 Found 2009 hits with Last Name = 'morgan' and Initial = 'm'
Found 2009 hits with Last Name = 'morgan' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50366620

(RESINIFERATOXIN)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3[C@H]4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)ccc1O |r,t:10,35,TLB:23:15:12:24.25.26,THB:16:15:12:24.25.26| Show InChI InChI=1S/C37H40O9/c1-21(2)35-17-23(4)37-27(33(35)44-36(45-35,46-37)19-24-9-7-6-8-10-24)14-26(18-34(41)30(37)13-22(3)32(34)40)20-43-31(39)16-25-11-12-28(38)29(15-25)42-5/h6-15,23,27,30,33,38,41H,1,16-20H2,2-5H3/t23-,27+,30-,33-,34-,35+,36?,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0426 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50247744
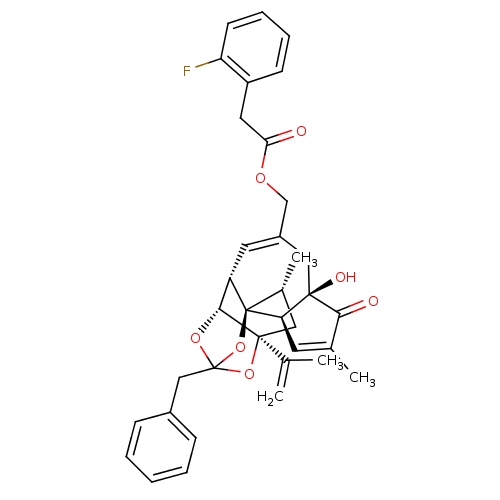
(CHEMBL504725 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...)Show SMILES C[C@@H]1C[C@]2(OC3(Cc4ccccc4)O[C@@H]2[C@@H]2C=C(COC(=O)Cc4ccccc4F)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]12O3)C(C)=C |r,t:18,37,TLB:6:5:15:3.2.1,THB:4:5:15:3.2.1| Show InChI InChI=1S/C36H37FO7/c1-21(2)34-17-23(4)36-27(32(34)42-35(43-34,44-36)19-24-10-6-5-7-11-24)15-25(18-33(40)29(36)14-22(3)31(33)39)20-41-30(38)16-26-12-8-9-13-28(26)37/h5-15,23,27,29,32,40H,1,16-20H2,2-4H3/t23-,27+,29-,32-,33-,34+,35?,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50247741
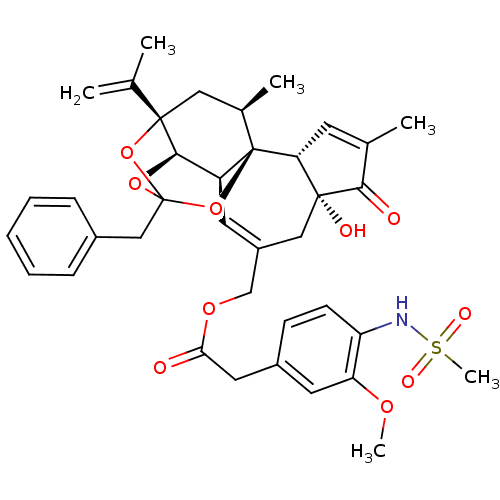
(CHEMBL503101 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3[C@H]4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)ccc1NS(C)(=O)=O |r,t:10,35,TLB:23:15:12:24.25.26,THB:16:15:12:24.25.26| Show InChI InChI=1S/C38H43NO10S/c1-22(2)36-18-24(4)38-28(34(36)47-37(48-36,49-38)20-25-10-8-7-9-11-25)15-27(19-35(42)31(38)14-23(3)33(35)41)21-46-32(40)17-26-12-13-29(30(16-26)45-5)39-50(6,43)44/h7-16,24,28,31,34,39,42H,1,17-21H2,2-6H3/t24-,28+,31-,34-,35-,36+,37?,38-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.228 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50247749
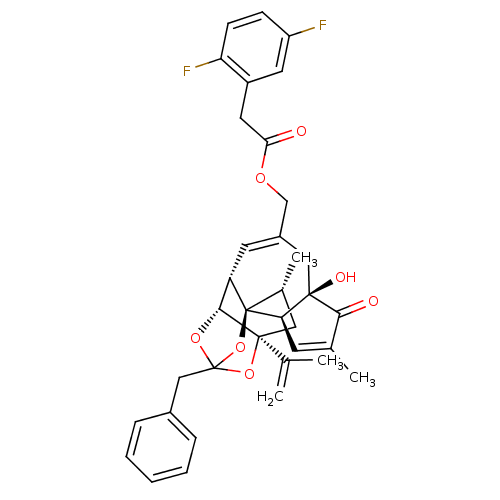
(CHEMBL510583 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...)Show SMILES C[C@@H]1C[C@]2(OC3(Cc4ccccc4)O[C@@H]2[C@@H]2C=C(COC(=O)Cc4cc(F)ccc4F)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]12O3)C(C)=C |r,t:18,38,TLB:6:5:15:3.2.1,THB:4:5:15:3.2.1| Show InChI InChI=1S/C36H36F2O7/c1-20(2)34-16-22(4)36-27(32(34)43-35(44-34,45-36)18-23-8-6-5-7-9-23)13-24(17-33(41)29(36)12-21(3)31(33)40)19-42-30(39)15-25-14-26(37)10-11-28(25)38/h5-14,22,27,29,32,41H,1,15-19H2,2-4H3/t22-,27+,29-,32-,33-,34+,35?,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50385670
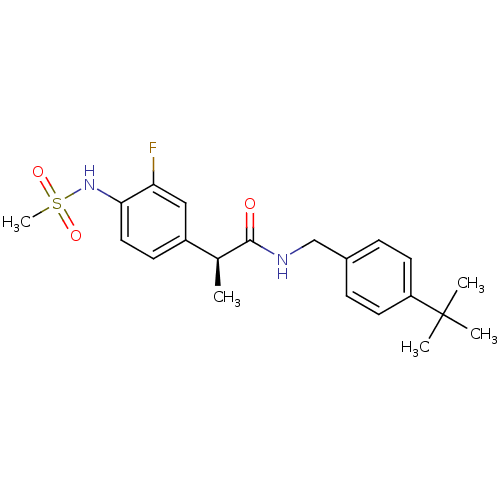
(CHEMBL2042399)Show SMILES C[C@H](C(=O)NCc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C21H27FN2O3S/c1-14(16-8-11-19(18(22)12-16)24-28(5,26)27)20(25)23-13-15-6-9-17(10-7-15)21(2,3)4/h6-12,14,24H,13H2,1-5H3,(H,23,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor expressed in CHO cells assessed as decrease in capsaicin-induced intracellular 45Ca2+ uptake after 1 hr b... |
Bioorg Med Chem 20: 215-24 (2011)
Article DOI: 10.1016/j.bmc.2011.11.008
BindingDB Entry DOI: 10.7270/Q2MG7QJJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541196

(CHEMBL4639090)Show InChI InChI=1S/C12H15BrN2/c1-8-7-15-5-4-14-6-9-2-3-10(13)11(8)12(9)15/h2-3,8,14H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50319419
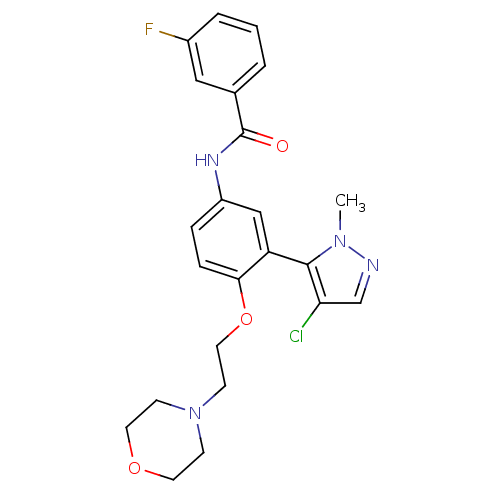
