 Found 106 hits with Last Name = 'nakamura' and Initial = 'r'
Found 106 hits with Last Name = 'nakamura' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Androgen receptor
(Homo sapiens (Human)) | BDBM50317662
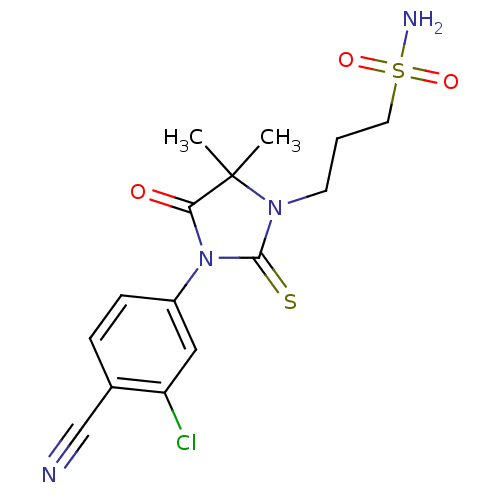
(3-[3-(3-Chloro-4-cyanophenyl)-5,5-dimethyl-4-oxo-2...)Show SMILES CC1(C)N(CCCS(N)(=O)=O)C(=S)N(C1=O)c1ccc(C#N)c(Cl)c1 Show InChI InChI=1S/C15H17ClN4O3S2/c1-15(2)13(21)20(11-5-4-10(9-17)12(16)8-11)14(24)19(15)6-3-7-25(18,22)23/h4-5,8H,3,6-7H2,1-2H3,(H2,18,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at AR in human bicalutamide-resistant LNCAP cells assessed as inhibition of cell proliferation after 6 days |
Bioorg Med Chem 18: 8150-7 (2010)
Article DOI: 10.1016/j.bmc.2010.10.023
BindingDB Entry DOI: 10.7270/Q29S1S1P |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50331872

(5-[3-(4-Cyano-3-trifluoromethylphenyl)-5,5-dimethy...)Show SMILES CC1(C)N(C(=S)N(C1=O)c1ccc(C#N)c(c1)C(F)(F)F)c1cncc(c1)S(N)(=O)=O Show InChI InChI=1S/C18H14F3N5O3S2/c1-17(2)15(27)25(11-4-3-10(7-22)14(6-11)18(19,20)21)16(30)26(17)12-5-13(9-24-8-12)31(23,28)29/h3-6,8-9H,1-2H3,(H2,23,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at AR in human bicalutamide-resistant LNCAP cells assessed as inhibition of cell proliferation after 6 days |
Bioorg Med Chem 18: 8150-7 (2010)
Article DOI: 10.1016/j.bmc.2010.10.023
BindingDB Entry DOI: 10.7270/Q29S1S1P |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50331871

(5-[3-(3-Chloro-4-cyanophenyl)-5,5-dimethyl-4-oxo-2...)Show SMILES CC1(C)N(C(=S)N(C1=O)c1ccc(C#N)c(Cl)c1)c1cncc(c1)S(N)(=O)=O Show InChI InChI=1S/C17H14ClN5O3S2/c1-17(2)15(24)22(11-4-3-10(7-19)14(18)6-11)16(27)23(17)12-5-13(9-21-8-12)28(20,25)26/h3-6,8-9H,1-2H3,(H2,20,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at AR in human bicalutamide-resistant LNCAP cells assessed as inhibition of cell proliferation after 6 days |
Bioorg Med Chem 18: 8150-7 (2010)
Article DOI: 10.1016/j.bmc.2010.10.023
BindingDB Entry DOI: 10.7270/Q29S1S1P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50596488
(CHEMBL5179953) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128570
BindingDB Entry DOI: 10.7270/Q2ZW1QZW |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50331870

(4-[3-(4-Cyano-3-trifluoromethylphenyl)-5,5-dimethy...)Show SMILES CC1(C)N(C(=S)N(C1=O)c1ccc(C#N)c(c1)C(F)(F)F)c1ccnc(c1)S(N)(=O)=O Show InChI InChI=1S/C18H14F3N5O3S2/c1-17(2)15(27)25(11-4-3-10(9-22)13(7-11)18(19,20)21)16(30)26(17)12-5-6-24-14(8-12)31(23,28)29/h3-8H,1-2H3,(H2,23,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at AR in human bicalutamide-resistant LNCAP cells assessed as inhibition of cell proliferation after 6 days |
Bioorg Med Chem 18: 8150-7 (2010)
Article DOI: 10.1016/j.bmc.2010.10.023
BindingDB Entry DOI: 10.7270/Q29S1S1P |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50331866

(3-[3-(3-Chloro-4-cyanophenyl)-5,5-dimethyl-4-oxo-2...)Show SMILES CC1(C)N(C(=S)N(C1=O)c1ccc(C#N)c(Cl)c1)c1cccc(c1)S(N)(=O)=O Show InChI InChI=1S/C18H15ClN4O3S2/c1-18(2)16(24)22(12-7-6-11(10-20)15(19)9-12)17(27)23(18)13-4-3-5-14(8-13)28(21,25)26/h3-9H,1-2H3,(H2,21,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at AR in human bicalutamide-resistant LNCAP cells assessed as inhibition of cell proliferation after 6 days |
Bioorg Med Chem 18: 8150-7 (2010)
Article DOI: 10.1016/j.bmc.2010.10.023
BindingDB Entry DOI: 10.7270/Q29S1S1P |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50331869

