t14-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length N-terminal GST-tagged BTK (2 to 659 residues) expressed in baculovirus expression system using FITC-AHA-E... |
Bioorg Med Chem Lett 28: 3419-3424 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.033
BindingDB Entry DOI: 10.7270/Q218395S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length N-terminal GST-tagged BTK (2 to 659 residues) expressed in baculovirus expression system using FITC-AHA-E... |
Bioorg Med Chem Lett 28: 3419-3424 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.033
BindingDB Entry DOI: 10.7270/Q218395S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full length BTK using FITC-AHA-EEPLYWSFPAKKK-NH2 as substrate after 90 mins by microfluid mobility shift assay |
Bioorg Med Chem Lett 28: 2939-2944 (2018)
Article DOI: 10.1016/j.bmcl.2018.07.008
BindingDB Entry DOI: 10.7270/Q2QR50TC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50519156
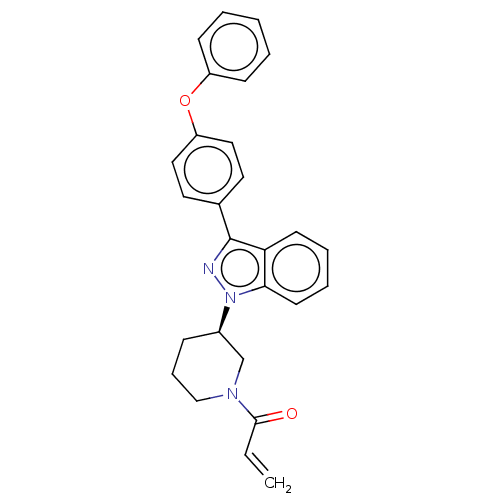
(CHEMBL4466205)Show SMILES C=CC(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2)c2ccccc12 |r| Show InChI InChI=1S/C27H25N3O2/c1-2-26(31)29-18-8-9-21(19-29)30-25-13-7-6-12-24(25)27(28-30)20-14-16-23(17-15-20)32-22-10-4-3-5-11-22/h2-7,10-17,21H,1,8-9,18-19H2/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM291635
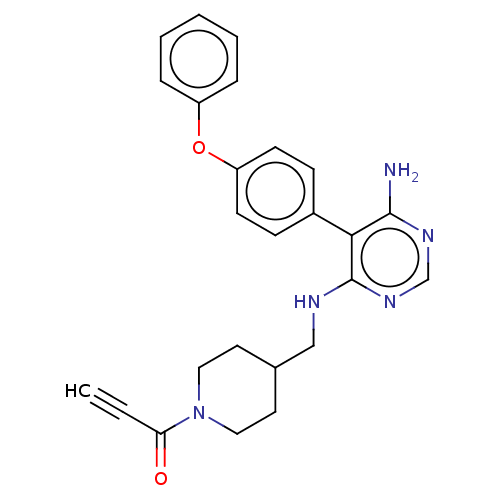
