 Found 33 hits with Last Name = 'nakajima' and Initial = 'n'
Found 33 hits with Last Name = 'nakajima' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Solute carrier family 22 member 6
(Rattus norvegicus) | BDBM50339185
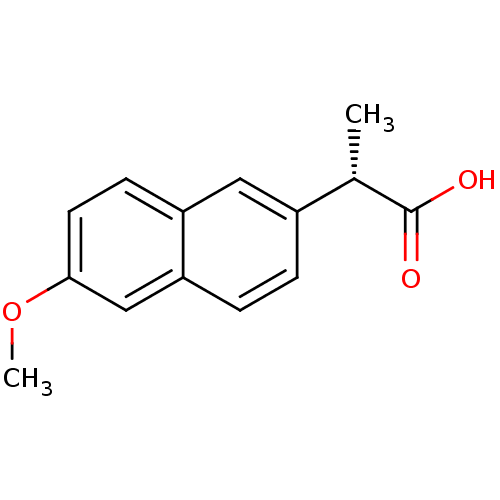
((2S)-2-(6-methoxynaphthalen-2-yl)propanoic acid | ...)Show InChI InChI=1S/C14H14O3/c1-9(14(15)16)10-3-4-12-8-13(17-2)6-5-11(12)7-10/h3-9H,1-2H3,(H,15,16)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of PAH uptake in Xenopus laevis oocytes |
Mol Pharmacol 55: 847-54 (1999)
BindingDB Entry DOI: 10.7270/Q28053WW |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 6
(Rattus norvegicus) | BDBM50009859
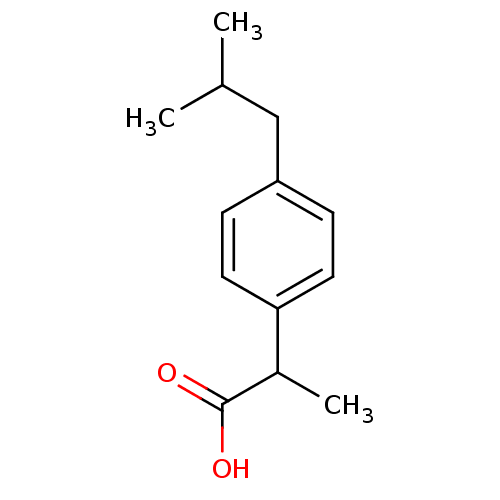
((+-)-2-(p-isobutylphenyl)propionic acid | (+-)-alp...)Show InChI InChI=1S/C13H18O2/c1-9(2)8-11-4-6-12(7-5-11)10(3)13(14)15/h4-7,9-10H,8H2,1-3H3,(H,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of PAH uptake in Xenopus laevis oocytes |
Mol Pharmacol 55: 847-54 (1999)
BindingDB Entry DOI: 10.7270/Q28053WW |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 6
(Rattus norvegicus) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of PAH uptake in Xenopus laevis oocytes |
Mol Pharmacol 55: 847-54 (1999)
BindingDB Entry DOI: 10.7270/Q28053WW |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 6
(Rattus norvegicus) | BDBM50328021
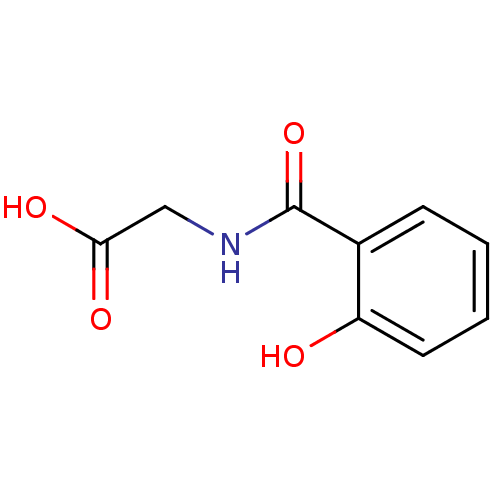
((2-Hydroxy-benzoylamino)-acetic acid | 2-(2-hydrox...)Show InChI InChI=1S/C9H9NO4/c11-7-4-2-1-3-6(7)9(14)10-5-8(12)13/h1-4,11H,5H2,(H,10,14)(H,12,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of PAH uptake in Xenopus laevis oocytes |
Mol Pharmacol 55: 847-54 (1999)
BindingDB Entry DOI: 10.7270/Q28053WW |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 6
(Rattus norvegicus) | BDBM81194