(6-[3-(4-Cyano-3-trifluoromethylphenyl)-5,5-dimethy...)Show SMILES CC1(C)N(C(=S)N(C1=O)c1ccc(C#N)c(c1)C(F)(F)F)c1cccc(n1)S(N)(=O)=O Show InChI InChI=1S/C18H14F3N5O3S2/c1-17(2)15(27)25(11-7-6-10(9-22)12(8-11)18(19,20)21)16(30)26(17)13-4-3-5-14(24-13)31(23,28)29/h3-8H,1-2H3,(H2,23,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at AR in human bicalutamide-resistant LNCAP cells assessed as inhibition of cell proliferation after 6 days |
Bioorg Med Chem 18: 8150-7 (2010)
Article DOI: 10.1016/j.bmc.2010.10.023
BindingDB Entry DOI: 10.7270/Q29S1S1P |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50331868

(6-[3-(3-Chloro-4-cyanophenyl)-5,5-dimethyl-4-oxo-2...)Show SMILES CC1(C)N(C(=S)N(C1=O)c1ccc(C#N)c(Cl)c1)c1cccc(n1)S(N)(=O)=O Show InChI InChI=1S/C17H14ClN5O3S2/c1-17(2)15(24)22(11-7-6-10(9-19)12(18)8-11)16(27)23(17)13-4-3-5-14(21-13)28(20,25)26/h3-8H,1-2H3,(H2,20,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at AR in human bicalutamide-resistant LNCAP cells assessed as inhibition of cell proliferation after 6 days |
Bioorg Med Chem 18: 8150-7 (2010)
Article DOI: 10.1016/j.bmc.2010.10.023
BindingDB Entry DOI: 10.7270/Q29S1S1P |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50331867
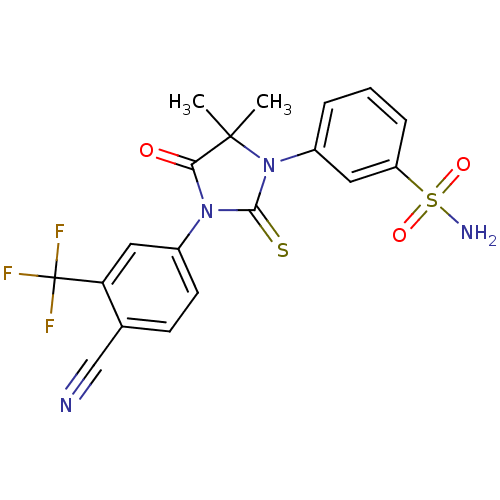
(3-[3-(4-Cyano-3-trifluoromethylphenyl)-5,5-dimethy...)Show SMILES CC1(C)N(C(=S)N(C1=O)c1ccc(C#N)c(c1)C(F)(F)F)c1cccc(c1)S(N)(=O)=O Show InChI InChI=1S/C19H15F3N4O3S2/c1-18(2)16(27)25(12-7-6-11(10-23)15(9-12)19(20,21)22)17(30)26(18)13-4-3-5-14(8-13)31(24,28)29/h3-9H,1-2H3,(H2,24,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at AR in human bicalutamide-resistant LNCAP cells assessed as inhibition of cell proliferation after 6 days |
Bioorg Med Chem 18: 8150-7 (2010)
Article DOI: 10.1016/j.bmc.2010.10.023
BindingDB Entry DOI: 10.7270/Q29S1S1P |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50331865
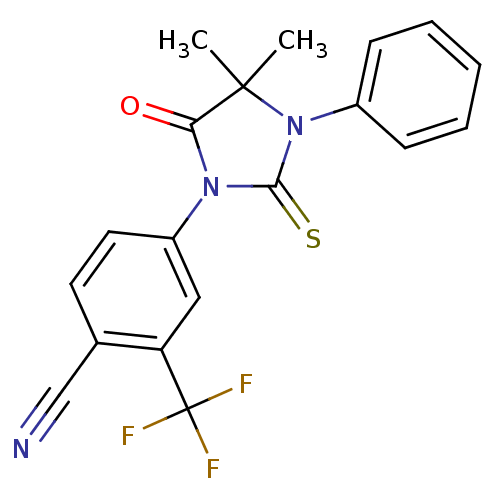
(4-(3-Phenyl-4,4-dimethyl-5-oxo-2-thioxoimidazolidi...)Show SMILES CC1(C)N(C(=S)N(C1=O)c1ccc(C#N)c(c1)C(F)(F)F)c1ccccc1 Show InChI InChI=1S/C19H14F3N3OS/c1-18(2)16(26)24(17(27)25(18)13-6-4-3-5-7-13)14-9-8-12(11-23)15(10-14)19(20,21)22/h3-10H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at AR in human bicalutamide-resistant LNCAP cells assessed as inhibition of cell proliferation after 6 days |
Bioorg Med Chem 18: 8150-7 (2010)
Article DOI: 10.1016/j.bmc.2010.10.023
BindingDB Entry DOI: 10.7270/Q29S1S1P |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50317662

