

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50251556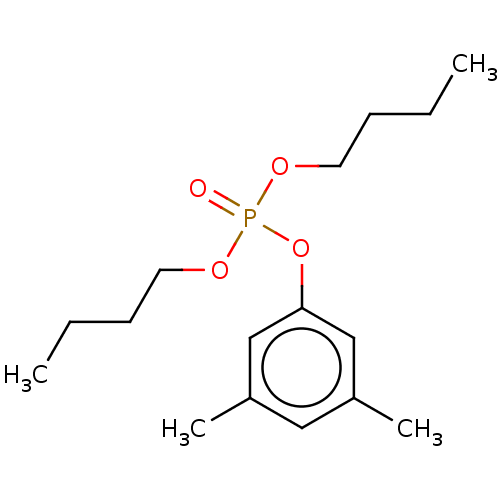 (CHEMBL4082512) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California State University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine as substrate by spectrophotometry | Bioorg Med Chem 25: 3171-3181 (2017) Article DOI: 10.1016/j.bmc.2017.04.002 BindingDB Entry DOI: 10.7270/Q2NV9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50251557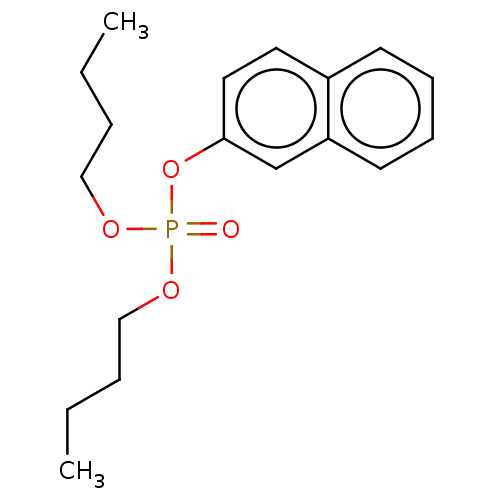 (CHEMBL4062422) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California State University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine as substrate by spectrophotometry | Bioorg Med Chem 25: 3171-3181 (2017) Article DOI: 10.1016/j.bmc.2017.04.002 BindingDB Entry DOI: 10.7270/Q2NV9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50251528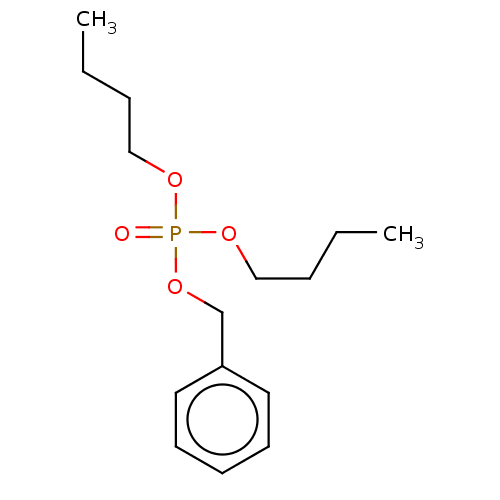 (CHEMBL4089303) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California State University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine as substrate by spectrophotometry | Bioorg Med Chem 25: 3171-3181 (2017) Article DOI: 10.1016/j.bmc.2017.04.002 BindingDB Entry DOI: 10.7270/Q2NV9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM50427221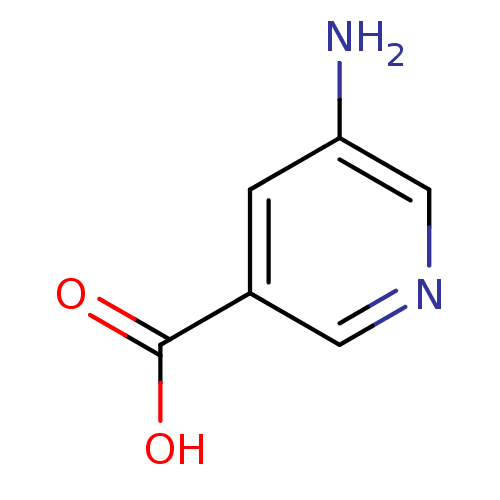 (5-Aminonicotinic Acid | CHEMBL1491941) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Binding affinity to human recombinant DDO | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RCG38204, isoform CRA_d (Rattus norvegicus) | BDBM50427221 (5-Aminonicotinic Acid | CHEMBL1491941) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 5.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of rat recombinant DDO using D-Asp | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50251512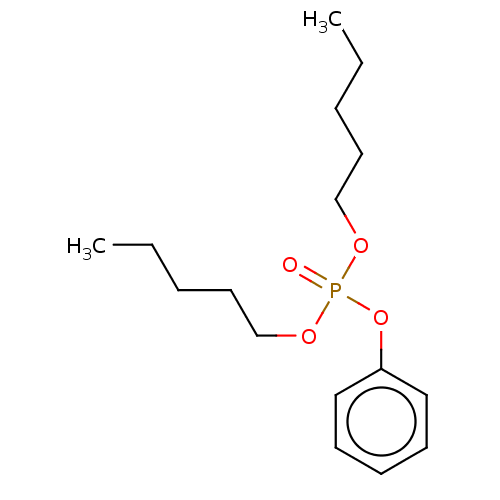 (CHEMBL4060321) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California State University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine as substrate by spectrophotometry | Bioorg Med Chem 25: 3171-3181 (2017) Article DOI: 10.1016/j.bmc.2017.04.002 BindingDB Entry DOI: 10.7270/Q2NV9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50251524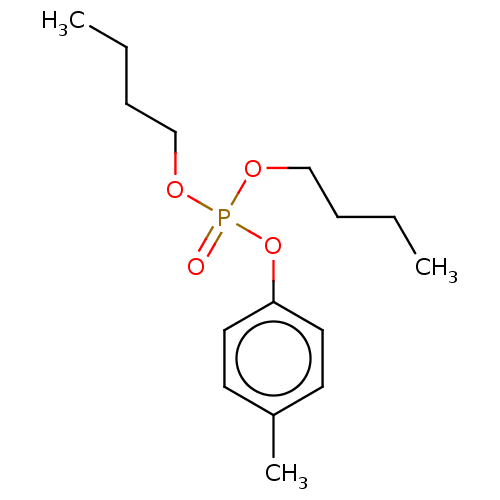 (CHEMBL4070069) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California