(MLS002154125 | OXYPHENBUTAZONE | SMR001233432 | ci...)Show InChI InChI=1S/C19H20N2O3/c1-2-3-9-17-18(23)20(14-7-5-4-6-8-14)21(19(17)24)15-10-12-16(22)13-11-15/h4-8,10-13,22,24H,2-3,9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of PAH uptake in Xenopus laevis oocytes |
Mol Pharmacol 55: 847-54 (1999)
BindingDB Entry DOI: 10.7270/Q28053WW |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 6
(Rattus norvegicus) | BDBM85245
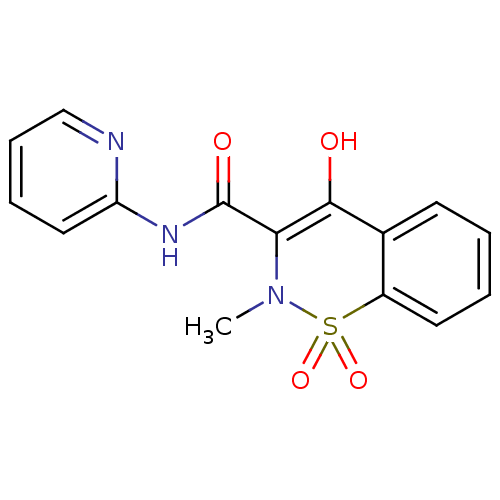
(CAS_36322-90-4 | NSC_4856 | Piroxicam)Show SMILES CN1C(C(=O)Nc2ccccn2)=C(O)c2ccccc2S1(=O)=O |t:12| Show InChI InChI=1S/C15H13N3O4S/c1-18-13(15(20)17-12-8-4-5-9-16-12)14(19)10-6-2-3-7-11(10)23(18,21)22/h2-9,19H,1H3,(H,16,17,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of PAH uptake in Xenopus laevis oocytes |
Mol Pharmacol 55: 847-54 (1999)
BindingDB Entry DOI: 10.7270/Q28053WW |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 6
(Rattus norvegicus) | BDBM26193
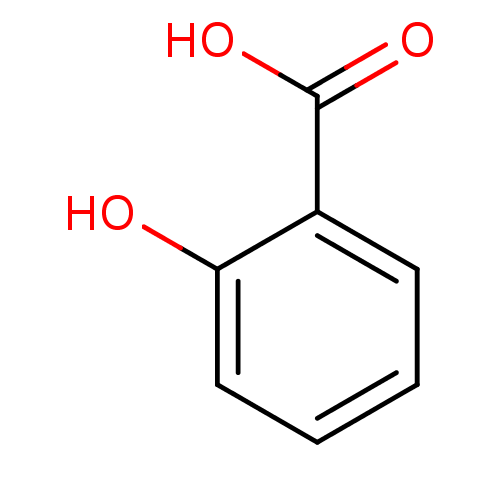
(2-Hydroxybenzoate, I | 2-hydroxybenzoic acid | CHE...)Show InChI InChI=1S/C7H6O3/c8-6-4-2-1-3-5(6)7(9)10/h1-4,8H,(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of PAH uptake in Xenopus laevis oocytes |
Mol Pharmacol 55: 847-54 (1999)
BindingDB Entry DOI: 10.7270/Q28053WW |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 6
(Rattus norvegicus) | BDBM50420241