(3-[3-(3-Chloro-4-cyanophenyl)-5,5-dimethyl-4-oxo-2...)Show SMILES CC1(C)N(CCCS(N)(=O)=O)C(=S)N(C1=O)c1ccc(C#N)c(Cl)c1 Show InChI InChI=1S/C15H17ClN4O3S2/c1-15(2)13(21)20(11-5-4-10(9-17)12(16)8-11)14(24)19(15)6-3-7-25(18,22)23/h4-5,8H,3,6-7H2,1-2H3,(H2,18,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR expressed in human HeLa cells co-transfected with MMTV-Luc-Hyg after 48 hrs by transient-luciferase reporter gene ass... |
Bioorg Med Chem 18: 8150-7 (2010)
Article DOI: 10.1016/j.bmc.2010.10.023
BindingDB Entry DOI: 10.7270/Q29S1S1P |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18525
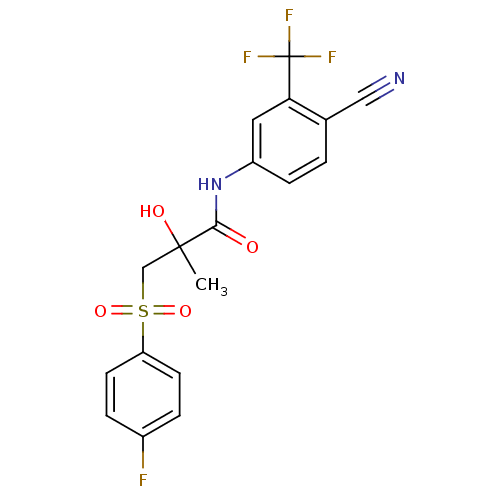
(Bicalutamide | CHEMBL409 | N-[4-cyano-3-(trifluoro...)Show SMILES CC(O)(CS(=O)(=O)c1ccc(F)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C18H14F4N2O4S/c1-17(26,10-29(27,28)14-6-3-12(19)4-7-14)16(25)24-13-5-2-11(9-23)15(8-13)18(20,21)22/h2-8,26H,10H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR expressed in human HeLa cells co-transfected with MMTV-Luc-Hyg after 48 hrs by transient-luciferase reporter gene ass... |
Bioorg Med Chem 18: 8150-7 (2010)
Article DOI: 10.1016/j.bmc.2010.10.023
BindingDB Entry DOI: 10.7270/Q29S1S1P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50331865

(4-(3-Phenyl-4,4-dimethyl-5-oxo-2-thioxoimidazolidi...)Show SMILES CC1(C)N(C(=S)N(C1=O)c1ccc(C#N)c(c1)C(F)(F)F)c1ccccc1 Show InChI InChI=1S/C19H14F3N3OS/c1-18(2)16(26)24(17(27)25(18)13-6-4-3-5-7-13)14-9-8-12(11-23)15(10-14)19(20,21)22/h3-10H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR expressed in human HeLa cells co-transfected with MMTV-Luc-Hyg after 48 hrs by transient-luciferase reporter gene ass... |
Bioorg Med Chem 18: 8150-7 (2010)
Article DOI: 10.1016/j.bmc.2010.10.023
BindingDB Entry DOI: 10.7270/Q29S1S1P |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50331867

(3-[3-(4-Cyano-3-trifluoromethylphenyl)-5,5-dimethy...)Show SMILES CC1(C)N(C(=S)N(C1=O)c1ccc(C#N)c(c1)C(F)(F)F)c1cccc(c1)S(N)(=O)=O Show InChI InChI=1S/C19H15F3N4O3S2/c1-18(2)16(27)25(12-7-6-11(10-23)15(9-12)19(20,21)22)17(30)26(18)13-4-3-5-14(8-13)31(24,28)29/h3-9H,1-2H3,(H2,24,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR expressed in human HeLa cells co-transfected with MMTV-Luc-Hyg after 48 hrs by transient-luciferase reporter gene ass... |
Bioorg Med Chem 18: 8150-7 (2010)
Article DOI: 10.1016/j.bmc.2010.10.023
BindingDB Entry DOI: 10.7270/Q29S1S1P |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50331866

(3-[3-(3-Chloro-4-cyanophenyl)-5,5-dimethyl-4-oxo-2...)Show SMILES CC1(C)N(C(=S)N(C1=O)c1ccc(C#N)c(Cl)c1)c1cccc(c1)S(N)(=O)=O Show InChI InChI=1S/C18H15ClN4O3S2/c1-18(2)16(24)22(12-7-6-11(10-20)15(19)9-12)17(27)23(18)13-4-3-5-14(8-13)28(21,25)26/h3-9H,1-2H3,(H2,21,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR expressed in human HeLa cells co-transfected with MMTV-Luc-Hyg after 48 hrs by transient-luciferase reporter gene ass... |
Bioorg Med Chem 18: 8150-7 (2010)
Article DOI: 10.1016/j.bmc.2010.10.023
BindingDB Entry DOI: 10.7270/Q29S1S1P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50596489
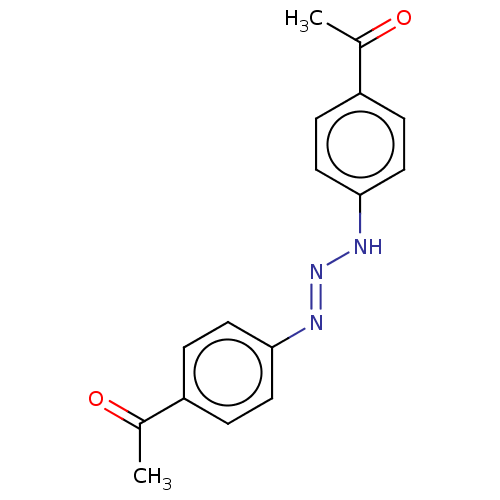
(CHEMBL5194993) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128570
BindingDB Entry DOI: 10.7270/Q2ZW1QZW |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50331868

(6-[3-(3-Chloro-4-cyanophenyl)-5,5-dimethyl-4-oxo-2...)Show SMILES CC1(C)N(C(=S)N(C1=O)c1ccc(C#N)c(Cl)c1)c1cccc(n1)S(N)(=O)=O Show InChI InChI=1S/C17H14ClN5O3S2/c1-17(2)15(24)22(11-7-6-10(9-19)12(18)8-11)16(27)23(17)13-4-3-5-14(21-13)28(20,25)26/h3-8H,1-2H3,(H2,20,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR expressed in human HeLa cells co-transfected with MMTV-Luc-Hyg after 48 hrs by transient-luciferase reporter gene ass... |
Bioorg Med Chem 18: 8150-7 (2010)
Article DOI: 10.1016/j.bmc.2010.10.023
BindingDB Entry DOI: 10.7270/Q29S1S1P |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18525