(CHEMBL1086075 | N-[3-(4-Chloro-2-methyl-2H-pyrazol...)Show SMILES Cn1ncc(Cl)c1-c1cc(NC(=O)c2cccc(F)c2)ccc1OCCN1CCOCC1 |(-5.98,-34.34,;-5.66,-35.84,;-6.69,-36.99,;-5.92,-38.32,;-4.41,-38,;-3.26,-39.03,;-4.25,-36.47,;-2.92,-35.7,;-1.59,-36.48,;-.25,-35.7,;1.09,-36.47,;2.42,-35.7,;2.42,-34.16,;3.75,-36.47,;3.75,-38,;5.08,-38.77,;6.41,-38,;6.41,-36.45,;7.74,-35.67,;5.07,-35.69,;-.26,-34.15,;-1.59,-33.39,;-2.92,-34.16,;-4.25,-33.39,;-4.25,-31.85,;-5.59,-31.08,;-5.59,-29.54,;-4.25,-28.77,;-4.25,-27.24,;-5.58,-26.46,;-6.92,-27.23,;-6.92,-28.77,)| Show InChI InChI=1S/C23H24ClFN4O3/c1-28-22(20(24)15-26-28)19-14-18(27-23(30)16-3-2-4-17(25)13-16)5-6-21(19)32-12-9-29-7-10-31-11-8-29/h2-6,13-15H,7-12H2,1H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells by scintillation counting |
J Med Chem 53: 4412-21 (2010)
Article DOI: 10.1021/jm100044a
BindingDB Entry DOI: 10.7270/Q2KK9BZV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50319414
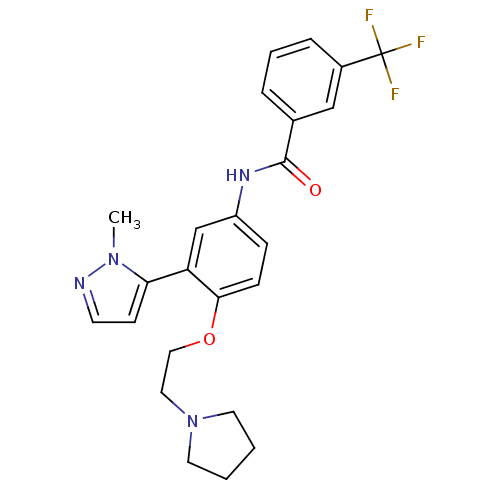
(CHEMBL1085155 | [3-(2-Methyl-2H-pyrazol-3-yl)-4-(2...)Show SMILES Cn1nccc1-c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1OCCN1CCCC1 Show InChI InChI=1S/C24H25F3N4O2/c1-30-21(9-10-28-30)20-16-19(7-8-22(20)33-14-13-31-11-2-3-12-31)29-23(32)17-5-4-6-18(15-17)24(25,26)27/h4-10,15-16H,2-3,11-14H2,1H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells by scintillation counting |
J Med Chem 53: 4412-21 (2010)
Article DOI: 10.1021/jm100044a
BindingDB Entry DOI: 10.7270/Q2KK9BZV |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20286
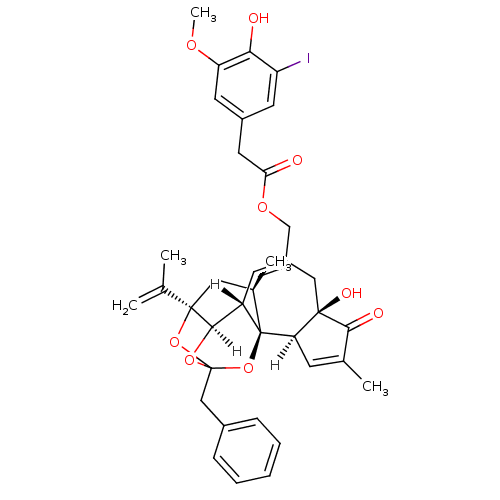
(5-I-RTX | 5-iodoresiniferatoxin | [(1R,2R,6R,10S,1...)Show SMILES [H][C@]12OC3(Cc4ccccc4)O[C@]1(C[C@@H](C)[C@]1(O3)[C@]3([H])C=C(C)C(=O)[C@@]3(O)CC(COC(=O)Cc3cc(I)c(O)c(OC)c3)=C[C@@]21[H])C(C)=C |c:48,t:23,TLB:11:3:12.14.13:45,THB:4:3:12.14.13:45| Show InChI InChI=1S/C37H39IO9/c1-20(2)35-16-22(4)37-26(33(35)45-36(46-35,47-37)18-23-9-7-6-8-10-23)12-25(17-34(42)29(37)11-21(3)32(34)41)19-44-30(39)15-24-13-27(38)31(40)28(14-24)43-5/h6-14,22,26,29,33,40,42H,1,15-19H2,2-5H3/t22-,26+,29-,33-,34-,35-,36?,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.610 | -54.7 | n/a | n/a | 12.2 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
J Med Chem 51: 57-67 (2008)
Article DOI: 10.1021/jm701049p
BindingDB Entry DOI: 10.7270/Q2222S1N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50319421
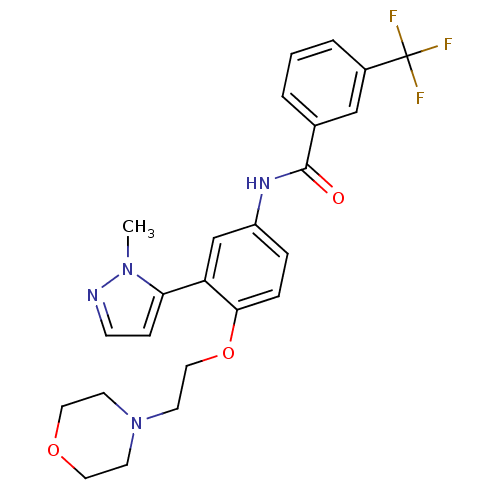
(CHEMBL1086317 | N-[3-(2-Methyl-2H-pyrazol-3-yl)-4-...)Show SMILES Cn1nccc1-c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1OCCN1CCOCC1 Show InChI InChI=1S/C24H25F3N4O3/c1-30-21(7-8-28-30)20-16-19(5-6-22(20)34-14-11-31-9-12-33-13-10-31)29-23(32)17-3-2-4-18(15-17)24(25,26)27/h2-8,15-16H,9-14H2,1H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells by scintillation counting |
J Med Chem 53: 4412-21 (2010)
Article DOI: 10.1021/jm100044a
BindingDB Entry DOI: 10.7270/Q2KK9BZV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541195
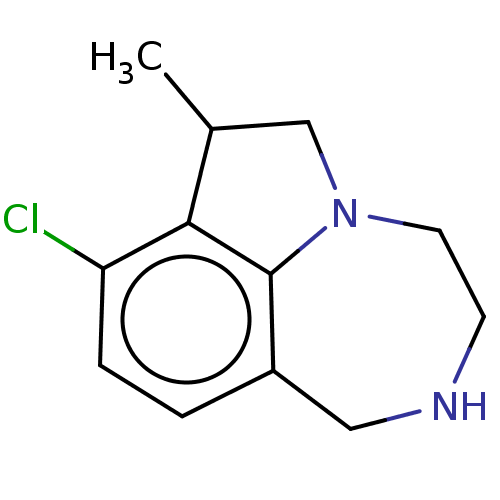
(CHEMBL4634942)Show InChI InChI=1S/C12H15ClN2/c1-8-7-15-5-4-14-6-9-2-3-10(13)11(8)12(9)15/h2-3,8,14H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541197
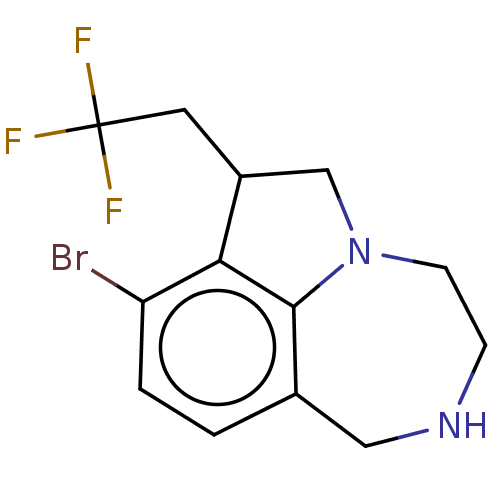
(CHEMBL4646572)Show InChI InChI=1S/C13H14BrF3N2/c14-10-2-1-8-6-18-3-4-19-7-9(5-13(15,16)17)11(10)12(8)19/h1-2,9,18H,3-7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50247742
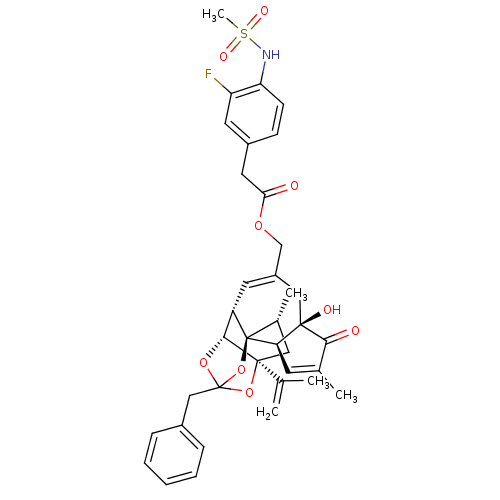