(ASPIRIN | CHEMBL447221)Show InChI InChI=1S/C9H8O4/c1-6(10)13-8-5-3-2-4-7(8)9(11)12/h2-5H,1H3,(H,11,12)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of PAH uptake in Xenopus laevis oocytes |
Mol Pharmacol 55: 847-54 (1999)
BindingDB Entry DOI: 10.7270/Q28053WW |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 6
(Rattus norvegicus) | BDBM50420191
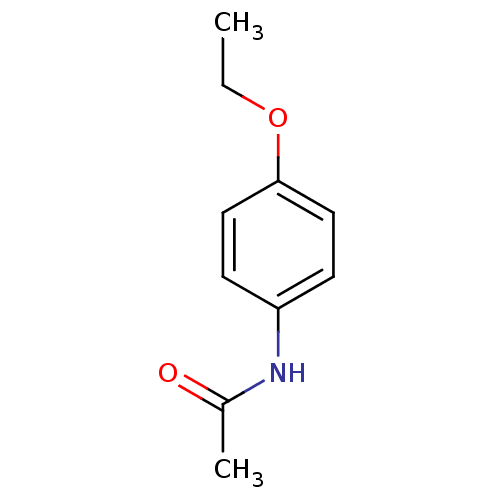
(ACETPHENETIDIN | Acetophenetidin | PHENACETIN)Show InChI InChI=1S/C10H13NO2/c1-3-13-10-6-4-9(5-7-10)11-8(2)12/h4-7H,3H2,1-2H3,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
| PubMed
| 4.88E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of PAH uptake in Xenopus laevis oocytes |
Mol Pharmacol 55: 847-54 (1999)
BindingDB Entry DOI: 10.7270/Q28053WW |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 6
(Rattus norvegicus) | BDBM26197
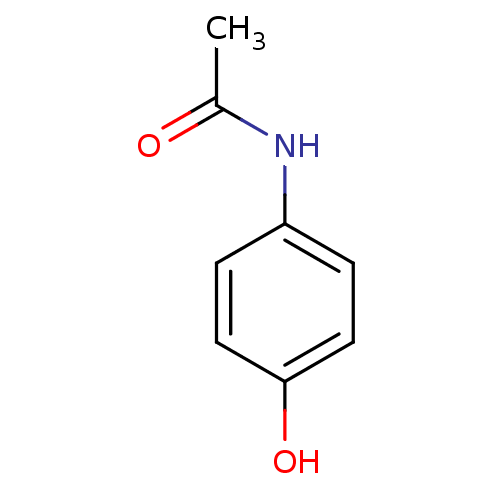
(CHEMBL112 | N-(4-hydroxyphenyl)acetamide | Norco |...)Show InChI InChI=1S/C8H9NO2/c1-6(10)9-7-2-4-8(11)5-3-7/h2-5,11H,1H3,(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PubMed
| 2.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of PAH uptake in Xenopus laevis oocytes |
Mol Pharmacol 55: 847-54 (1999)
BindingDB Entry DOI: 10.7270/Q28053WW |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50342601

(CHEMBL1255901 | Huperzine A)Show SMILES C\C=C1/[C@@H]2Cc3[nH]c(=O)ccc3[C@]1(N)CC(C)=C2 |r,c:18,TLB:1:2:11.5.4:17.14.15| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Acetylcholinesterase (AChE). |
Bioorg Med Chem Lett 6: 1927-1930 (1996)
Article DOI: 10.1016/0960-894X(96)00337-X
BindingDB Entry DOI: 10.7270/Q2P84CD1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM19264

((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...)Show InChI InChI=1S/C17H20O6/c1-9(5-7-13(18)19)4-6-11-15(20)14-12(8-23-17(14)21)10(2)16(11)22-3/h4,20H,5-8H2,1-3H3,(H,18,19)/b9-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 expressed in Escherichia coli strain BL21(DE3) after 60 mins |
Bioorg Med Chem 18: 8106-11 (2010)
Article DOI: 10.1016/j.bmc.2010.09.004
BindingDB Entry DOI: 10.7270/Q2Q52PWJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM19264

((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...)Show InChI InChI=1S/C17H20O6/c1-9(5-7-13(18)19)4-6-11-15(20)14-12(8-23-17(14)21)10(2)16(11)22-3/h4,20H,5-8H2,1-3H3,(H,18,19)/b9-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH1 expressed in Escherichia coli strain BL21(DE3) after 60 mins |
Bioorg Med Chem 18: 8106-11 (2010)
Article DOI: 10.1016/j.bmc.2010.09.004
BindingDB Entry DOI: 10.7270/Q2Q52PWJ |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50054017
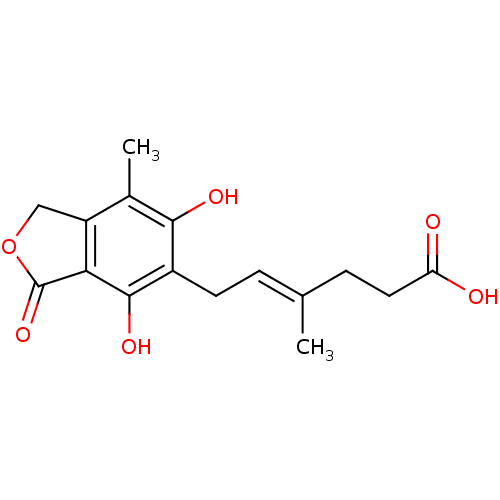
((E)-6-(4,6-Dihydroxy-7-methyl-3-oxo-1,3-dihydro-is...)Show InChI InChI=1S/C16H18O6/c1-8(4-6-12(17)18)3-5-10-14(19)9(2)11-7-22-16(21)13(11)15(10)20/h3,19-20H,4-7H2,1-2H3,(H,17,18)/b8-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH1 expressed in Escherichia coli strain BL21(DE3) after 60 mins |
Bioorg Med Chem 18: 8106-11 (2010)
Article DOI: 10.1016/j.bmc.2010.09.004
BindingDB Entry DOI: 10.7270/Q2Q52PWJ |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50054017