(Bicalutamide | CHEMBL409 | N-[4-cyano-3-(trifluoro...)Show SMILES CC(O)(CS(=O)(=O)c1ccc(F)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C18H14F4N2O4S/c1-17(26,10-29(27,28)14-6-3-12(19)4-7-14)16(25)24-13-5-2-11(9-23)15(8-13)18(20,21)22/h2-8,26H,10H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at AR in human bicalutamide-resistant LNCAP cells assessed as inhibition of cell proliferation after 6 days |
Bioorg Med Chem 18: 8150-7 (2010)
Article DOI: 10.1016/j.bmc.2010.10.023
BindingDB Entry DOI: 10.7270/Q29S1S1P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50331869

(6-[3-(4-Cyano-3-trifluoromethylphenyl)-5,5-dimethy...)Show SMILES CC1(C)N(C(=S)N(C1=O)c1ccc(C#N)c(c1)C(F)(F)F)c1cccc(n1)S(N)(=O)=O Show InChI InChI=1S/C18H14F3N5O3S2/c1-17(2)15(27)25(11-7-6-10(9-22)12(8-11)18(19,20)21)16(30)26(17)13-4-3-5-14(24-13)31(23,28)29/h3-8H,1-2H3,(H2,23,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR expressed in human HeLa cells co-transfected with MMTV-Luc-Hyg after 48 hrs by transient-luciferase reporter gene ass... |
Bioorg Med Chem 18: 8150-7 (2010)
Article DOI: 10.1016/j.bmc.2010.10.023
BindingDB Entry DOI: 10.7270/Q29S1S1P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50596490
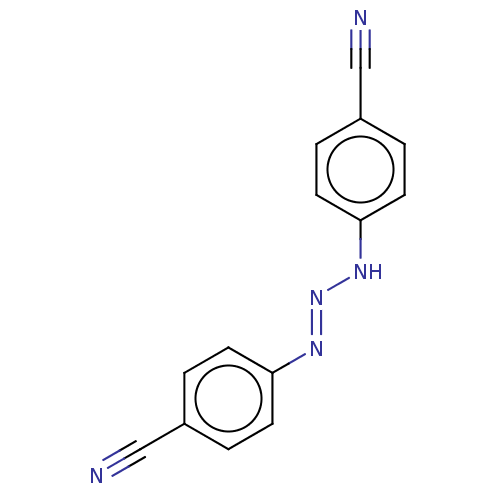
(CHEMBL5173529) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128570
BindingDB Entry DOI: 10.7270/Q2ZW1QZW |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50331872

(5-[3-(4-Cyano-3-trifluoromethylphenyl)-5,5-dimethy...)Show SMILES CC1(C)N(C(=S)N(C1=O)c1ccc(C#N)c(c1)C(F)(F)F)c1cncc(c1)S(N)(=O)=O Show InChI InChI=1S/C18H14F3N5O3S2/c1-17(2)15(27)25(11-4-3-10(7-22)14(6-11)18(19,20)21)16(30)26(17)12-5-13(9-24-8-12)31(23,28)29/h3-6,8-9H,1-2H3,(H2,23,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR expressed in human HeLa cells co-transfected with MMTV-Luc-Hyg after 48 hrs by transient-luciferase reporter gene ass... |
Bioorg Med Chem 18: 8150-7 (2010)
Article DOI: 10.1016/j.bmc.2010.10.023
BindingDB Entry DOI: 10.7270/Q29S1S1P |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50331870

(4-[3-(4-Cyano-3-trifluoromethylphenyl)-5,5-dimethy...)Show SMILES CC1(C)N(C(=S)N(C1=O)c1ccc(C#N)c(c1)C(F)(F)F)c1ccnc(c1)S(N)(=O)=O Show InChI InChI=1S/C18H14F3N5O3S2/c1-17(2)15(27)25(11-4-3-10(9-22)13(7-11)18(19,20)21)16(30)26(17)12-5-6-24-14(8-12)31(23,28)29/h3-8H,1-2H3,(H2,23,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR expressed in human HeLa cells co-transfected with MMTV-Luc-Hyg after 48 hrs by transient-luciferase reporter gene ass... |
Bioorg Med Chem 18: 8150-7 (2010)
Article DOI: 10.1016/j.bmc.2010.10.023
BindingDB Entry DOI: 10.7270/Q29S1S1P |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50184757
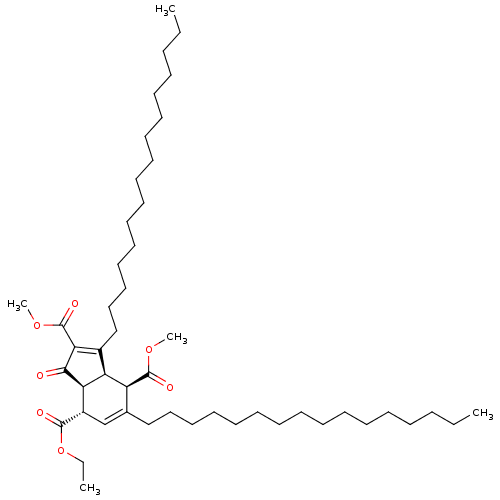
((3aR,4R,7S,7aS)-7-ethyl 2,4-dimethyl 3,5-dihexadec...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(=O)OCC |c:16,48| Show InChI InChI=1S/C48H82O7/c1-6-9-11-13-15-17-19-21-23-25-27-29-31-33-35-38-37-40(46(50)55-8-3)43-42(41(38)47(51)53-4)39(44(45(43)49)48(52)54-5)36-34-32-30-28-26-24-22-20-18-16-14-12-10-7-2/h37,40-43H,6-36H2,1-5H3/t40-,41-,42-,43+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase beta |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184755
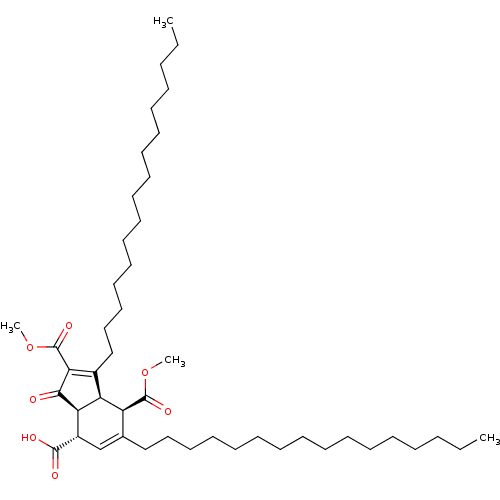
((3aS,4S,7R,7aR)-1,6-dihexadecyl-2,7-bis(methoxycar...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(O)=O |c:16,48| Show InChI InChI=1S/C46H78O7/c1-5-7-9-11-13-15-17-19-21-23-25-27-29-31-33-36-35-38(44(48)49)41-40(39(36)45(50)52-3)37(42(43(41)47)46(51)53-4)34-32-30-28-26-24-22-20-18-16-14-12-10-8-6-2/h35,38-41H,5-34H2,1-4H3,(H,48,49)/t38-,39-,40-,41+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184758
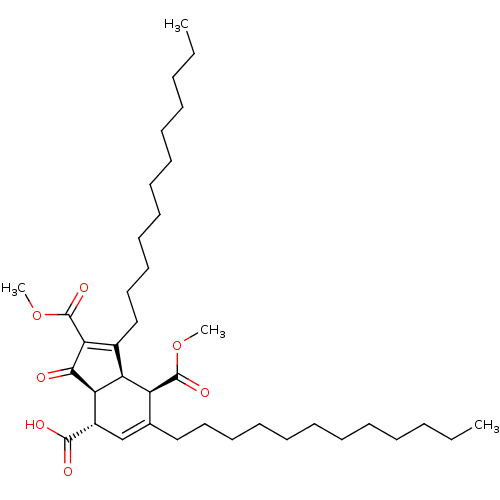
((3aS,4S,7R,7aR)-1,6-didodecyl-2,7-bis(methoxycarbo...)Show SMILES CCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC)C(CCCCCCCCCCCC)=C[C@@H]2C(O)=O |c:12,40| Show InChI InChI=1S/C38H62O7/c1-5-7-9-11-13-15-17-19-21-23-25-28-27-30(36(40)41)33-32(31(28)37(42)44-3)29(34(35(33)39)38(43)45-4)26-24-22-20-18-16-14-12-10-8-6-2/h27,30-33H,5-26H2,1-4H3,(H,40,41)/t30-,31-,32-,33+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50331871