(CHEMBL509154 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...)Show SMILES C[C@@H]1C[C@]2(OC3(Cc4ccccc4)O[C@@H]2[C@@H]2C=C(COC(=O)Cc4ccc(NS(C)(=O)=O)c(F)c4)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]12O3)C(C)=C |r,t:18,42,TLB:6:5:15:3.2.1,THB:4:5:15:3.2.1| Show InChI InChI=1S/C37H40FNO9S/c1-21(2)35-17-23(4)37-27(33(35)46-36(47-35,48-37)19-24-9-7-6-8-10-24)14-26(18-34(42)30(37)13-22(3)32(34)41)20-45-31(40)16-25-11-12-29(28(38)15-25)39-49(5,43)44/h6-15,23,27,30,33,39,42H,1,16-20H2,2-5H3/t23-,27+,30-,33-,34-,35+,36?,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50247748
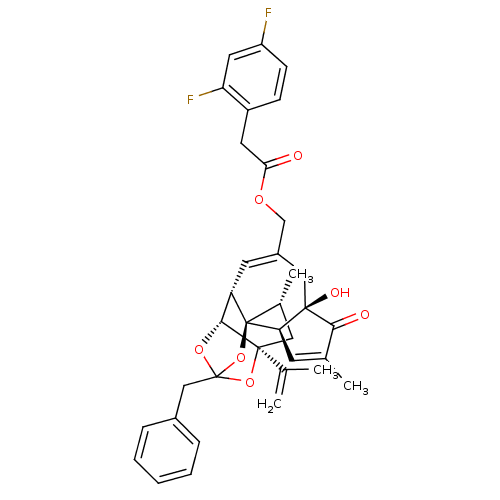
(CHEMBL510228 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...)Show SMILES C[C@@H]1C[C@]2(OC3(Cc4ccccc4)O[C@@H]2[C@@H]2C=C(COC(=O)Cc4ccc(F)cc4F)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]12O3)C(C)=C |r,t:18,38,TLB:6:5:15:3.2.1,THB:4:5:15:3.2.1| Show InChI InChI=1S/C36H36F2O7/c1-20(2)34-16-22(4)36-27(32(34)43-35(44-34,45-36)18-23-8-6-5-7-9-23)13-24(17-33(41)29(36)12-21(3)31(33)40)19-42-30(39)14-25-10-11-26(37)15-28(25)38/h5-13,15,22,27,29,32,41H,1,14,16-19H2,2-4H3/t22-,27+,29-,32-,33-,34+,35?,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541204

(CHEMBL4636977)Show InChI InChI=1S/C13H17BrN2/c1-13(2)8-16-6-5-15-7-9-3-4-10(14)11(13)12(9)16/h3-4,15H,5-8H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50247751
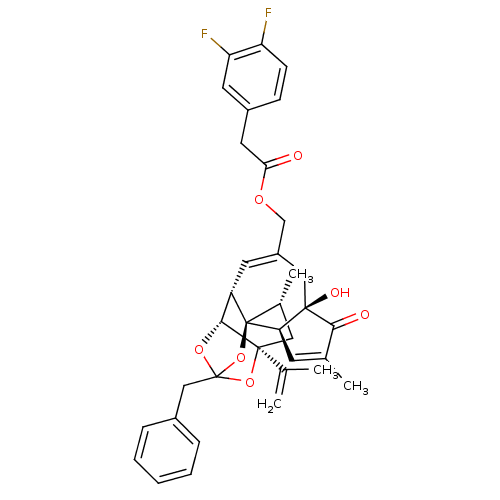
(CHEMBL449201 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...)Show SMILES C[C@@H]1C[C@]2(OC3(Cc4ccccc4)O[C@@H]2[C@@H]2C=C(COC(=O)Cc4ccc(F)c(F)c4)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]12O3)C(C)=C |r,t:18,38,TLB:6:5:15:3.2.1,THB:4:5:15:3.2.1| Show InChI InChI=1S/C36H36F2O7/c1-20(2)34-16-22(4)36-26(32(34)43-35(44-34,45-36)18-23-8-6-5-7-9-23)13-25(17-33(41)29(36)12-21(3)31(33)40)19-42-30(39)15-24-10-11-27(37)28(38)14-24/h5-14,22,26,29,32,41H,1,15-19H2,2-4H3/t22-,26+,29-,32-,33-,34+,35?,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(RAT) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from recombinant rat IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation count... |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541205

(CHEMBL4635105)Show InChI InChI=1S/C13H17ClN2/c1-13(2)8-16-6-5-15-7-9-3-4-10(14)11(13)12(9)16/h3-4,15H,5-8H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50319432

(CHEMBL1082474 | N-{4-[2-(2-Hydroxyethylamino)ethox...)Show SMILES COc1cccc(c1)C(=O)Nc1ccc(OCCNCCO)c(c1)-c1ccnn1C Show InChI InChI=1S/C22H26N4O4/c1-26-20(8-9-24-26)19-15-17(6-7-21(19)30-13-11-23-10-12-27)25-22(28)16-4-3-5-18(14-16)29-2/h3-9,14-15,23,27H,10-13H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells by scintillation counting |
J Med Chem 53: 4412-21 (2010)
Article DOI: 10.1021/jm100044a
BindingDB Entry DOI: 10.7270/Q2KK9BZV |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20314
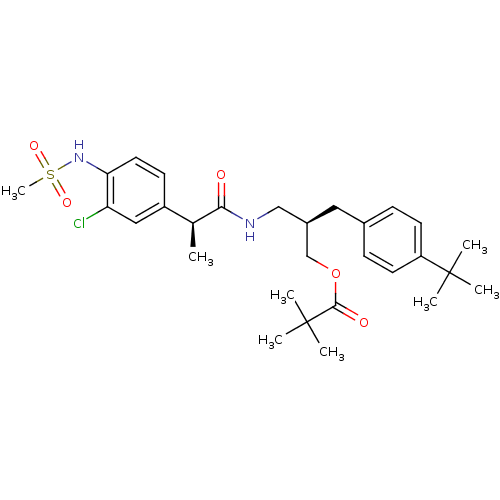
((2R)-2-[(4-tert-butylphenyl)methyl]-3-[(2S)-2-(3-c...)Show SMILES C[C@H](C(=O)NC[C@H](COC(=O)C(C)(C)C)Cc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(Cl)c1 |r| Show InChI InChI=1S/C29H41ClN2O5S/c1-19(22-11-14-25(24(30)16-22)32-38(8,35)36)26(33)31-17-21(18-37-27(34)29(5,6)7)15-20-9-12-23(13-10-20)28(2,3)4/h9-14,16,19,21,32H,15,17-18H2,1-8H3,(H,31,33)/t19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.83 | -51.9 | n/a | n/a | 5.20 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
J Med Chem 51: 57-67 (2008)
Article DOI: 10.1021/jm701049p
BindingDB Entry DOI: 10.7270/Q2222S1N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50319413