((E)-6-(4,6-Dihydroxy-7-methyl-3-oxo-1,3-dihydro-is...)Show InChI InChI=1S/C16H18O6/c1-8(4-6-12(17)18)3-5-10-14(19)9(2)11-7-22-16(21)13(11)15(10)20/h3,19-20H,4-7H2,1-2H3,(H,17,18)/b8-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 expressed in Escherichia coli strain BL21(DE3) after 60 mins |
Bioorg Med Chem 18: 8106-11 (2010)
Article DOI: 10.1016/j.bmc.2010.09.004
BindingDB Entry DOI: 10.7270/Q2Q52PWJ |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50228085

(CHEMBL238461 | N-hydroxy-6-(4-hydroxy-6-methoxy-7-...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1C\C=C(/C)CCC(=O)NO Show InChI InChI=1S/C17H21NO6/c1-9(5-7-13(19)18-22)4-6-11-15(20)14-12(8-24-17(14)21)10(2)16(11)23-3/h4,20,22H,5-8H2,1-3H3,(H,18,19)/b9-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH1 expressed in Escherichia coli strain BL21(DE3) after 60 mins |
Bioorg Med Chem 18: 8106-11 (2010)
Article DOI: 10.1016/j.bmc.2010.09.004
BindingDB Entry DOI: 10.7270/Q2Q52PWJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50287625
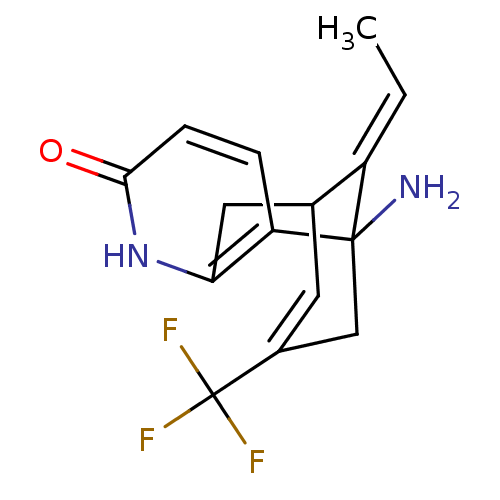
(1-Amino-13-eth-(E)-ylidene-11-trifluoromethyl-6-az...)Show SMILES C\C=C1/C2Cc3[nH]c(=O)ccc3C1(N)CC(=C2)C(F)(F)F |c:17,TLB:10:11:2:15.14.16,THB:6:5:2:15.14.16| Show InChI InChI=1S/C15H15F3N2O/c1-2-10-8-5-9(15(16,17)18)7-14(10,19)11-3-4-13(21)20-12(11)6-8/h2-5,8H,6-7,19H2,1H3,(H,20,21)/b10-2+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Acetylcholinesterase (AChE). |
Bioorg Med Chem Lett 6: 1927-1930 (1996)
Article DOI: 10.1016/0960-894X(96)00337-X
BindingDB Entry DOI: 10.7270/Q2P84CD1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50228085

(CHEMBL238461 | N-hydroxy-6-(4-hydroxy-6-methoxy-7-...)Show SMILES COc1c(C)c2COC(=O)c2c(O)c1C\C=C(/C)CCC(=O)NO Show InChI InChI=1S/C17H21NO6/c1-9(5-7-13(19)18-22)4-6-11-15(20)14-12(8-24-17(14)21)10(2)16(11)23-3/h4,20,22H,5-8H2,1-3H3,(H,18,19)/b9-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 expressed in Escherichia coli strain BL21(DE3) after 60 mins |
Bioorg Med Chem 18: 8106-11 (2010)
Article DOI: 10.1016/j.bmc.2010.09.004
BindingDB Entry DOI: 10.7270/Q2Q52PWJ |
More data for this
Ligand-Target Pair | |
Prolyl oligopeptidase family protein
(Flavobacterium psychrophilum (strain JIP02/86 / AT...) | BDBM50455076