(5-[3-(3-Chloro-4-cyanophenyl)-5,5-dimethyl-4-oxo-2...)Show SMILES CC1(C)N(C(=S)N(C1=O)c1ccc(C#N)c(Cl)c1)c1cncc(c1)S(N)(=O)=O Show InChI InChI=1S/C17H14ClN5O3S2/c1-17(2)15(24)22(11-4-3-10(7-19)14(18)6-11)16(27)23(17)12-5-13(9-21-8-12)28(20,25)26/h3-6,8-9H,1-2H3,(H2,20,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR expressed in human HeLa cells co-transfected with MMTV-Luc-Hyg after 48 hrs by transient-luciferase reporter gene ass... |
Bioorg Med Chem 18: 8150-7 (2010)
Article DOI: 10.1016/j.bmc.2010.10.023
BindingDB Entry DOI: 10.7270/Q29S1S1P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50596487
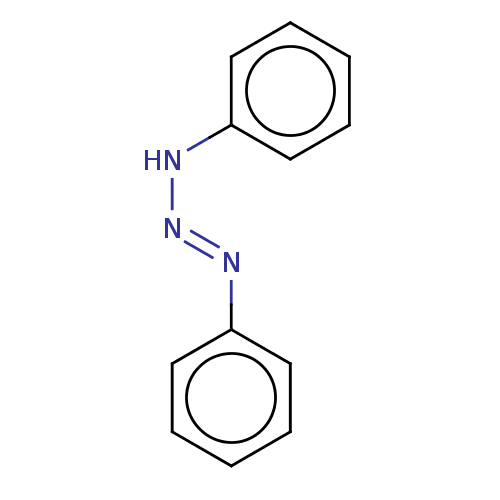
(CHEMBL573540) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128570
BindingDB Entry DOI: 10.7270/Q2ZW1QZW |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50184758

((3aS,4S,7R,7aR)-1,6-didodecyl-2,7-bis(methoxycarbo...)Show SMILES CCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC)C(CCCCCCCCCCCC)=C[C@@H]2C(O)=O |c:12,40| Show InChI InChI=1S/C38H62O7/c1-5-7-9-11-13-15-17-19-21-23-25-28-27-30(36(40)41)33-32(31(28)37(42)44-3)29(34(35(33)39)38(43)45-4)26-24-22-20-18-16-14-12-10-8-6-2/h27,30-33H,5-26H2,1-4H3,(H,40,41)/t30-,31-,32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase beta |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184757