(CHEMBL1084895 | N-[3-(2-Methyl-2H-pyrazol-3-yl)-4-...)Show SMILES Cn1nccc1-c1cc(NC(=O)c2ccc(cc2)C(F)(F)F)ccc1OCCN1CCCC1 Show InChI InChI=1S/C24H25F3N4O2/c1-30-21(10-11-28-30)20-16-19(8-9-22(20)33-15-14-31-12-2-3-13-31)29-23(32)17-4-6-18(7-5-17)24(25,26)27/h4-11,16H,2-3,12-15H2,1H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells by scintillation counting |
J Med Chem 53: 4412-21 (2010)
Article DOI: 10.1021/jm100044a
BindingDB Entry DOI: 10.7270/Q2KK9BZV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50319428
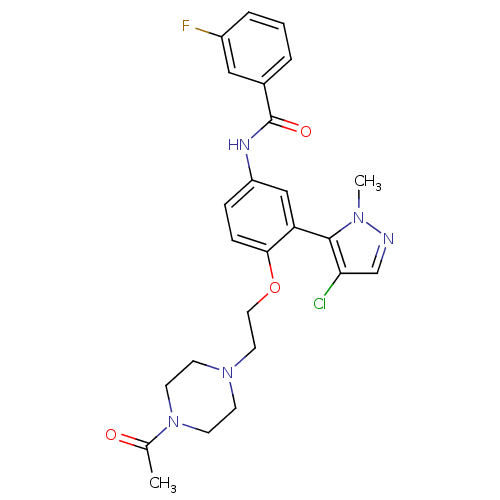
(CHEMBL1085628 | N-{4-[2-(4-Acetylpiperazin-1-yl)et...)Show SMILES CC(=O)N1CCN(CCOc2ccc(NC(=O)c3cccc(F)c3)cc2-c2c(Cl)cnn2C)CC1 |(-8.55,-30.04,;-9.88,-30.81,;-11.22,-30.03,;-9.89,-32.35,;-8.56,-33.12,;-8.56,-34.66,;-9.9,-35.43,;-9.9,-36.97,;-8.56,-37.74,;-8.56,-39.28,;-7.23,-40.05,;-5.9,-39.28,;-4.56,-40.04,;-4.56,-41.59,;-3.22,-42.36,;-1.89,-41.59,;-1.89,-40.05,;-.56,-42.36,;-.56,-43.89,;.77,-44.66,;2.1,-43.89,;2.1,-42.34,;3.43,-41.56,;.76,-41.58,;-5.9,-42.36,;-7.23,-41.59,;-8.56,-42.36,;-8.72,-43.89,;-7.57,-44.91,;-10.23,-44.21,;-11,-42.88,;-9.97,-41.73,;-10.28,-40.22,;-11.25,-34.66,;-11.24,-33.12,)| Show InChI InChI=1S/C25H27ClFN5O3/c1-17(33)32-10-8-31(9-11-32)12-13-35-23-7-6-20(15-21(23)24-22(26)16-28-30(24)2)29-25(34)18-4-3-5-19(27)14-18/h3-7,14-16H,8-13H2,1-2H3,(H,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells by scintillation counting |
J Med Chem 53: 4412-21 (2010)
Article DOI: 10.1021/jm100044a
BindingDB Entry DOI: 10.7270/Q2KK9BZV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50319433

(CHEMBL1082795 | N-[4-(2-Aminoethoxy)-3-(1-methyl-1...)Show SMILES COc1cccc(c1)C(=O)Nc1ccc(OCCN)c(c1)-c1ccnn1C Show InChI InChI=1S/C20H22N4O3/c1-24-18(8-10-22-24)17-13-15(6-7-19(17)27-11-9-21)23-20(25)14-4-3-5-16(12-14)26-2/h3-8,10,12-13H,9,11,21H2,1-2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells by scintillation counting |
J Med Chem 53: 4412-21 (2010)
Article DOI: 10.1021/jm100044a
BindingDB Entry DOI: 10.7270/Q2KK9BZV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50319420
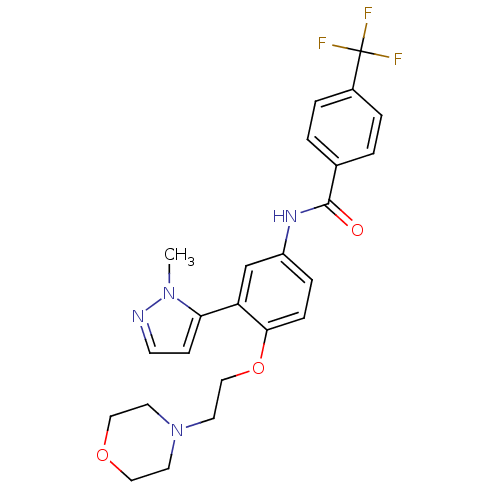
(CHEMBL1086316 | N-[3-(2-Methyl-2H-pyrazol-3-yl)-4-...)Show SMILES Cn1nccc1-c1cc(NC(=O)c2ccc(cc2)C(F)(F)F)ccc1OCCN1CCOCC1 Show InChI InChI=1S/C24H25F3N4O3/c1-30-21(8-9-28-30)20-16-19(6-7-22(20)34-15-12-31-10-13-33-14-11-31)29-23(32)17-2-4-18(5-3-17)24(25,26)27/h2-9,16H,10-15H2,1H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells by scintillation counting |
J Med Chem 53: 4412-21 (2010)
Article DOI: 10.1021/jm100044a
BindingDB Entry DOI: 10.7270/Q2KK9BZV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541194
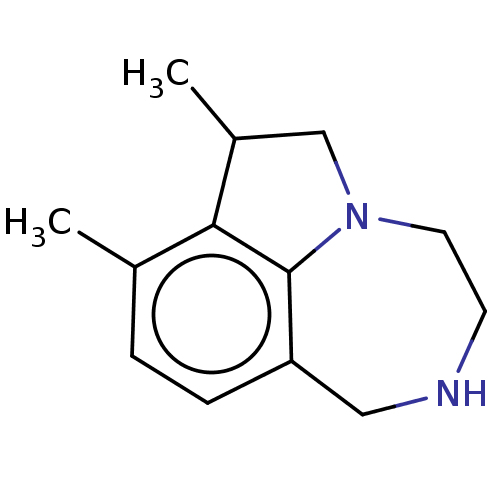
(CHEMBL4646618)Show InChI InChI=1S/C13H18N2/c1-9-3-4-11-7-14-5-6-15-8-10(2)12(9)13(11)15/h3-4,10,14H,5-8H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50247745
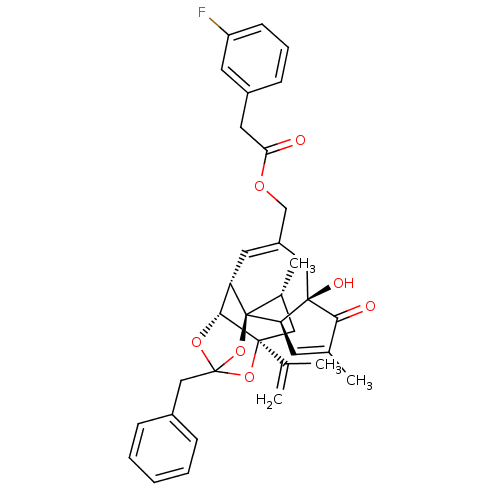
(CHEMBL455555 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...)Show SMILES C[C@@H]1C[C@]2(OC3(Cc4ccccc4)O[C@@H]2[C@@H]2C=C(COC(=O)Cc4cccc(F)c4)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]12O3)C(C)=C |r,t:18,37,TLB:6:5:15:3.2.1,THB:4:5:15:3.2.1| Show InChI InChI=1S/C36H37FO7/c1-21(2)34-17-23(4)36-28(32(34)42-35(43-34,44-36)19-24-9-6-5-7-10-24)15-26(18-33(40)29(36)13-22(3)31(33)39)20-41-30(38)16-25-11-8-12-27(37)14-25/h5-15,23,28-29,32,40H,1,16-20H2,2-4H3/t23-,28+,29-,32-,33-,34+,35?,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50247750
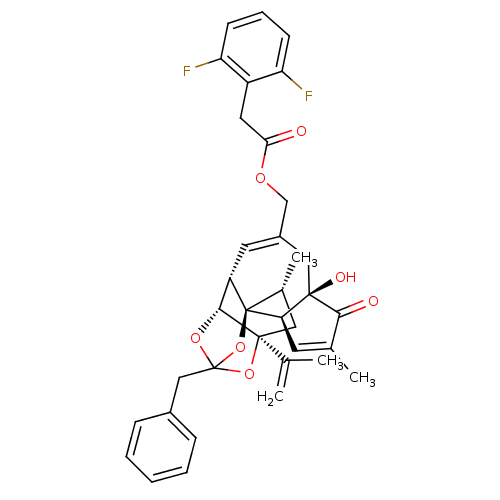