(CHEMBL4214751)Show SMILES CC(=O)Oc1c(OC(C)=O)c2c3cc(O)c(O)cc3oc2c2oc3cc(O)c(O)cc3c12 Show InChI InChI=1S/C22H14O10/c1-7(23)29-19-17-9-3-11(25)13(27)5-15(9)31-21(17)22-18(20(19)30-8(2)24)10-4-12(26)14(28)6-16(10)32-22/h3-6,25-28H,1-2H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN CSRS
Curated by ChEMBL
| Assay Description
Inhibition of Flavobacterium POP preincubated for 5 mins followed by Z-Gly-Pro-pNA substrate measured after 30 mins |
Bioorg Med Chem Lett 28: 930-933 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.054
BindingDB Entry DOI: 10.7270/Q26M39DX |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50331099
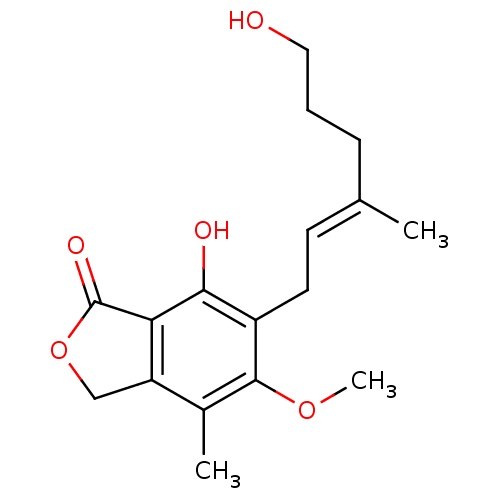
(7-Hydroxy-6-((E)-6-hydroxy-3-methyl-hex-2-enyl)-5-...)Show InChI InChI=1S/C17H22O5/c1-10(5-4-8-18)6-7-12-15(19)14-13(9-22-17(14)20)11(2)16(12)21-3/h6,18-19H,4-5,7-9H2,1-3H3/b10-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH1 expressed in Escherichia coli strain BL21(DE3) after 60 mins |
Bioorg Med Chem 18: 8106-11 (2010)
Article DOI: 10.1016/j.bmc.2010.09.004
BindingDB Entry DOI: 10.7270/Q2Q52PWJ |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50331099