((3aR,4R,7S,7aS)-7-ethyl 2,4-dimethyl 3,5-dihexadec...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(=O)OCC |c:16,48| Show InChI InChI=1S/C48H82O7/c1-6-9-11-13-15-17-19-21-23-25-27-29-31-33-35-38-37-40(46(50)55-8-3)43-42(41(38)47(51)53-4)39(44(45(43)49)48(52)54-5)36-34-32-30-28-26-24-22-20-18-16-14-12-10-7-2/h37,40-43H,6-36H2,1-5H3/t40-,41-,42-,43+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50184756
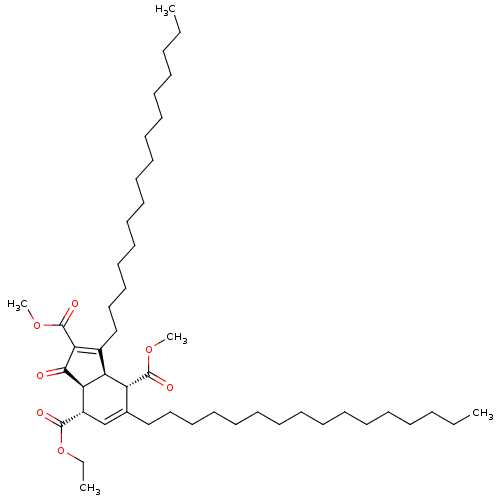
((3aR,4S,7S,7aS)-7-ethyl 2,4-dimethyl 3,5-dihexadec...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@H](C(=O)OC)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(=O)OCC |c:16,48| Show InChI InChI=1S/C48H82O7/c1-6-9-11-13-15-17-19-21-23-25-27-29-31-33-35-38-37-40(46(50)55-8-3)43-42(41(38)47(51)53-4)39(44(45(43)49)48(52)54-5)36-34-32-30-28-26-24-22-20-18-16-14-12-10-7-2/h37,40-43H,6-36H2,1-5H3/t40-,41+,42-,43+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase beta |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA nucleotidylexotransferase
(Homo sapiens (Human)) | BDBM50184755

((3aS,4S,7R,7aR)-1,6-dihexadecyl-2,7-bis(methoxycar...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(O)=O |c:16,48| Show InChI InChI=1S/C46H78O7/c1-5-7-9-11-13-15-17-19-21-23-25-27-29-31-33-36-35-38(44(48)49)41-40(39(36)45(50)52-3)37(42(43(41)47)46(51)53-4)34-32-30-28-26-24-22-20-18-16-14-12-10-8-6-2/h35,38-41H,5-34H2,1-4H3,(H,48,49)/t38-,39-,40-,41+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human TdT |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A1
(Homo sapiens (Human)) | BDBM50596488

(CHEMBL5179953) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128570
BindingDB Entry DOI: 10.7270/Q2ZW1QZW |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50184755

((3aS,4S,7R,7aR)-1,6-dihexadecyl-2,7-bis(methoxycar...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(O)=O |c:16,48| Show InChI InChI=1S/C46H78O7/c1-5-7-9-11-13-15-17-19-21-23-25-27-29-31-33-36-35-38(44(48)49)41-40(39(36)45(50)52-3)37(42(43(41)47)46(51)53-4)34-32-30-28-26-24-22-20-18-16-14-12-10-8-6-2/h35,38-41H,5-34H2,1-4H3,(H,48,49)/t38-,39-,40-,41+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase beta |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184752
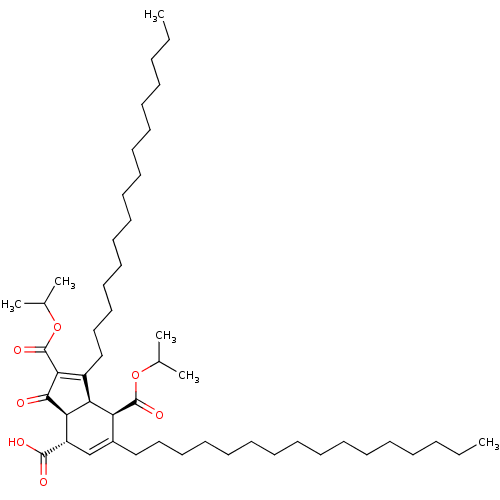
((3aS,4S,7R,7aR)-1,6-dihexadecyl-2,7-bis(isopropoxy...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC(C)C)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC(C)C)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(O)=O |c:16,52| Show InChI InChI=1S/C50H86O7/c1-7-9-11-13-15-17-19-21-23-25-27-29-31-33-35-40-37-42(48(52)53)45-44(43(40)49(54)56-38(3)4)41(46(47(45)51)50(55)57-39(5)6)36-34-32-30-28-26-24-22-20-18-16-14-12-10-8-2/h37-39,42-45H,7-36H2,1-6H3,(H,52,53)/t42-,43-,44-,45+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184756

((3aR,4S,7S,7aS)-7-ethyl 2,4-dimethyl 3,5-dihexadec...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@H](C(=O)OC)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(=O)OCC |c:16,48| Show InChI InChI=1S/C48H82O7/c1-6-9-11-13-15-17-19-21-23-25-27-29-31-33-35-38-37-40(46(50)55-8-3)43-42(41(38)47(51)53-4)39(44(45(43)49)48(52)54-5)36-34-32-30-28-26-24-22-20-18-16-14-12-10-7-2/h37,40-43H,6-36H2,1-5H3/t40-,41+,42-,43+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184754
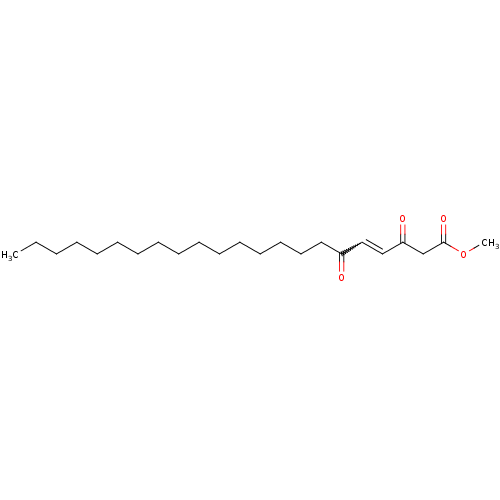
((E)-methyl-3,6-dioxo-4-docosenoate | CHEMBL425779)Show SMILES CCCCCCCCCCCCCCCCC(=O)C=CC(=O)CC(=O)OC |w:18.17| Show InChI InChI=1S/C23H40O4/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-21(24)18-19-22(25)20-23(26)27-2/h18-19H,3-17,20H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A1
(Homo sapiens (Human)) | BDBM50596489