(1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)...)Show SMILES Nc1ncnc(NCC2CCN(CC2)C(=O)C#C)c1-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C25H25N5O2/c1-2-22(31)30-14-12-18(13-15-30)16-27-25-23(24(26)28-17-29-25)19-8-10-21(11-9-19)32-20-6-4-3-5-7-20/h1,3-11,17-18H,12-16H2,(H3,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK... |
J Med Chem 62: 7643-7655 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00794
BindingDB Entry DOI: 10.7270/Q21R6TXT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50571038
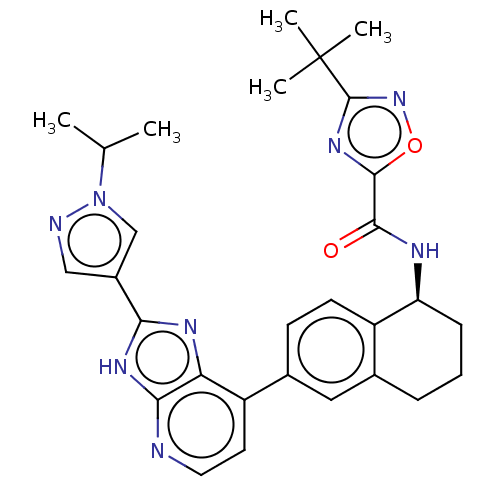
(CHEMBL4868813)Show SMILES CC(C)n1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc2[C@H](CCCc2c1)NC(=O)c1nc(no1)C(C)(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514956
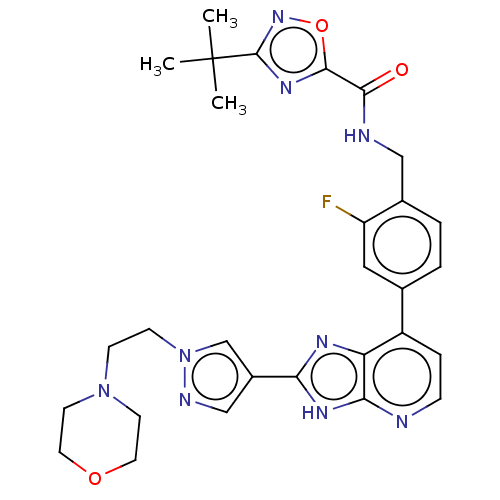
(US11098041, Example 187)Show SMILES CC(C)(C)c1noc(n1)C(=O)NCc1ccc(cc1F)-c1ccnc2[nH]c(nc12)-c1cnn(CCN2CCOCC2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50466325
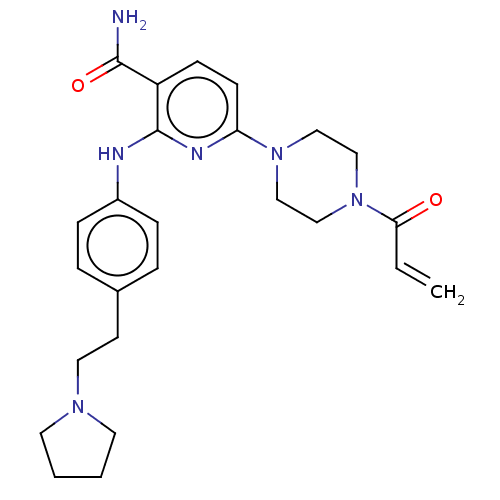
(CHEMBL4279430)Show SMILES NC(=O)c1ccc(nc1Nc1ccc(CCN2CCCC2)cc1)N1CCN(CC1)C(=O)C=C Show InChI InChI=1S/C25H32N6O2/c1-2-23(32)31-17-15-30(16-18-31)22-10-9-21(24(26)33)25(28-22)27-20-7-5-19(6-8-20)11-14-29-12-3-4-13-29/h2,5-10H,1,3-4,11-18H2,(H2,26,33)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length N-terminal GST-tagged BTK (2 to 659 residues) expressed in baculovirus expression system using FITC-AHA-E... |
Bioorg Med Chem Lett 28: 3419-3424 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.033
BindingDB Entry DOI: 10.7270/Q218395S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514887
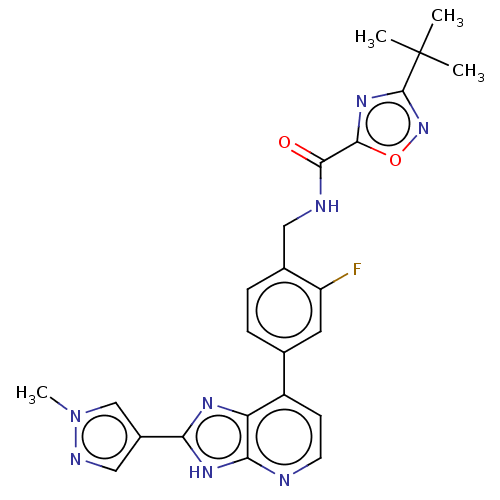