(CHEMBL455155 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...)Show SMILES C[C@@H]1C[C@]2(OC3(Cc4ccccc4)O[C@@H]2[C@@H]2C=C(COC(=O)Cc4c(F)cccc4F)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]12O3)C(C)=C |r,t:18,38,TLB:6:5:15:3.2.1,THB:4:5:15:3.2.1| Show InChI InChI=1S/C36H36F2O7/c1-20(2)34-16-22(4)36-26(32(34)43-35(44-34,45-36)18-23-9-6-5-7-10-23)14-24(17-33(41)29(36)13-21(3)31(33)40)19-42-30(39)15-25-27(37)11-8-12-28(25)38/h5-14,22,26,29,32,41H,1,15-19H2,2-4H3/t22-,26+,29-,32-,33-,34+,35?,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541199
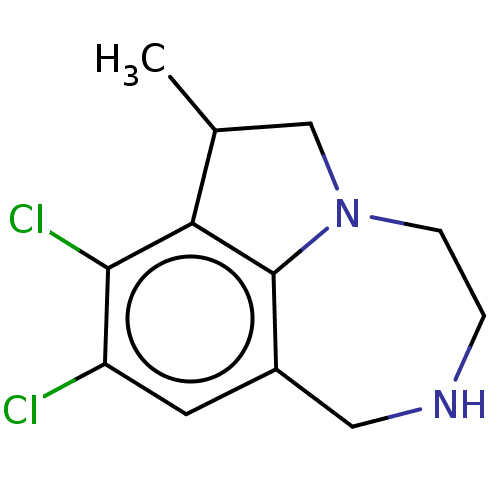
(CHEMBL4632473)Show InChI InChI=1S/C12H14Cl2N2/c1-7-6-16-3-2-15-5-8-4-9(13)11(14)10(7)12(8)16/h4,7,15H,2-3,5-6H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50314073
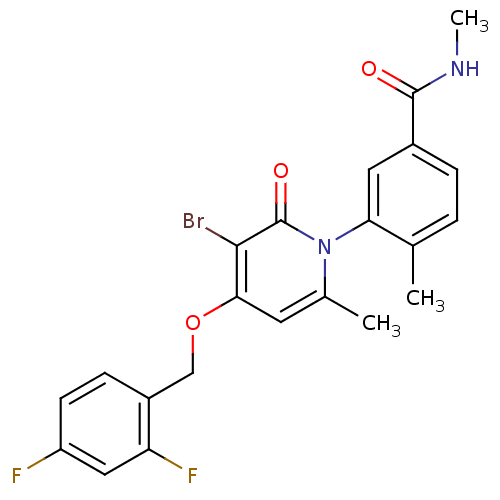
(3-(3-bromo-4-(2,4-difluorobenzyloxy)-6-methyl-2-ox...)Show SMILES CNC(=O)c1ccc(C)c(c1)-n1c(C)cc(OCc2ccc(F)cc2F)c(Br)c1=O |(29.32,-4.45,;29.32,-2.91,;27.99,-2.14,;26.65,-2.91,;27.99,-.6,;29.32,.17,;29.31,1.72,;27.98,2.48,;27.97,4.02,;26.66,1.71,;26.65,.17,;25.32,2.48,;25.32,4.03,;26.65,4.8,;23.98,4.79,;22.65,4.02,;21.32,4.79,;19.99,4.02,;18.65,4.79,;18.65,6.33,;17.32,7.1,;16,6.33,;14.66,7.1,;15.99,4.78,;17.32,4.02,;17.33,2.48,;22.65,2.48,;21.32,1.71,;23.99,1.71,;23.99,.17,)| Show InChI InChI=1S/C22H19BrF2N2O3/c1-12-4-5-14(21(28)26-3)9-18(12)27-13(2)8-19(20(23)22(27)29)30-11-15-6-7-16(24)10-17(15)25/h4-10H,11H2,1-3H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha kinase |
Bioorg Med Chem Lett 21: 3856-60 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.006
BindingDB Entry DOI: 10.7270/Q2G73F2S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541208

(CHEMBL4634785)Show SMILES [H][C@@]12CCC[C@]1([H])C1=CC=CC3CNCCN(C2)C13 |r,c:10,t:8| Show InChI InChI=1S/C15H22N2/c1-3-11-9-16-7-8-17-10-12-4-2-5-13(12)14(6-1)15(11)17/h1,3,6,11-13,15-16H,2,4-5,7-10H2/t11?,12-,13-,15?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from recombinant human 5HT2C receptor expressed in CHO cells measured after 120 ... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235385

(APD-811 | Ralinepag)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(Cl)cc2)CC1 |r,wU:6.5,wD:9.9,(-11.29,-9.03,;-9.96,-9.8,;-9.96,-11.34,;-8.62,-9.03,;-7.29,-9.8,;-5.96,-9.03,;-4.62,-9.8,;-3.29,-9.03,;-1.96,-9.8,;-1.96,-11.34,;-.62,-12.11,;.71,-11.34,;2.04,-12.11,;2.04,-13.65,;3.38,-11.34,;3.38,-9.8,;2.04,-9.03,;2.04,-7.49,;3.38,-6.72,;4.71,-7.49,;4.71,-9.03,;4.71,-12.11,;6.05,-11.34,;7.38,-12.11,;7.38,-13.65,;8.71,-14.42,;6.05,-14.42,;4.71,-13.65,;-3.29,-12.11,;-4.62,-11.34,)| Show InChI InChI=1S/C23H26ClNO5/c24-19-10-12-21(13-11-19)25(20-4-2-1-3-5-20)23(28)30-15-18-8-6-17(7-9-18)14-29-16-22(26)27/h1-5,10-13,17-18H,6-9,14-16H2,(H,26,27)/t17-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from recombinant human IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation cou... |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50247746
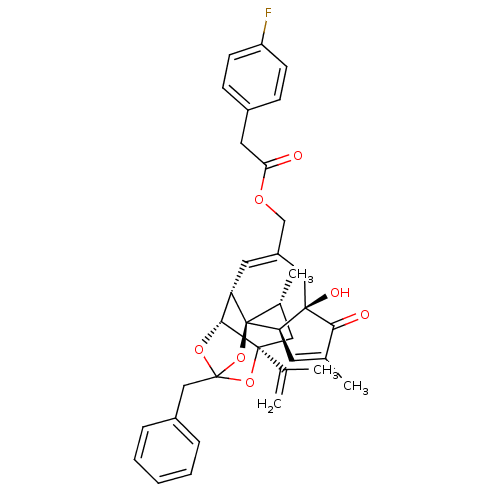
(CHEMBL502769 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...)Show SMILES C[C@@H]1C[C@]2(OC3(Cc4ccccc4)O[C@@H]2[C@@H]2C=C(COC(=O)Cc4ccc(F)cc4)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]12O3)C(C)=C |r,t:18,37,TLB:6:5:15:3.2.1,THB:4:5:15:3.2.1| Show InChI InChI=1S/C36H37FO7/c1-21(2)34-17-23(4)36-28(32(34)42-35(43-34,44-36)19-25-8-6-5-7-9-25)15-26(18-33(40)29(36)14-22(3)31(33)39)20-41-30(38)16-24-10-12-27(37)13-11-24/h5-15,23,28-29,32,40H,1,16-20H2,2-4H3/t23-,28+,29-,32-,33-,34+,35?,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from recombinant human IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation cou... |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20311