(CHEMBL5194993) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128570
BindingDB Entry DOI: 10.7270/Q2ZW1QZW |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50143526

(CHEMBL366792 | Untenone A)Show SMILES CCCCCCCCCCCCCCCC[C@]1(O)C=CC(=O)[C@H]1C(=O)OC |c:18| Show InChI InChI=1S/C23H40O4/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-18-23(26)19-17-20(24)21(23)22(25)27-2/h17,19,21,26H,3-16,18H2,1-2H3/t21-,23-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50184752

((3aS,4S,7R,7aR)-1,6-dihexadecyl-2,7-bis(isopropoxy...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC(C)C)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC(C)C)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(O)=O |c:16,52| Show InChI InChI=1S/C50H86O7/c1-7-9-11-13-15-17-19-21-23-25-27-29-31-33-35-40-37-42(48(52)53)45-44(43(40)49(54)56-38(3)4)41(46(47(45)51)50(55)57-39(5)6)36-34-32-30-28-26-24-22-20-18-16-14-12-10-8-2/h37-39,42-45H,7-36H2,1-6H3,(H,52,53)/t42-,43-,44-,45+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase beta |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184761
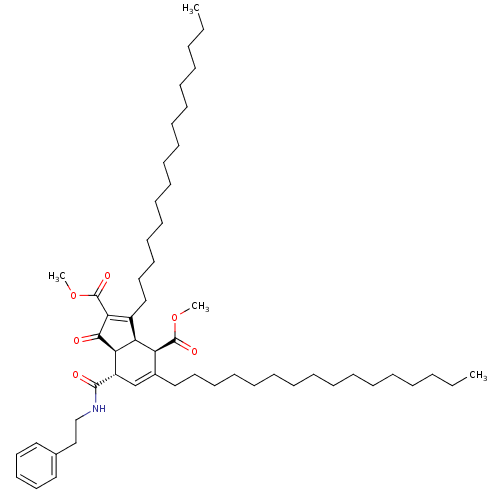
((3aR,4R,7S,7aS)-dimethyl 3,5-dihexadecyl-1-oxo-7-(...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(=O)NCCc1ccccc1 |c:16,48| Show InChI InChI=1S/C54H87NO6/c1-5-7-9-11-13-15-17-19-21-23-25-27-29-34-38-44-42-46(52(57)55-41-40-43-36-32-31-33-37-43)49-48(47(44)53(58)60-3)45(50(51(49)56)54(59)61-4)39-35-30-28-26-24-22-20-18-16-14-12-10-8-6-2/h31-33,36-37,42,46-49H,5-30,34-35,38-41H2,1-4H3,(H,55,57)/t46-,47-,48-,49+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184750
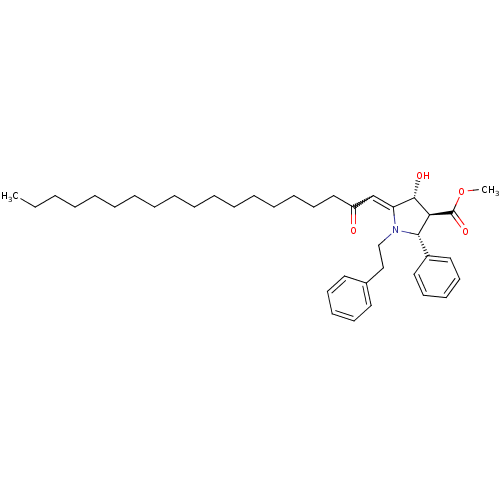
((2R,3S,4R)-4-hydroxy-5-[2-oxo-nonadec-ylidene]-1-p...)Show SMILES CCCCCCCCCCCCCCCCCC(=O)C=C1[C@H](O)[C@H]([C@@H](N1CCc1ccccc1)c1ccccc1)C(=O)OC |w:19.18| Show InChI InChI=1S/C39H57NO4/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-23-28-34(41)31-35-38(42)36(39(43)44-2)37(33-26-21-18-22-27-33)40(35)30-29-32-24-19-17-20-25-32/h17-22,24-27,31,36-38,42H,3-16,23,28-30H2,1-2H3/t36-,37-,38-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A1
(Homo sapiens (Human)) | BDBM50596487

(CHEMBL573540) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128570
BindingDB Entry DOI: 10.7270/Q2ZW1QZW |
More data for this
Ligand-Target Pair | |
DNA nucleotidylexotransferase
(Homo sapiens (Human)) | BDBM50184758

((3aS,4S,7R,7aR)-1,6-didodecyl-2,7-bis(methoxycarbo...)Show SMILES CCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC)C(CCCCCCCCCCCC)=C[C@@H]2C(O)=O |c:12,40| Show InChI InChI=1S/C38H62O7/c1-5-7-9-11-13-15-17-19-21-23-25-28-27-30(36(40)41)33-32(31(28)37(42)44-3)29(34(35(33)39)38(43)45-4)26-24-22-20-18-16-14-12-10-8-6-2/h27,30-33H,5-26H2,1-4H3,(H,40,41)/t30-,31-,32-,33+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human TdT |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50184761

((3aR,4R,7S,7aS)-dimethyl 3,5-dihexadecyl-1-oxo-7-(...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(=O)NCCc1ccccc1 |c:16,48| Show InChI InChI=1S/C54H87NO6/c1-5-7-9-11-13-15-17-19-21-23-25-27-29-34-38-44-42-46(52(57)55-41-40-43-36-32-31-33-37-43)49-48(47(44)53(58)60-3)45(50(51(49)56)54(59)61-4)39-35-30-28-26-24-22-20-18-16-14-12-10-8-6-2/h31-33,36-37,42,46-49H,5-30,34-35,38-41H2,1-4H3,(H,55,57)/t46-,47-,48-,49+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase beta |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50184748