(7-Hydroxy-6-((E)-6-hydroxy-3-methyl-hex-2-enyl)-5-...)Show InChI InChI=1S/C17H22O5/c1-10(5-4-8-18)6-7-12-15(19)14-13(9-22-17(14)20)11(2)16(12)21-3/h6,18-19H,4-5,7-9H2,1-3H3/b10-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 expressed in Escherichia coli strain BL21(DE3) after 60 mins |
Bioorg Med Chem 18: 8106-11 (2010)
Article DOI: 10.1016/j.bmc.2010.09.004
BindingDB Entry DOI: 10.7270/Q2Q52PWJ |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50455075
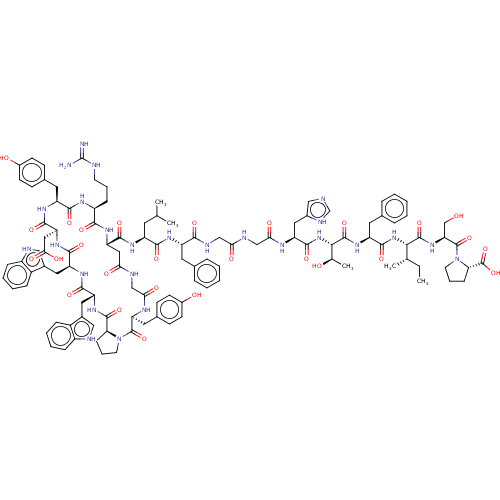
(CHEMBL4209844)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CC(=O)NCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N2CCC[C@H]2C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1)[C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C113H142N26O27/c1-6-61(4)95(108(161)135-87(58-140)111(164)139-41-19-30-89(139)112(165)166)136-105(158)80(44-64-22-11-8-12-23-64)134-109(162)96(62(5)141)137-106(159)83(49-69-54-116-59-123-69)124-92(146)57-121-91(145)55-122-97(150)78(43-63-20-9-7-10-21-63)128-99(152)77(42-60(2)3)127-103(156)84-50-90(144)120-56-93(147)125-86(46-66-33-37-71(143)38-34-66)110(163)138-40-18-29-88(138)107(160)133-82(48-68-53-119-75-27-16-14-25-73(68)75)102(155)130-81(47-67-52-118-74-26-15-13-24-72(67)74)101(154)132-85(51-94(148)149)104(157)129-79(45-65-31-35-70(142)36-32-65)100(153)126-76(98(151)131-84)28-17-39-117-113(114)115/h7-16,20-27,31-38,52-54,59-62,76-89,95-96,118-119,140-143H,6,17-19,28-30,39-51,55-58H2,1-5H3,(H,116,123)(H,120,144)(H,121,145)(H,122,150)(H,124,146)(H,125,147)(H,126,153)(H,127,156)(H,128,152)(H,129,157)(H,130,155)(H,131,151)(H,132,154)(H,133,160)(H,134,162)(H,135,161)(H,136,158)(H,137,159)(H,148,149)(H,165,166)(H4,114,115,117)/t61-,62+,76-,77-,78-,79-,80-,81-,82+,83-,84-,85-,86-,87-,88-,89-,95-,96-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN CSRS
Curated by ChEMBL
| Assay Description
Inhibition of prolyl oligopeptidase (unknown origin) |
Bioorg Med Chem Lett 28: 930-933 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.054
BindingDB Entry DOI: 10.7270/Q26M39DX |
More data for this
Ligand-Target Pair | |
Prolyl oligopeptidase family protein
(Flavobacterium psychrophilum (strain JIP02/86 / AT...) | BDBM50269566
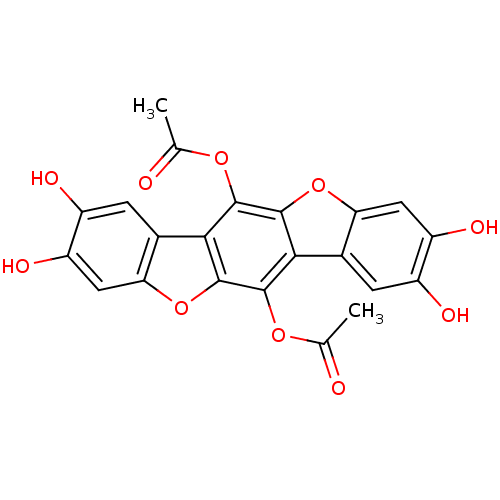
(CHEMBL458248 | polyozellin)Show SMILES CC(=O)Oc1c2oc3cc(O)c(O)cc3c2c(OC(C)=O)c2oc3cc(O)c(O)cc3c12 Show InChI InChI=1S/C22H14O10/c1-7(23)29-19-17-9-3-11(25)13(27)5-15(9)32-22(17)20(30-8(2)24)18-10-4-12(26)14(28)6-16(10)31-21(18)19/h3-6,25-28H,1-2H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN CSRS
Curated by ChEMBL
| Assay Description
Inhibition of Flavobacterium POP preincubated for 5 mins followed by Z-Gly-Pro-pNA substrate measured after 30 mins |
Bioorg Med Chem Lett 28: 930-933 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.054
BindingDB Entry DOI: 10.7270/Q26M39DX |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50331100
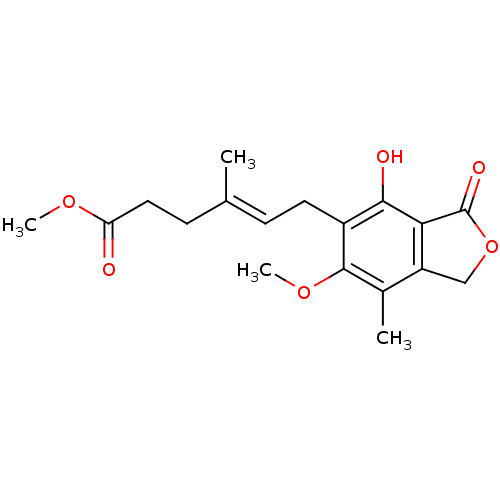
(CHEMBL237760 | methyl 6-(4-hydroxy-6-methoxy-7-met...)Show SMILES COC(=O)CC\C(C)=C\Cc1c(O)c2C(=O)OCc2c(C)c1OC Show InChI InChI=1S/C18H22O6/c1-10(6-8-14(19)22-3)5-7-12-16(20)15-13(9-24-18(15)21)11(2)17(12)23-4/h5,20H,6-9H2,1-4H3/b10-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 expressed in Escherichia coli strain BL21(DE3) after 60 mins |
Bioorg Med Chem 18: 8106-11 (2010)
Article DOI: 10.1016/j.bmc.2010.09.004
BindingDB Entry DOI: 10.7270/Q2Q52PWJ |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50331100