(US11098041, Example 112)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc(CNC(=O)c2nc(no2)C(C)(C)C)c(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50571039
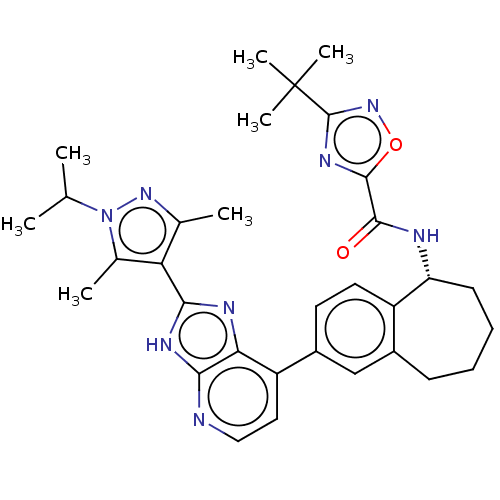
(CHEMBL4877846)Show SMILES CC(C)n1nc(C)c(c1C)-c1nc2c(ccnc2[nH]1)-c1ccc2[C@@H](CCCCc2c1)NC(=O)c1nc(no1)C(C)(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514891
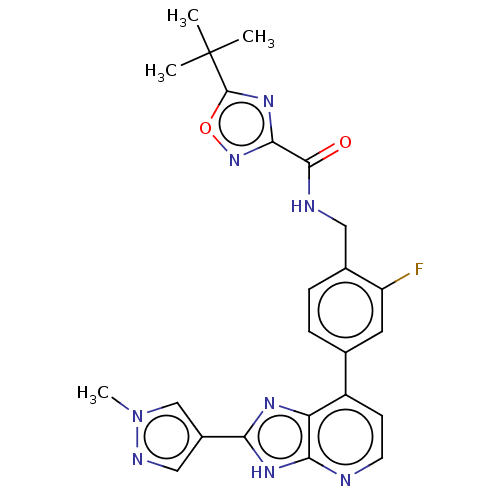
(US11098041, Example 116)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc(CNC(=O)c2noc(n2)C(C)(C)C)c(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514935
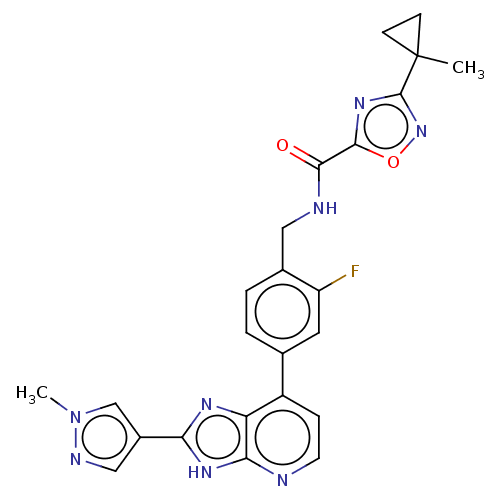
(US11098041, Example 165)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc(CNC(=O)c2nc(no2)C2(C)CC2)c(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50466328
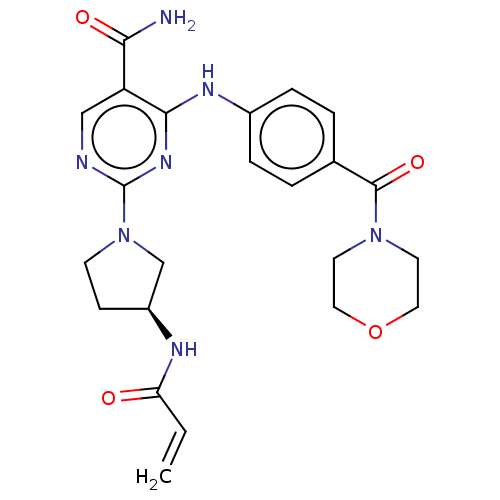
(CHEMBL4290540)Show SMILES NC(=O)c1cnc(nc1Nc1ccc(cc1)C(=O)N1CCOCC1)N1CC[C@@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C23H27N7O4/c1-2-19(31)26-17-7-8-30(14-17)23-25-13-18(20(24)32)21(28-23)27-16-5-3-15(4-6-16)22(33)29-9-11-34-12-10-29/h2-6,13,17H,1,7-12,14H2,(H2,24,32)(H,26,31)(H,25,27,28)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length N-terminal GST-tagged BTK (2 to 659 residues) expressed in baculovirus expression system using FITC-AHA-E... |
Bioorg Med Chem Lett 28: 3419-3424 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.033
BindingDB Entry DOI: 10.7270/Q218395S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514904
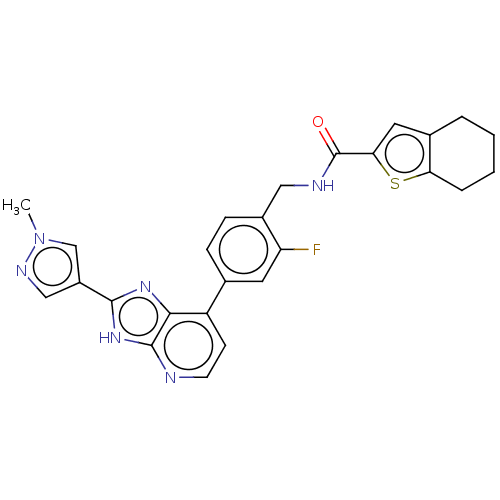