((2S,3S,4R)-methyl 2-ethyl-4-hydroxy-5-(2-oxononade...)Show SMILES CCCCCCCCCCCCCCCCCC(=O)C=C1[C@H](O)[C@H]([C@H](CC)N1CCc1ccccc1)C(=O)OC |w:19.18| Show InChI InChI=1S/C35H57NO4/c1-4-6-7-8-9-10-11-12-13-14-15-16-17-18-22-25-30(37)28-32-34(38)33(35(39)40-3)31(5-2)36(32)27-26-29-23-20-19-21-24-29/h19-21,23-24,28,31,33-34,38H,4-18,22,25-27H2,1-3H3/t31-,33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase beta |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50184754

((E)-methyl-3,6-dioxo-4-docosenoate | CHEMBL425779)Show SMILES CCCCCCCCCCCCCCCCC(=O)C=CC(=O)CC(=O)OC |w:18.17| Show InChI InChI=1S/C23H40O4/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-21(24)18-19-22(25)20-23(26)27-2/h18-19H,3-17,20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase beta |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50184750

((2R,3S,4R)-4-hydroxy-5-[2-oxo-nonadec-ylidene]-1-p...)Show SMILES CCCCCCCCCCCCCCCCCC(=O)C=C1[C@H](O)[C@H]([C@@H](N1CCc1ccccc1)c1ccccc1)C(=O)OC |w:19.18| Show InChI InChI=1S/C39H57NO4/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-23-28-34(41)31-35-38(42)36(39(43)44-2)37(33-26-21-18-22-27-33)40(35)30-29-32-24-19-17-20-25-32/h17-22,24-27,31,36-38,42H,3-16,23,28-30H2,1-2H3/t36-,37-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase beta |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184748

((2S,3S,4R)-methyl 2-ethyl-4-hydroxy-5-(2-oxononade...)Show SMILES CCCCCCCCCCCCCCCCCC(=O)C=C1[C@H](O)[C@H]([C@H](CC)N1CCc1ccccc1)C(=O)OC |w:19.18| Show InChI InChI=1S/C35H57NO4/c1-4-6-7-8-9-10-11-12-13-14-15-16-17-18-22-25-30(37)28-32-34(38)33(35(39)40-3)31(5-2)36(32)27-26-29-23-20-19-21-24-29/h19-21,23-24,28,31,33-34,38H,4-18,22,25-27H2,1-3H3/t31-,33-,34-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50184759
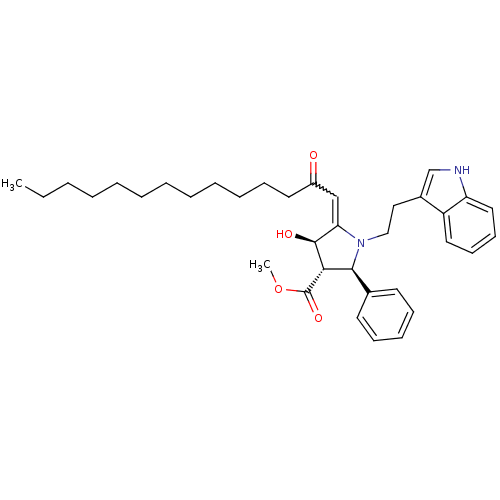
((2R,3S,4R)-methyl 1-(2-(1H-indol-3-yl)ethyl)-4-hyd...)Show SMILES CCCCCCCCCCCCC(=O)C=C1[C@H](O)[C@H]([C@@H](N1CCc1c[nH]c2ccccc12)c1ccccc1)C(=O)OC |w:14.13| Show InChI InChI=1S/C36H48N2O4/c1-3-4-5-6-7-8-9-10-11-15-20-29(39)25-32-35(40)33(36(41)42-2)34(27-18-13-12-14-19-27)38(32)24-23-28-26-37-31-22-17-16-21-30(28)31/h12-14,16-19,21-22,25-26,33-35,37,40H,3-11,15,20,23-24H2,1-2H3/t33-,34-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase beta |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA nucleotidylexotransferase
(Homo sapiens (Human)) | BDBM50184753
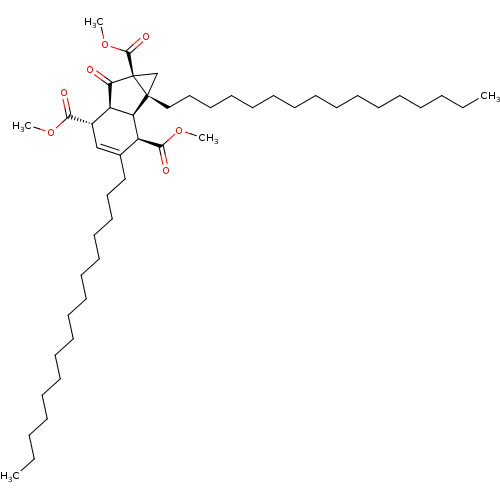
((1R,1bR,2R,5S,5aS)-1a,3-dihexadecyl-6-oxo-1a,1b,2,...)Show SMILES CCCCCCCCCCCCCCCCC1=C[C@@H]([C@@H]2[C@H]([C@H]1C(=O)OC)[C@@]1(CCCCCCCCCCCCCCCC)C[C@@]1(C(=O)OC)C2=O)C(=O)OC |t:16| Show InChI InChI=1S/C48H82O7/c1-6-8-10-12-14-16-18-20-22-24-26-28-30-32-34-38-36-39(44(50)53-3)41-42(40(38)45(51)54-4)47(37-48(47,43(41)49)46(52)55-5)35-33-31-29-27-25-23-21-19-17-15-13-11-9-7-2/h36,39-42H,6-35,37H2,1-5H3/t39-,40-,41+,42-,47+,48-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human TdT |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data