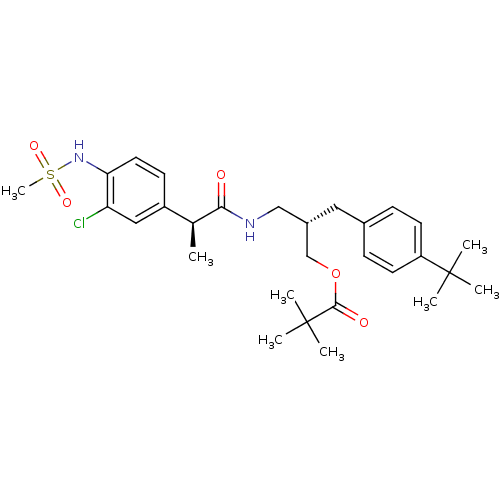
((2S)-2-[(4-tert-butylphenyl)methyl]-3-[(2S)-2-(3-c...)Show SMILES C[C@H](C(=O)NC[C@@H](COC(=O)C(C)(C)C)Cc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(Cl)c1 |r| Show InChI InChI=1S/C29H41ClN2O5S/c1-19(22-11-14-25(24(30)16-22)32-38(8,35)36)26(33)31-17-21(18-37-27(34)29(5,6)7)15-20-9-12-23(13-10-20)28(2,3)4/h9-14,16,19,21,32H,15,17-18H2,1-8H3,(H,31,33)/t19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.29 | -50.4 | n/a | n/a | 12.1 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
J Med Chem 51: 57-67 (2008)
Article DOI: 10.1021/jm701049p
BindingDB Entry DOI: 10.7270/Q2222S1N |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20330
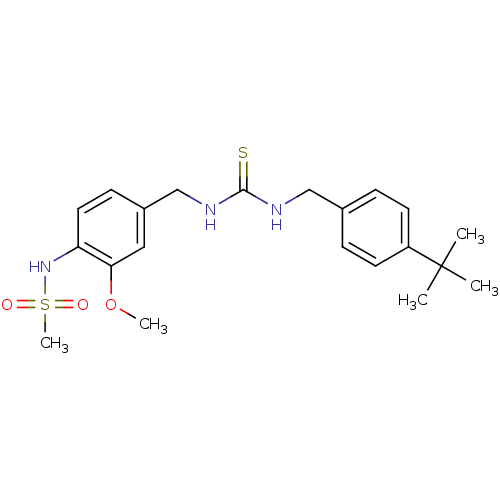
(3-[(4-tert-butylphenyl)methyl]-1-[(4-methanesulfon...)Show SMILES COc1cc(CNC(=S)NCc2ccc(cc2)C(C)(C)C)ccc1NS(C)(=O)=O Show InChI InChI=1S/C21H29N3O3S2/c1-21(2,3)17-9-6-15(7-10-17)13-22-20(28)23-14-16-8-11-18(19(12-16)27-4)24-29(5,25)26/h6-12,24H,13-14H2,1-5H3,(H2,22,23,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity towards rat TRPV1 expressed in CHO cells |
Bioorg Med Chem Lett 15: 4143-50 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.006
BindingDB Entry DOI: 10.7270/Q2JH3KQB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20330

(3-[(4-tert-butylphenyl)methyl]-1-[(4-methanesulfon...)Show SMILES COc1cc(CNC(=S)NCc2ccc(cc2)C(C)(C)C)ccc1NS(C)(=O)=O Show InChI InChI=1S/C21H29N3O3S2/c1-21(2,3)17-9-6-15(7-10-17)13-22-20(28)23-14-16-8-11-18(19(12-16)27-4)24-29(5,25)26/h6-12,24H,13-14H2,1-5H3,(H2,22,23,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity for rat TRPV1 expressed in CHO cells |
Bioorg Med Chem Lett 15: 4136-42 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.009
BindingDB Entry DOI: 10.7270/Q23B5ZN0 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50247747
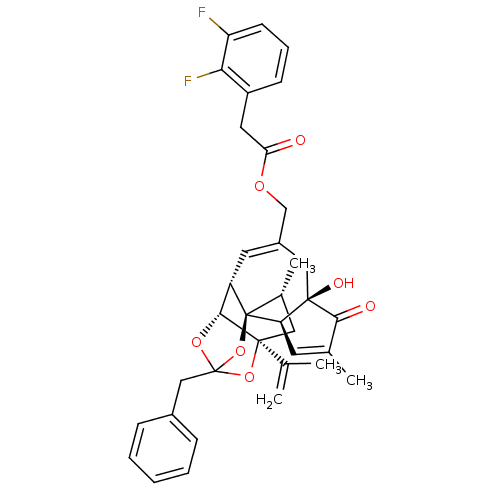
(CHEMBL509331 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...)Show SMILES C[C@@H]1C[C@]2(OC3(Cc4ccccc4)O[C@@H]2[C@@H]2C=C(COC(=O)Cc4cccc(F)c4F)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]12O3)C(C)=C |r,t:18,38,TLB:6:5:15:3.2.1,THB:4:5:15:3.2.1| Show InChI InChI=1S/C36H36F2O7/c1-20(2)34-16-22(4)36-26(32(34)43-35(44-34,45-36)18-23-9-6-5-7-10-23)14-24(17-33(41)28(36)13-21(3)31(33)40)19-42-29(39)15-25-11-8-12-27(37)30(25)38/h5-14,22,26,28,32,41H,1,15-19H2,2-4H3/t22-,26+,28-,32-,33-,34+,35?,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
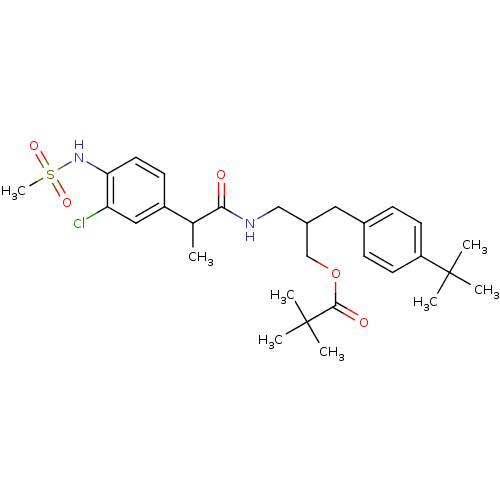
(Rattus norvegicus (rat)) | BDBM20291
(2-[(4-tert-butylphenyl)methyl]-3-[2-(3-chloro-4-me...)Show SMILES CC(C(=O)NCC(COC(=O)C(C)(C)C)Cc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(Cl)c1 Show InChI InChI=1S/C29H41ClN2O5S/c1-19(22-11-14-25(24(30)16-22)32-38(8,35)36)26(33)31-17-21(18-37-27(34)29(5,6)7)15-20-9-12-23(13-10-20)28(2,3)4/h9-14,16,19,21,32H,15,17-18H2,1-8H3,(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | -50.1 | n/a | n/a | 12.3 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
J Med Chem 51: 57-67 (2008)
Article DOI: 10.1021/jm701049p
BindingDB Entry DOI: 10.7270/Q2222S1N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50319422
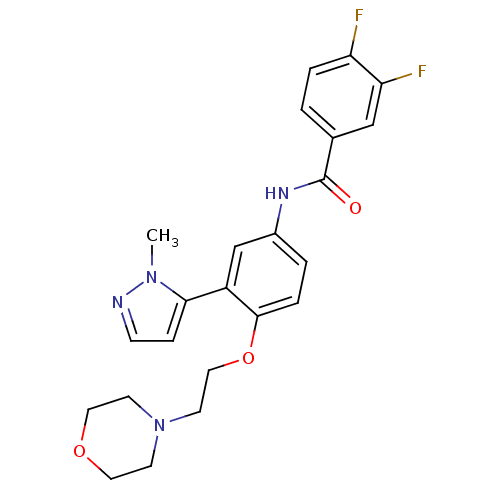
