

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (Sus scrofa) | BDBM50280989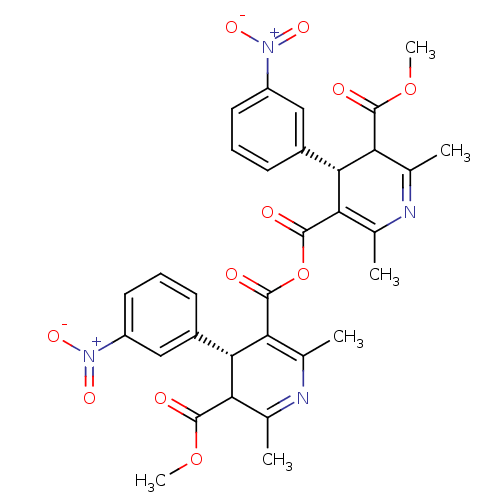 (2,6-dimethyl-5-methyloxycarbonyl-4-(3-nitrophenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its ability to inhibit the binding of [125I]-ET-1 to the Endothelin A receptor in porcine thoracic aorta membranes. | Bioorg Med Chem Lett 3: 2099-2104 (1993) Article DOI: 10.1016/S0960-894X(01)81025-8 BindingDB Entry DOI: 10.7270/Q2DV1JS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D2 (Homo sapiens (Human)) | BDBM123744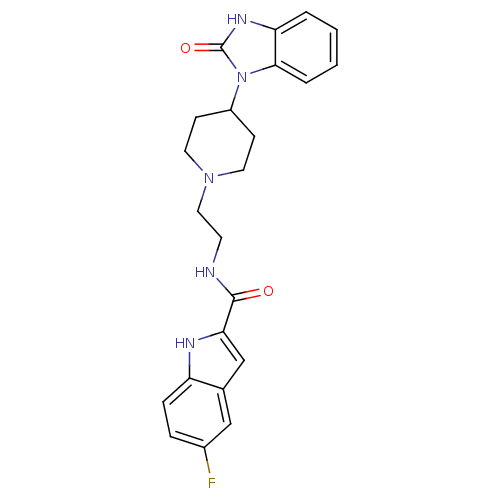 (5-Fluoro-N-(2-(4-(2-Oxo-2,3-dihydro-1H-benzo[d]imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PLD2 | Bioorg Med Chem Lett 17: 2310-1 (2007) Article DOI: 10.1016/j.bmcl.2007.01.059 BindingDB Entry DOI: 10.7270/Q21G0KXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D2 (Homo sapiens (Human)) | BDBM50206156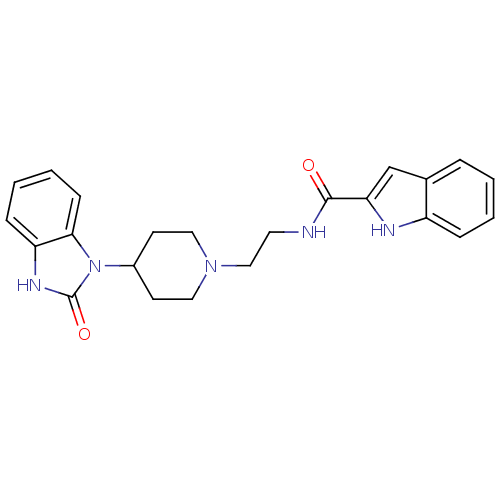 (CHEMBL246240 | N-(2-(4-(2-oxo-2,3-dihydrobenzo[d]i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PLD2 | Bioorg Med Chem Lett 17: 2310-1 (2007) Article DOI: 10.1016/j.bmcl.2007.01.059 BindingDB Entry DOI: 