(US11098041, Example 132)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc(CNC(=O)c2cc3CCCCc3s2)c(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50571033
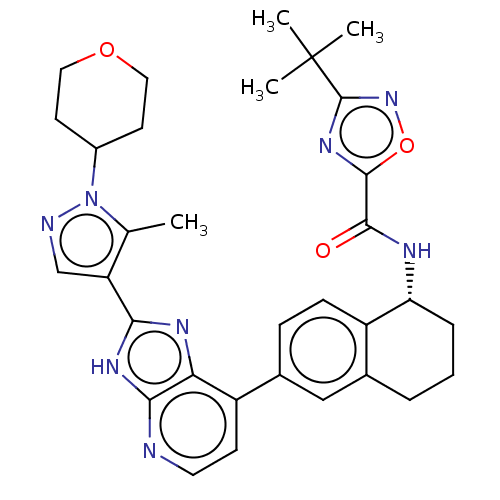
(CHEMBL4876326)Show SMILES Cc1c(cnn1C1CCOCC1)-c1nc2c(ccnc2[nH]1)-c1ccc2[C@@H](CCCc2c1)NC(=O)c1nc(no1)C(C)(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM514915

(US11098041, Example 144)Show SMILES Cn1cc(cn1)-c1nc2c(ccnc2[nH]1)-c1ccc(CNC(=O)c2cc(no2)C(C)(C)C)c(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50571037
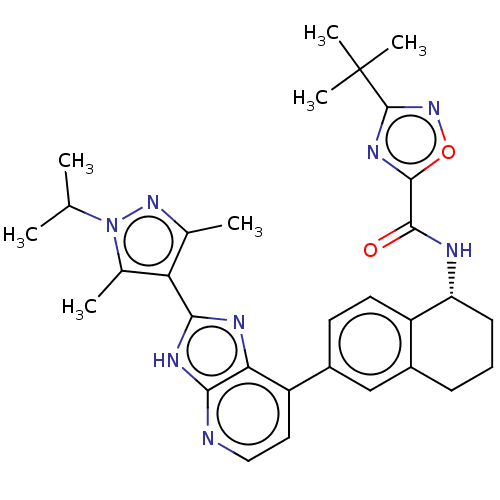
(CHEMBL4862532)Show SMILES CC(C)n1nc(C)c(c1C)-c1nc2c(ccnc2[nH]1)-c1ccc2[C@@H](CCCc2c1)NC(=O)c1nc(no1)C(C)(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116163
BindingDB Entry DOI: 10.7270/Q2K35ZF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50466206
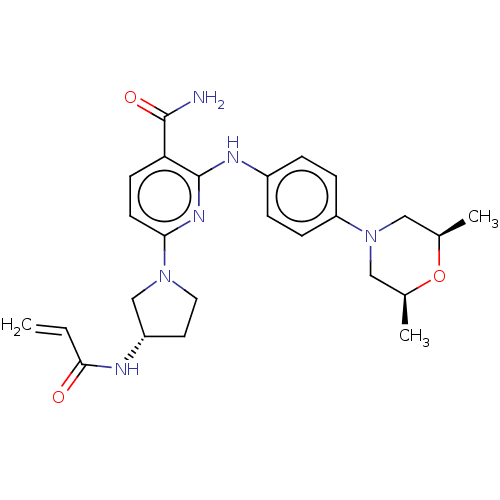
(CHEMBL4281335)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1ccc(Nc2nc(ccc2C(N)=O)N2CC[C@@H](C2)NC(=O)C=C)cc1 |r| Show InChI InChI=1S/C25H32N6O3/c1-4-23(32)27-19-11-12-30(15-19)22-10-9-21(24(26)33)25(29-22)28-18-5-7-20(8-6-18)31-13-16(2)34-17(3)14-31/h4-10,16-17,19H,1,11-15H2,2-3H3,(H2,26,33)(H,27,32)(H,28,29)/t16-,17+,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length N-terminal GST-tagged BTK (2 to 659 residues) expressed in baculovirus expression system using FITC-AHA-E... |
Bioorg Med Chem Lett 28: 3419-3424 (2018)
Article DOI: 10.1016/j.bmcl.2018.09.033
BindingDB Entry DOI: 10.7270/Q218395S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data