(3,4-Difluoro-N-[3-(2-methyl-2H-pyrazol-3-yl)-4-(2-...)Show SMILES Cn1nccc1-c1cc(NC(=O)c2ccc(F)c(F)c2)ccc1OCCN1CCOCC1 Show InChI InChI=1S/C23H24F2N4O3/c1-28-21(6-7-26-28)18-15-17(27-23(30)16-2-4-19(24)20(25)14-16)3-5-22(18)32-13-10-29-8-11-31-12-9-29/h2-7,14-15H,8-13H2,1H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells by scintillation counting |
J Med Chem 53: 4412-21 (2010)
Article DOI: 10.1021/jm100044a
BindingDB Entry DOI: 10.7270/Q2KK9BZV |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20313
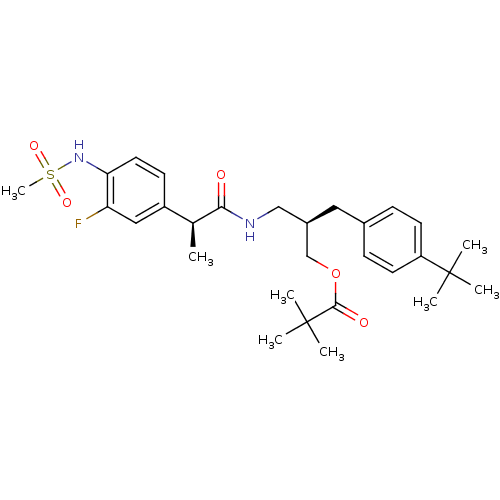
((2R)-2-[(4-tert-butylphenyl)methyl]-3-[(2S)-2-(3-f...)Show SMILES C[C@H](C(=O)NC[C@H](COC(=O)C(C)(C)C)Cc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C29H41FN2O5S/c1-19(22-11-14-25(24(30)16-22)32-38(8,35)36)26(33)31-17-21(18-37-27(34)29(5,6)7)15-20-9-12-23(13-10-20)28(2,3)4/h9-14,16,19,21,32H,15,17-18H2,1-8H3,(H,31,33)/t19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.12 | -49.8 | n/a | n/a | 0.580 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
J Med Chem 51: 57-67 (2008)
Article DOI: 10.1021/jm701049p
BindingDB Entry DOI: 10.7270/Q2222S1N |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50385670

(CHEMBL2042399)Show SMILES C[C@H](C(=O)NCc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C21H27FN2O3S/c1-14(16-8-11-19(18(22)12-16)24-28(5,26)27)20(25)23-13-15-6-9-17(10-7-15)21(2,3)4/h6-12,14,24H,13H2,1-5H3,(H,23,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV1 receptor expressed in CHO cells assessed as decrease in capsaicin-induced intracellular 45Ca2+ uptake after 1 hr by ... |
Bioorg Med Chem 20: 215-24 (2011)
Article DOI: 10.1016/j.bmc.2011.11.008
BindingDB Entry DOI: 10.7270/Q2MG7QJJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541207
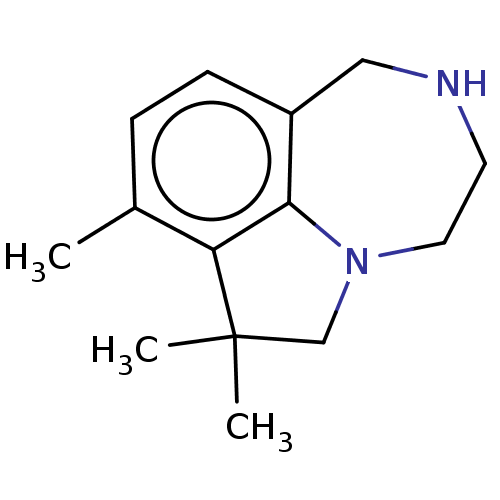
(CHEMBL4638879)Show InChI InChI=1S/C14H20N2/c1-10-4-5-11-8-15-6-7-16-9-14(2,3)12(10)13(11)16/h4-5,15H,6-9H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50319426

(CHEMBL1086555 | N-{4-[2-(4-Acetylpiperazin-1-yl)et...)Show SMILES CC(=O)N1CCN(CCOc2ccc(NC(=O)c3cccc(c3)C(F)(F)F)cc2-c2ccnn2C)CC1 Show InChI InChI=1S/C26H28F3N5O3/c1-18(35)34-12-10-33(11-13-34)14-15-37-24-7-6-21(17-22(24)23-8-9-30-32(23)2)31-25(36)19-4-3-5-20(16-19)26(27,28)29/h3-9,16-17H,10-15H2,1-2H3,(H,31,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells by scintillation counting |
J Med Chem 53: 4412-21 (2010)
Article DOI: 10.1021/jm100044a
BindingDB Entry DOI: 10.7270/Q2KK9BZV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50319429
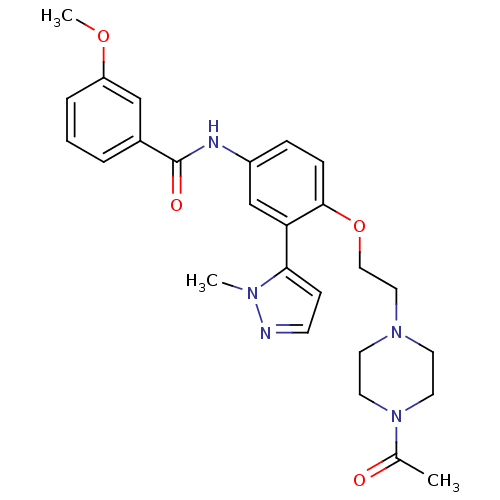
(CHEMBL1085854 | N-{4-[2-(4-Acetylpiperazin-1-yl)et...)Show SMILES COc1cccc(c1)C(=O)Nc1ccc(OCCN2CCN(CC2)C(C)=O)c(c1)-c1ccnn1C Show InChI InChI=1S/C26H31N5O4/c1-19(32)31-13-11-30(12-14-31)15-16-35-25-8-7-21(18-23(25)24-9-10-27-29(24)2)28-26(33)20-5-4-6-22(17-20)34-3/h4-10,17-18H,11-16H2,1-3H3,(H,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells by scintillation counting |
J Med Chem 53: 4412-21 (2010)
Article DOI: 10.1021/jm100044a
BindingDB Entry DOI: 10.7270/Q2KK9BZV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50319423
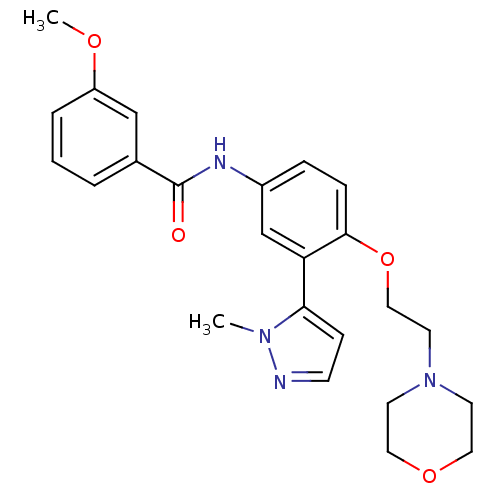
(3-Methoxy-N-[3-(2-methyl-2H-pyrazol-3-yl)-4-(2-mor...)Show SMILES COc1cccc(c1)C(=O)Nc1ccc(OCCN2CCOCC2)c(c1)-c1ccnn1C Show InChI InChI=1S/C24H28N4O4/c1-27-22(8-9-25-27)21-17-19(26-24(29)18-4-3-5-20(16-18)30-2)6-7-23(21)32-15-12-28-10-13-31-14-11-28/h3-9,16-17H,10-15H2,1-2H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells by scintillation counting |