10.7270/Q21G0KXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50280989 (2,6-dimethyl-5-methyloxycarbonyl-4-(3-nitrophenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 295 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its ability to inhibit the binding of [125I]-ET-1 to the Endothelin B receptor in rat cerebellum membranes. | Bioorg Med Chem Lett 3: 2099-2104 (1993) Article DOI: 10.1016/S0960-894X(01)81025-8 BindingDB Entry DOI: 10.7270/Q2DV1JS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50280990 (2,6-dimethyl-5-methyloxycarbonyl-4-(3-nitrophenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 422 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against Endothelin A receptor | Bioorg Med Chem Lett 3: 2099-2104 (1993) Article DOI: 10.1016/S0960-894X(01)81025-8 BindingDB Entry DOI: 10.7270/Q2DV1JS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C/alpha-1D/alpha-1S (Rattus norvegicus-RAT) | BDBM50280989 (2,6-dimethyl-5-methyloxycarbonyl-4-(3-nitrophenyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 592 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]PN-200-110 binding to L-type calcium channels in rat cortex membranes. | Bioorg Med Chem Lett 3: 2099-2104 (1993) Article DOI: 10.1016/S0960-894X(01)81025-8 BindingDB Entry DOI: 10.7270/Q2DV1JS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D2 (Homo sapiens (Human)) | BDBM50206153 (CHEMBL246032 | N-(2-(4-(2-oxo-2,3-dihydro-1H-benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PLD2 | Bioorg Med Chem Lett 17: 2310-1 (2007) Article DOI: 10.1016/j.bmcl.2007.01.059 BindingDB Entry DOI: 10.7270/Q21G0KXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D2 (Homo sapiens (Human)) | BDBM50206155 (CHEMBL246031 | N-(2-(4-(2-oxo-2,3-dihydrobenzo[d]i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PLD2 | Bioorg Med Chem Lett 17: 2310-1 (2007) Article DOI: 10.1016/j.bmcl.2007.01.059 BindingDB Entry DOI: 10.7270/Q21G0KXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D2 (Homo sapiens (Human)) | BDBM50206159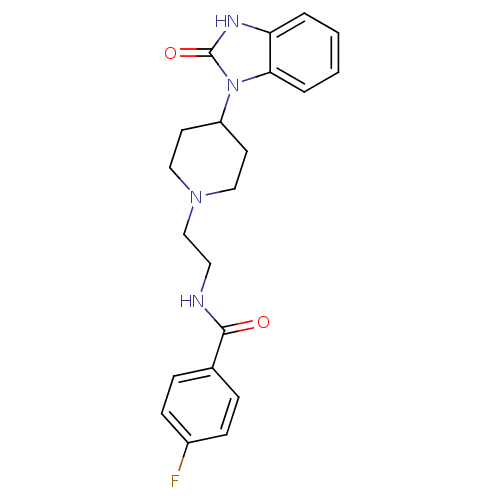 (4-fluoro-N-(2-(4-(2-oxo-2,3-dihydrobenzo[d]imidazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PLD2 | Bioorg Med Chem Lett 17: 2310-1 (2007) Article DOI: 10.1016/j.bmcl.2007.01.059 BindingDB Entry DOI: 10.7270/Q21G0KXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156655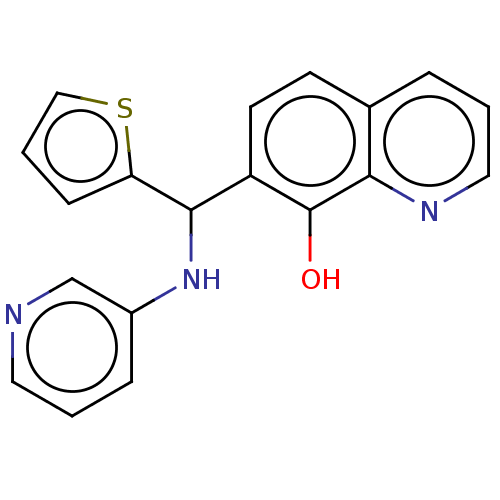 (US9023354, AD4-10315) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D2 (Homo sapiens (Human)) | BDBM50206160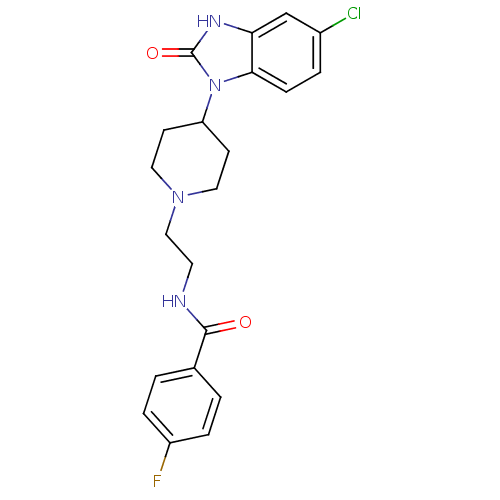 (CHEMBL245621 | Halopemide | Halopemide, 8 | N-(2-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PLD2 | Bioorg Med Chem Lett 17: 2310-1 (2007) Article DOI: 10.1016/j.bmcl.2007.01.059 BindingDB Entry DOI: 10.7270/Q21G0KXW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phospholipase D2 (Homo sapiens (Human)) | BDBM50206154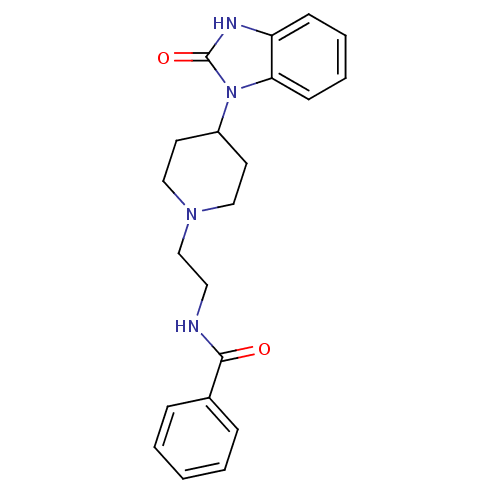 (CHEMBL245623 | N-(2-(4-(2-oxo-2,3-dihydrobenzo[d]i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PLD2 | Bioorg Med Chem Lett 17: 2310-1 (2007) Article DOI: 10.1016/j.bmcl.2007.01.059 BindingDB Entry DOI: 10.7270/Q21G0KXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D2 (Homo sapiens (Human)) | BDBM50206157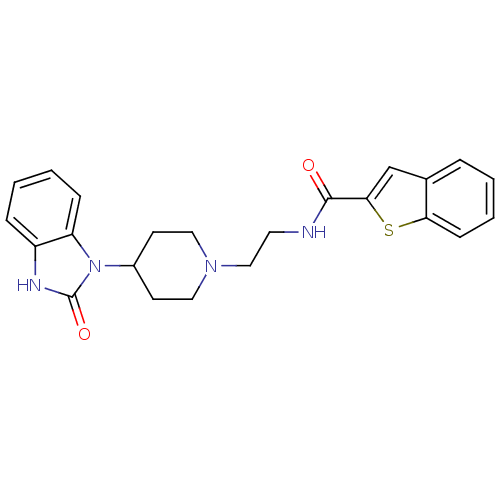 (CHEMBL398568 | N-(2-(4-(2-Oxo-2,3-dihydro-1H-benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PLD2 | Bioorg Med Chem Lett 17: 2310-1 (2007) Article DOI: 10.1016/j.bmcl.2007.01.059 BindingDB Entry DOI: 10.7270/Q21G0KXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156660 (US9023354, AD4-10963) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50280990 (2,6-dimethyl-5-methyloxycarbonyl-4-(3-nitrophenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against Endothelin B receptor | Bioorg Med Chem Lett 3: 2099-2104 (1993) Article DOI: 10.1016/S0960-894X(01)81025-8 BindingDB Entry DOI: 10.7270/Q2DV1JS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50447144 (CHEMBL3112895 | US9023354, AD4-12917) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM47305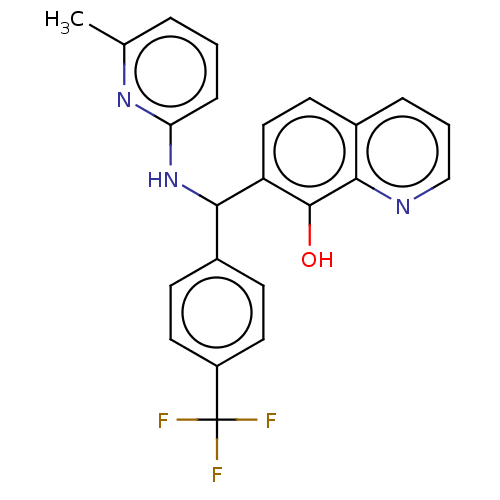 (BDBM156659 | US9023354, AD4-10483) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156422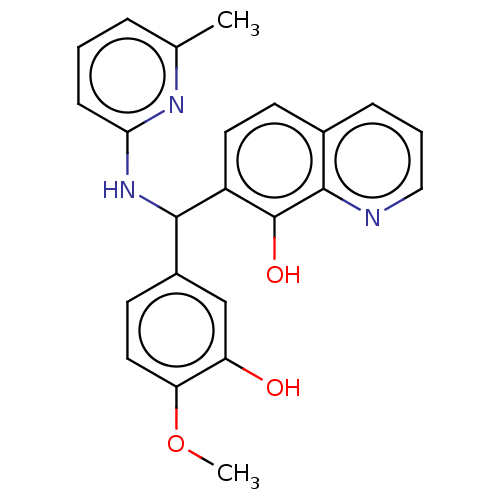 (US9023354, AD4-12909) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156414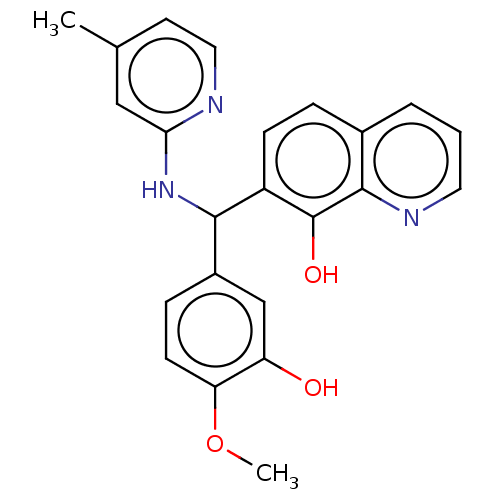 (US9023354, AD4-1505) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156419 (US9023354, AD4-12906) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM47305 (BDBM156659 | US9023354, AD4-10483) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156430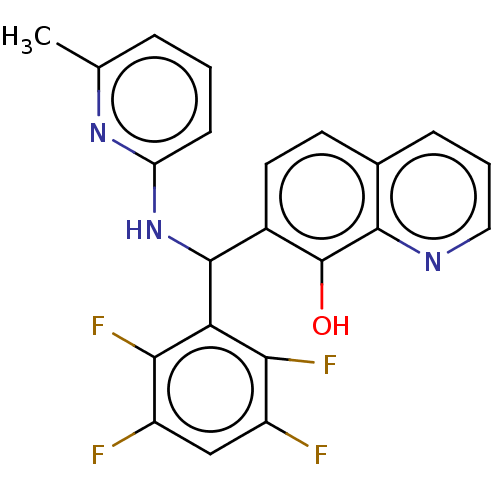 (US9023354, AD4-12918) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156506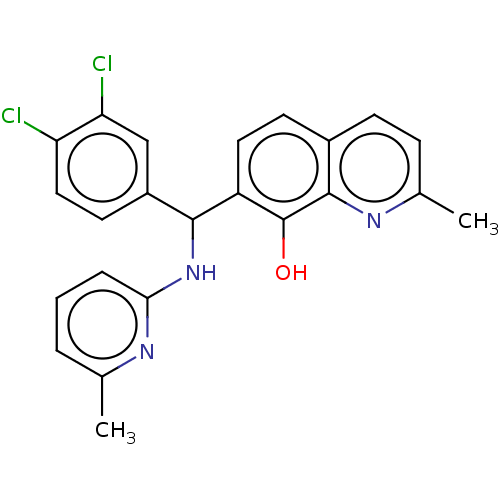 (US9023354, AD4-13080) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156654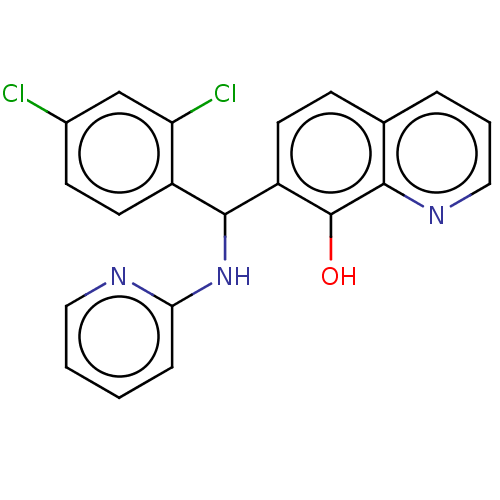 (US9023354, AD4-10484) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156423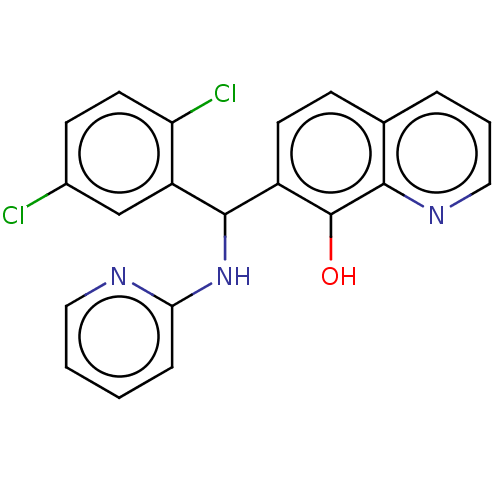 (US9023354, AD4-12910) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156492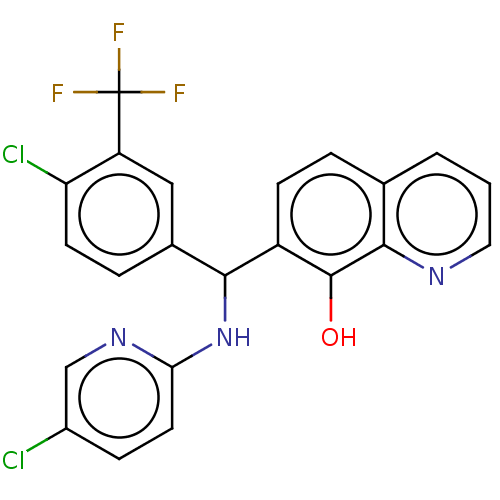 (US9023354, AD4-13067) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156495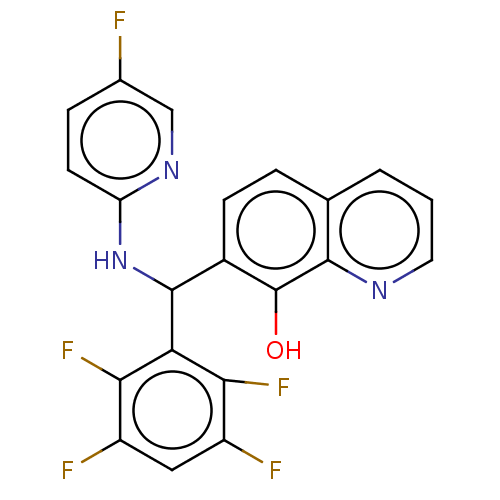 (US9023354, AD4-13070) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM47310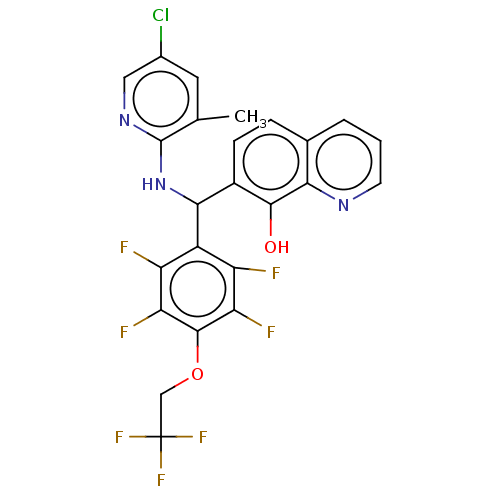 (US9023354, AD4-13243) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156479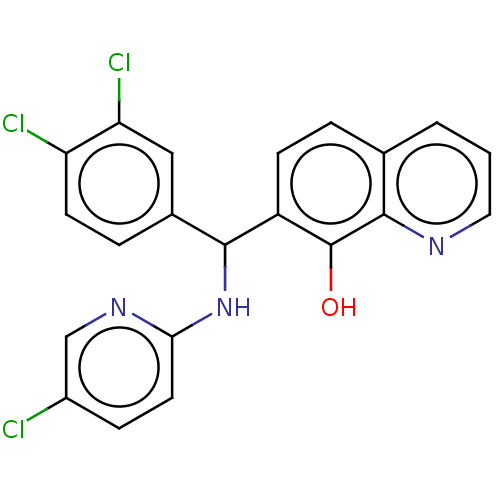 (US9023354, AD4-13054) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156540 (US9023354, AD4-13113) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156420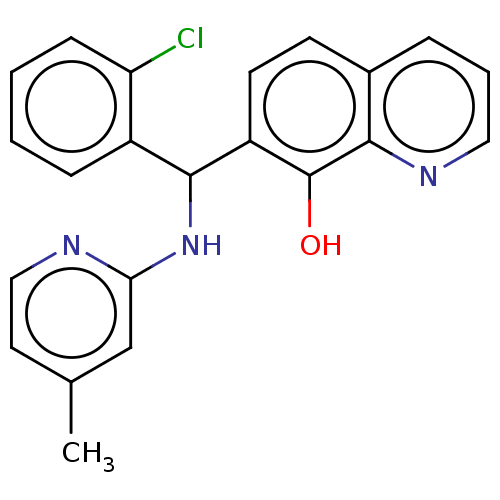 (US9023354, AD4-12907) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156415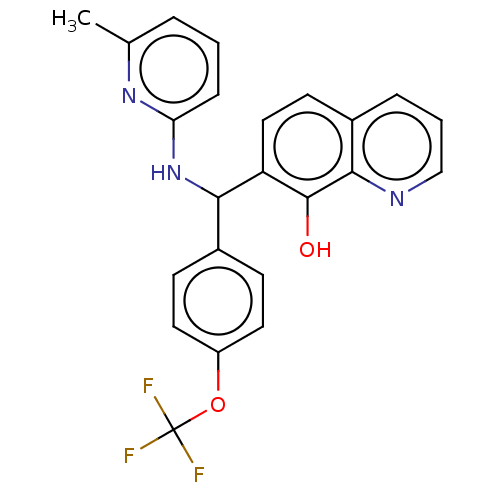 (US9023354, AD4-12902) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156626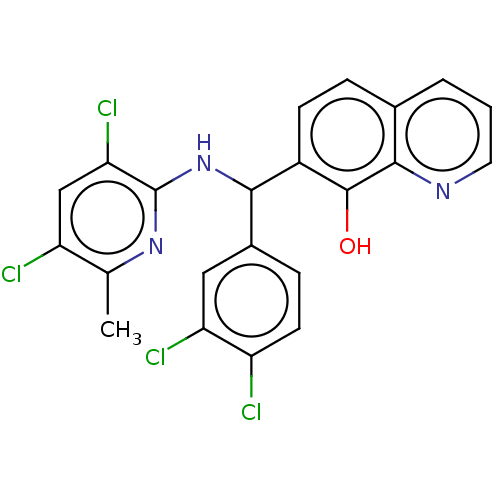 (US9023354, AD4-13202) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156527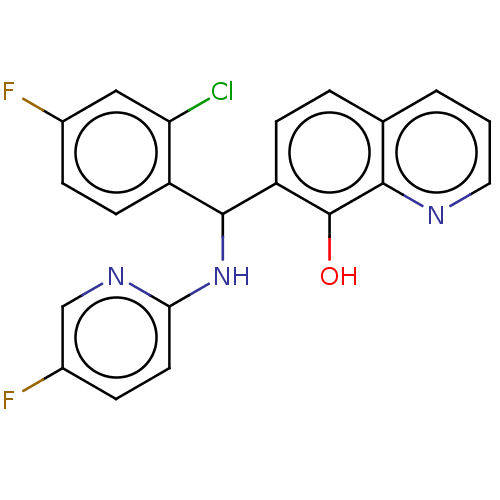 (US9023354, AD4-13099) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156620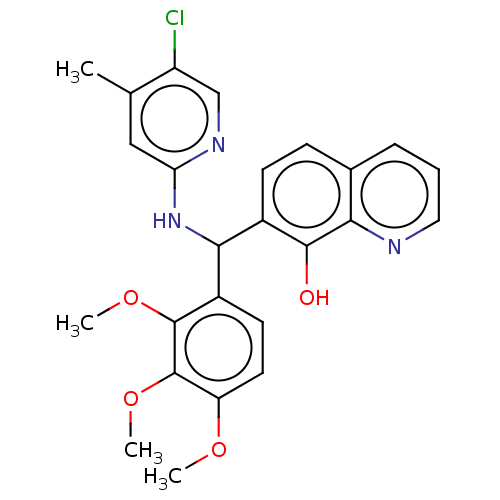 (US9023354, AD4-13196) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156478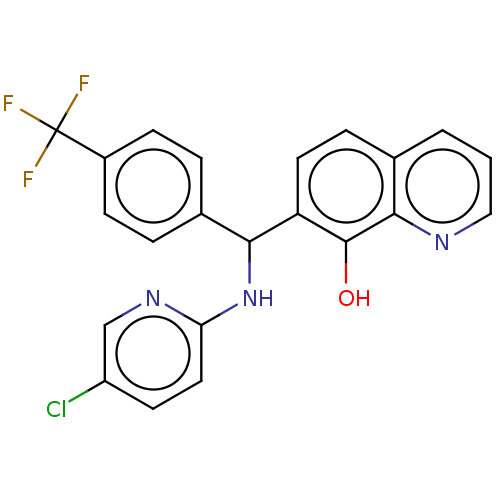 (US9023354, AD4-13053) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156543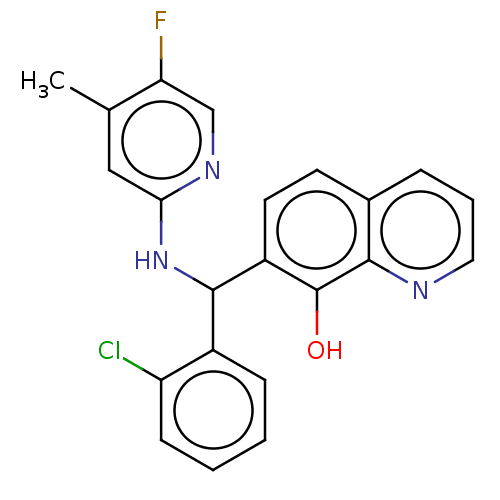 (US9023354, AD4-13116) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156632 (US9023354, AD4-13208) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156418 (US9023354, AD4-12905) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156517 (US9023354, AD4-13089) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM47298 (BDBM64616 | US9023354, AD4-10960) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM47300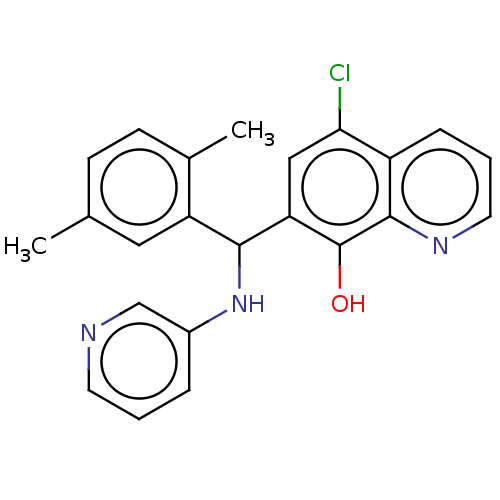 (US9023354, AD4-10944) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156416 (US9023354, AD4-12903) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM47307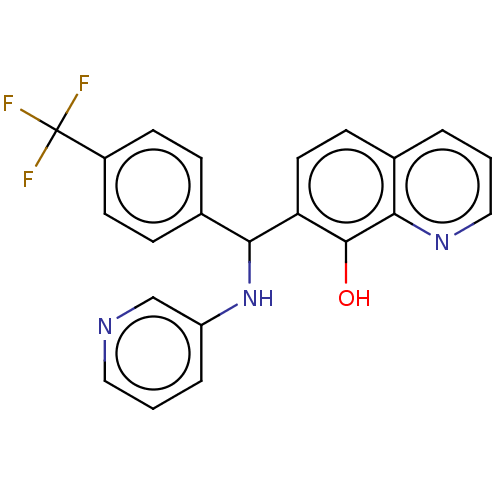 (BDBM50339143 | US9023354, AD4-10942) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM47303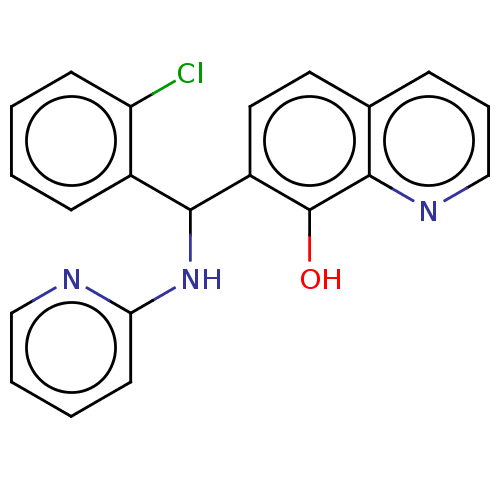 (US9023354, AD4-10482) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156621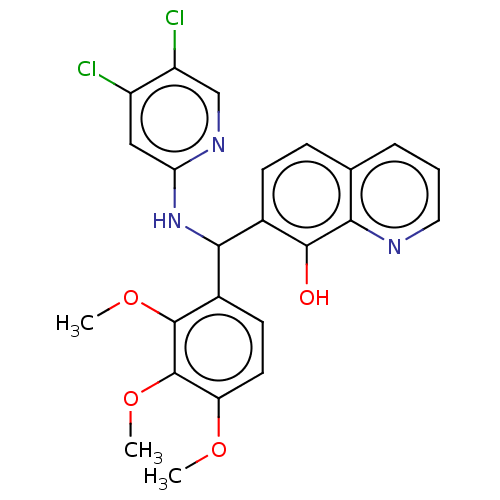 (US9023354, AD4-13197) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156635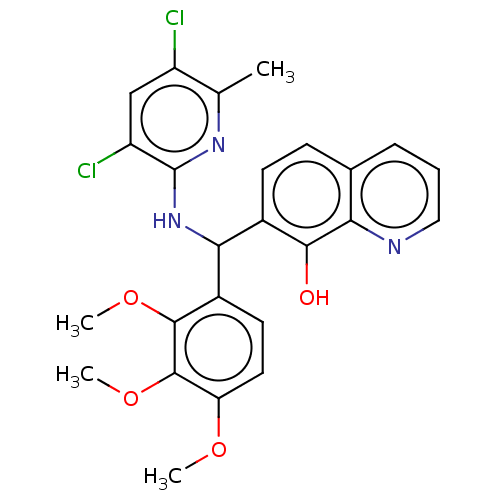 (US9023354, AD4-13210) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156528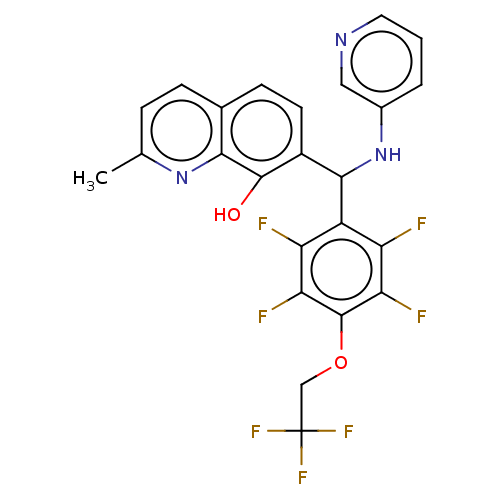 (US9023354, AD4-13101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156639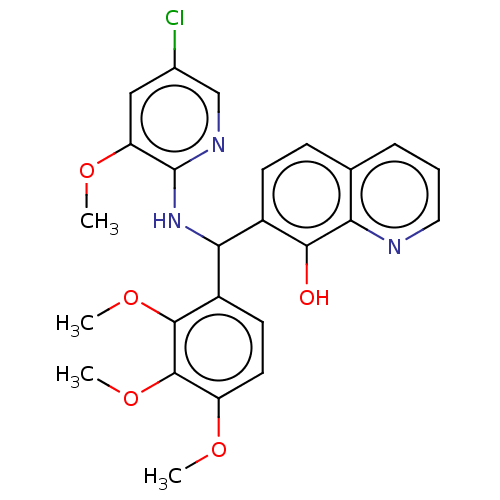 (US9023354, AD4-13214) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM156617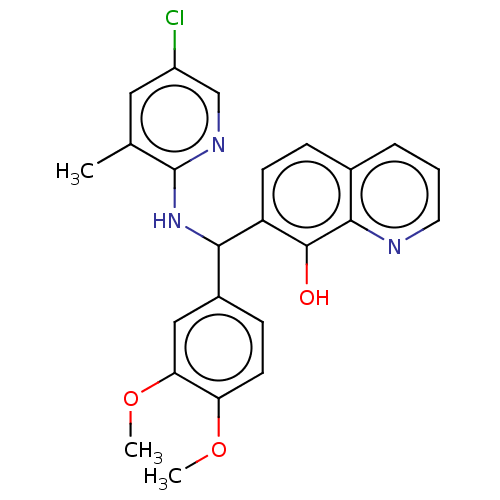 (US9023354, AD4-13193) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
TBA US Patent | Assay Description The following example describes an assay that measured the ability of compounds to inhibit the binding of p53 to MDM2 using the AlphaScreen assay tec... | US Patent US9023354 (2015) BindingDB Entry DOI: 10.7270/Q26M35KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 74 total ) | Next | Last >> |