(CHEMBL237760 | methyl 6-(4-hydroxy-6-methoxy-7-met...)Show SMILES COC(=O)CC\C(C)=C\Cc1c(O)c2C(=O)OCc2c(C)c1OC Show InChI InChI=1S/C18H22O6/c1-10(6-8-14(19)22-3)5-7-12-16(20)15-13(9-24-18(15)21)11(2)17(12)23-4/h5,20H,6-9H2,1-4H3/b10-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH1 expressed in Escherichia coli strain BL21(DE3) after 60 mins |
Bioorg Med Chem 18: 8106-11 (2010)
Article DOI: 10.1016/j.bmc.2010.09.004
BindingDB Entry DOI: 10.7270/Q2Q52PWJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50287624
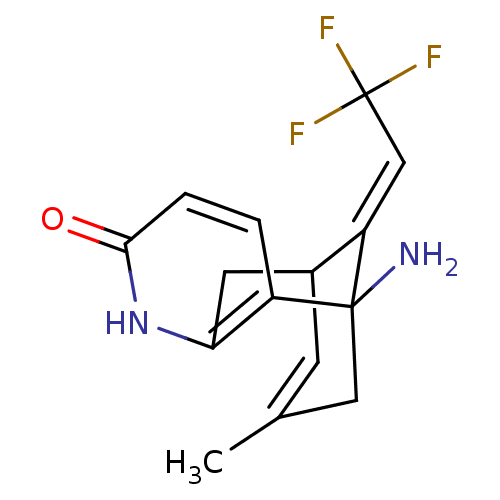
(1-Amino-13-eth-(E)-ylidene-11-trifluoromethyl-6-az...)Show SMILES CC1=CC2Cc3[nH]c(=O)ccc3C(N)(C1)\C2=C\C(F)(F)F |t:1,TLB:6:5:15:2.14.1,THB:10:11:15:2.14.1| Show InChI InChI=1S/C15H15F3N2O/c1-8-4-9-5-12-10(2-3-13(21)20-12)14(19,6-8)11(9)7-15(16,17)18/h2-4,7,9H,5-6,19H2,1H3,(H,20,21)/b11-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Acetylcholinesterase (AChE). |
Bioorg Med Chem Lett 6: 1927-1930 (1996)
Article DOI: 10.1016/0960-894X(96)00337-X
BindingDB Entry DOI: 10.7270/Q2P84CD1 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50269566

(CHEMBL458248 | polyozellin)Show SMILES CC(=O)Oc1c2oc3cc(O)c(O)cc3c2c(OC(C)=O)c2oc3cc(O)c(O)cc3c12 Show InChI InChI=1S/C22H14O10/c1-7(23)29-19-17-9-3-11(25)13(27)5-15(9)32-22(17)20(30-8(2)24)18-10-4-12(26)14(28)6-16(10)31-21(18)19/h3-6,25-28H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN CSRS
Curated by ChEMBL
| Assay Description
Inhibition of prolyl oligopeptidase (unknown origin) |
Bioorg Med Chem Lett 28: 930-933 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.054
BindingDB Entry DOI: 10.7270/Q26M39DX |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50287623

(1-Amino-13-[2,2,2-trifluoro-eth-(E)-ylidene]-11-tr...)Show SMILES NC12CC(=CC(Cc3[nH]c(=O)ccc13)\C2=C/C(F)(F)F)C(F)(F)F |c:3,TLB:8:7:14:3.2.4,THB:12:13:14:3.2.4| Show InChI InChI=1S/C15H12F6N2O/c16-14(17,18)6-10-7-3-8(15(19,20)21)5-13(10,22)9-1-2-12(24)23-11(9)4-7/h1-3,6-7H,4-5,22H2,(H,23,24)/b10-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Acetylcholinesterase (AChE). |
Bioorg Med Chem Lett 6: 1927-1930 (1996)
Article DOI: 10.1016/0960-894X(96)00337-X
BindingDB Entry DOI: 10.7270/Q2P84CD1 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50331098
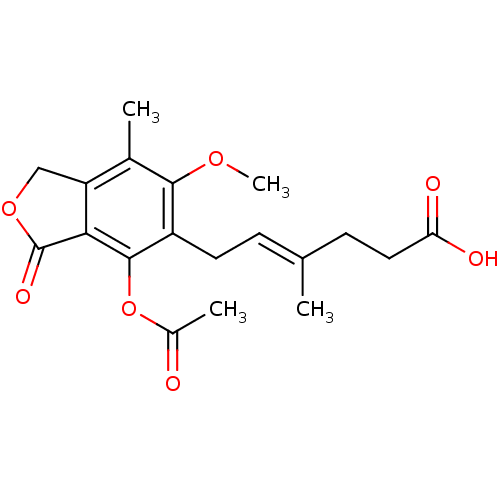
(6-(4-acetoxy-6-methoxy-7-methyl-3-oxo-1,3-dihydroi...)Show SMILES COc1c(C)c2COC(=O)c2c(OC(C)=O)c1C\C=C(/C)CCC(O)=O Show InChI InChI=1S/C19H22O7/c1-10(6-8-15(21)22)5-7-13-17(24-4)11(2)14-9-25-19(23)16(14)18(13)26-12(3)20/h5H,6-9H2,1-4H3,(H,21,22)/b10-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 expressed in Escherichia coli strain BL21(DE3) after 60 mins |
Bioorg Med Chem 18: 8106-11 (2010)
Article DOI: 10.1016/j.bmc.2010.09.004
BindingDB Entry DOI: 10.7270/Q2Q52PWJ |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50331098