J Med Chem 53: 4412-21 (2010)
Article DOI: 10.1021/jm100044a
BindingDB Entry DOI: 10.7270/Q2KK9BZV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50319417
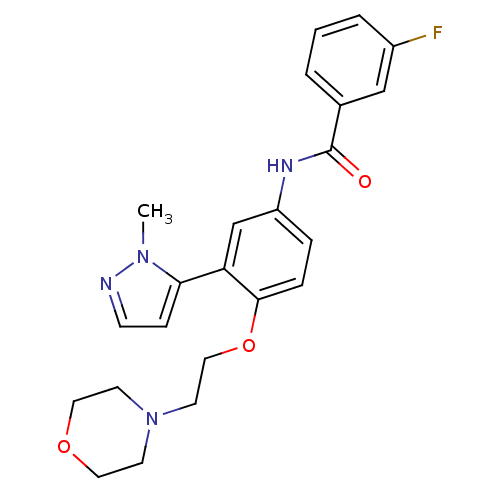
(3-Fluoro-N-[3-(2-methyl-2H-pyrazol-3-yl)-4-(2-morp...)Show SMILES Cn1nccc1-c1cc(NC(=O)c2cccc(F)c2)ccc1OCCN1CCOCC1 Show InChI InChI=1S/C23H25FN4O3/c1-27-21(7-8-25-27)20-16-19(26-23(29)17-3-2-4-18(24)15-17)5-6-22(20)31-14-11-28-9-12-30-13-10-28/h2-8,15-16H,9-14H2,1H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells by scintillation counting |
J Med Chem 53: 4412-21 (2010)
Article DOI: 10.1021/jm100044a
BindingDB Entry DOI: 10.7270/Q2KK9BZV |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50385656
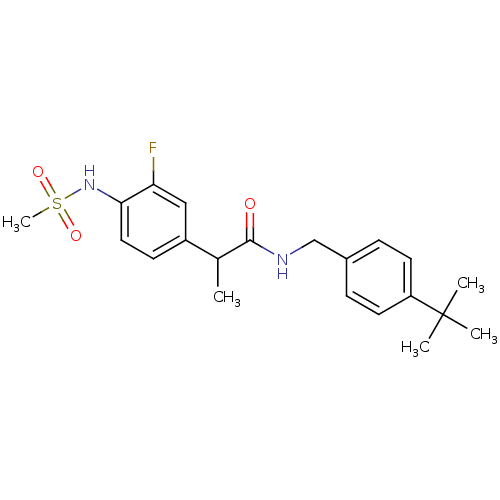
(CHEMBL2042264)Show SMILES CC(C(=O)NCc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C21H27FN2O3S/c1-14(16-8-11-19(18(22)12-16)24-28(5,26)27)20(25)23-13-15-6-9-17(10-7-15)21(2,3)4/h6-12,14,24H,13H2,1-5H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor expressed in CHO cells assessed as decrease in capsaicin-induced intracellular 45Ca2+ uptake after 1 hr b... |
Bioorg Med Chem 20: 215-24 (2011)
Article DOI: 10.1016/j.bmc.2011.11.008
BindingDB Entry DOI: 10.7270/Q2MG7QJJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50319430
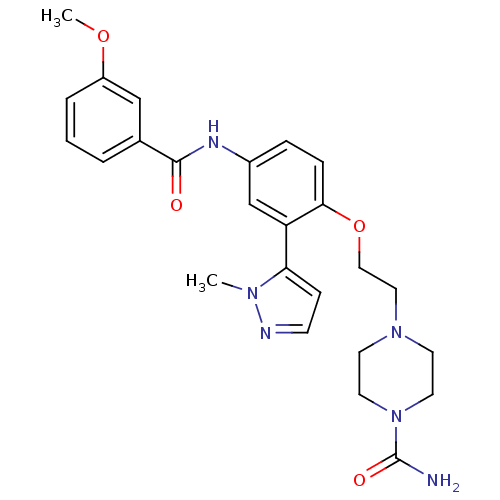
(4-{2-[4-(3-Methoxy-benzoylamino)-2-(2-methyl-2H-py...)Show SMILES COc1cccc(c1)C(=O)Nc1ccc(OCCN2CCN(CC2)C(N)=O)c(c1)-c1ccnn1C Show InChI InChI=1S/C25H30N6O4/c1-29-22(8-9-27-29)21-17-19(28-24(32)18-4-3-5-20(16-18)34-2)6-7-23(21)35-15-14-30-10-12-31(13-11-30)25(26)33/h3-9,16-17H,10-15H2,1-2H3,(H2,26,33)(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells by scintillation counting |
J Med Chem 53: 4412-21 (2010)
Article DOI: 10.1021/jm100044a
BindingDB Entry DOI: 10.7270/Q2KK9BZV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50319425

(CHEMBL1084865 | N-{3-(4-Chloro-2-methyl-2H-pyrazol...)Show SMILES Cn1ncc(Cl)c1-c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1OCCN1CCNC(=O)C1 |(-5.14,-22.2,;-4.83,-23.71,;-5.86,-24.85,;-5.09,-26.19,;-3.58,-25.86,;-2.43,-26.89,;-3.42,-24.34,;-2.09,-23.57,;-.75,-24.34,;.58,-23.57,;1.92,-24.34,;3.25,-23.57,;3.25,-22.03,;4.58,-24.33,;4.58,-25.87,;5.91,-26.64,;7.25,-25.87,;7.24,-24.32,;5.9,-23.56,;8.57,-23.54,;9.91,-24.3,;8.56,-22,;9.89,-22.76,;.58,-22.02,;-.76,-21.26,;-2.09,-22.03,;-3.42,-21.26,;-3.42,-19.72,;-4.76,-18.95,;-4.76,-17.41,;-3.42,-16.64,;-3.42,-15.1,;-4.75,-14.33,;-6.09,-15.09,;-7.42,-14.32,;-6.09,-16.64,)| Show InChI InChI=1S/C24H23ClF3N5O3/c1-32-22(19(25)13-30-32)18-12-17(31-23(35)15-3-2-4-16(11-15)24(26,27)28)5-6-20(18)36-10-9-33-8-7-29-21(34)14-33/h2-6,11-13H,7-10,14H2,1H3,(H,29,34)(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells by scintillation counting |
J Med Chem 53: 4412-21 (2010)
Article DOI: 10.1021/jm100044a
BindingDB Entry DOI: 10.7270/Q2KK9BZV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50346920
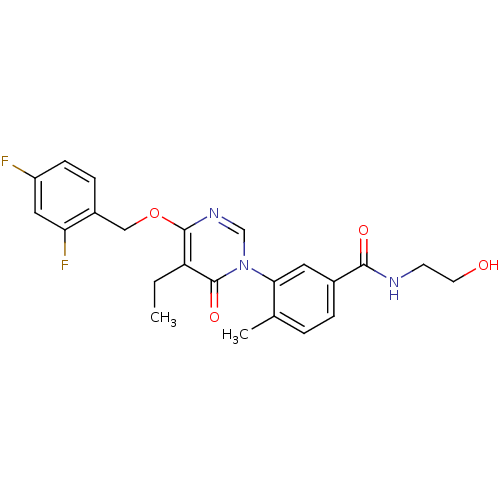
(CHEMBL1795686)Show SMILES CCc1c(OCc2ccc(F)cc2F)ncn(-c2cc(ccc2C)C(=O)NCCO)c1=O Show InChI InChI=1S/C23H23F2N3O4/c1-3-18-22(32-12-16-6-7-17(24)11-19(16)25)27-13-28(23(18)31)20-10-15(5-4-14(20)2)21(30)26-8-9-29/h4-7,10-11,13,29H,3,8-9,12H2,1-2H3,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38beta kinase |
Bioorg Med Chem Lett 21: 3856-60 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.006
BindingDB Entry DOI: 10.7270/Q2G73F2S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data