(6-(4-acetoxy-6-methoxy-7-methyl-3-oxo-1,3-dihydroi...)Show SMILES COc1c(C)c2COC(=O)c2c(OC(C)=O)c1C\C=C(/C)CCC(O)=O Show InChI InChI=1S/C19H22O7/c1-10(6-8-15(21)22)5-7-13-17(24-4)11(2)14-9-25-19(23)16(14)18(13)26-12(3)20/h5H,6-9H2,1-4H3,(H,21,22)/b10-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH1 expressed in Escherichia coli strain BL21(DE3) after 60 mins |
Bioorg Med Chem 18: 8106-11 (2010)
Article DOI: 10.1016/j.bmc.2010.09.004
BindingDB Entry DOI: 10.7270/Q2Q52PWJ |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 1
(Homo sapiens (Human)) | BDBM50331101
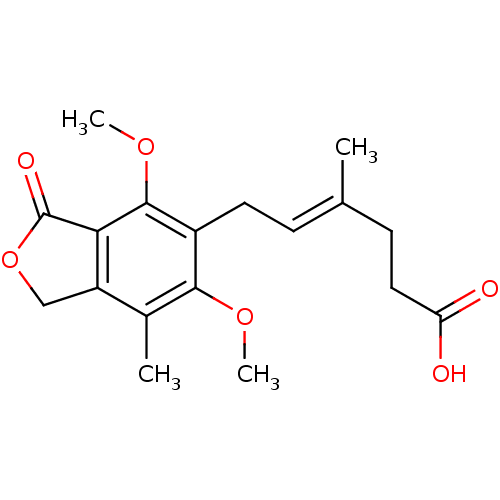
(6-(4,6-dimethoxy-7-methyl-3-oxo-1,3-dihydroisobenz...)Show SMILES COc1c2C(=O)OCc2c(C)c(OC)c1C\C=C(/C)CCC(O)=O Show InChI InChI=1S/C18H22O6/c1-10(6-8-14(19)20)5-7-12-16(22-3)11(2)13-9-24-18(21)15(13)17(12)23-4/h5H,6-9H2,1-4H3,(H,19,20)/b10-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH1 expressed in Escherichia coli strain BL21(DE3) after 60 mins |
Bioorg Med Chem 18: 8106-11 (2010)
Article DOI: 10.1016/j.bmc.2010.09.004
BindingDB Entry DOI: 10.7270/Q2Q52PWJ |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase 2
(Homo sapiens (Human)) | BDBM50331101

(6-(4,6-dimethoxy-7-methyl-3-oxo-1,3-dihydroisobenz...)Show SMILES COc1c2C(=O)OCc2c(C)c(OC)c1C\C=C(/C)CCC(O)=O Show InChI InChI=1S/C18H22O6/c1-10(6-8-14(19)20)5-7-12-16(22-3)11(2)13-9-24-18(21)15(13)17(12)23-4/h5H,6-9H2,1-4H3,(H,19,20)/b10-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human IMPDH2 expressed in Escherichia coli strain BL21(DE3) after 60 mins |
Bioorg Med Chem 18: 8106-11 (2010)
Article DOI: 10.1016/j.bmc.2010.09.004
BindingDB Entry DOI: 10.7270/Q2Q52PWJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM19264

((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...)Show InChI InChI=1S/C17H20O6/c1-9(5-7-13(18)19)4-6-11-15(20)14-12(8-23-17(14)21)10(2)16(11)22-3/h4,20H,5-8H2,1-3H3,(H,18,19)/b9-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma |
Bioorg Med Chem Lett 17: 4767-70 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.059
BindingDB Entry DOI: 10.7270/Q21R6Q65 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data