State University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine as substrate by spectrophotometry | Bioorg Med Chem 25: 3171-3181 (2017) Article DOI: 10.1016/j.bmc.2017.04.002 BindingDB Entry DOI: 10.7270/Q2NV9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Mus musculus) | BDBM50427221 (5-Aminonicotinic Acid | CHEMBL1491941) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 8.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant DDO using D-Asp | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50251525 (CHEMBL4103125) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California State University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine as substrate by spectrophotometry | Bioorg Med Chem 25: 3171-3181 (2017) Article DOI: 10.1016/j.bmc.2017.04.002 BindingDB Entry DOI: 10.7270/Q2NV9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50251526 (CHEMBL4060636) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California State University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine as substrate by spectrophotometry | Bioorg Med Chem 25: 3171-3181 (2017) Article DOI: 10.1016/j.bmc.2017.04.002 BindingDB Entry DOI: 10.7270/Q2NV9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50251558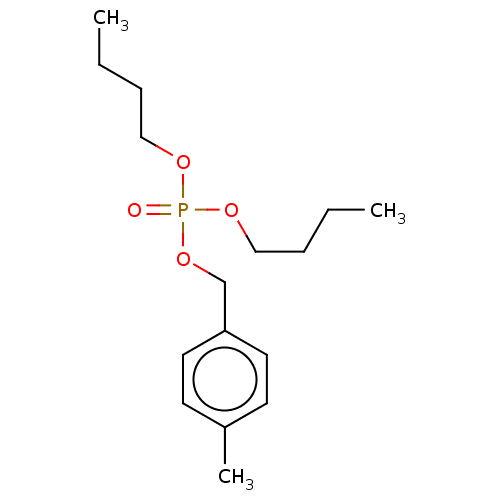 (CHEMBL4097681) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California State University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine as substrate by spectrophotometry | Bioorg Med Chem 25: 3171-3181 (2017) Article DOI: 10.1016/j.bmc.2017.04.002 BindingDB Entry DOI: 10.7270/Q2NV9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM50121995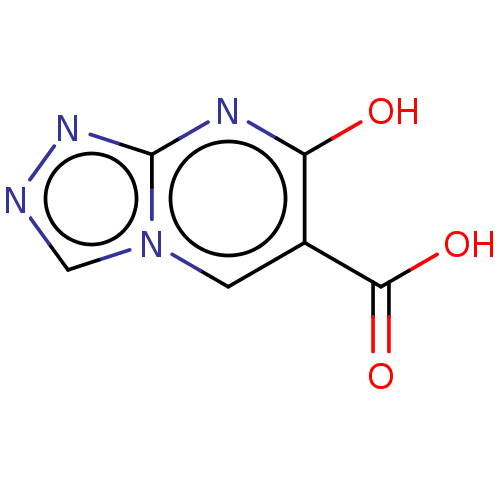 (CHEMBL3617316) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Binding affinity to human recombinant DDO | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50251523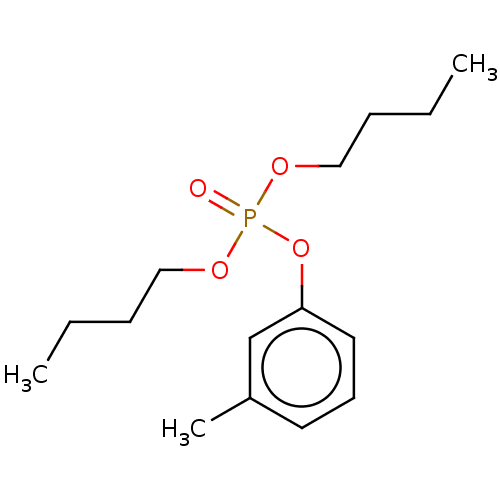 (CHEMBL4076133) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California State University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine as substrate by spectrophotometry | Bioorg Med Chem 25: 3171-3181 (2017) Article DOI: 10.1016/j.bmc.2017.04.002 BindingDB Entry DOI: 10.7270/Q2NV9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50251527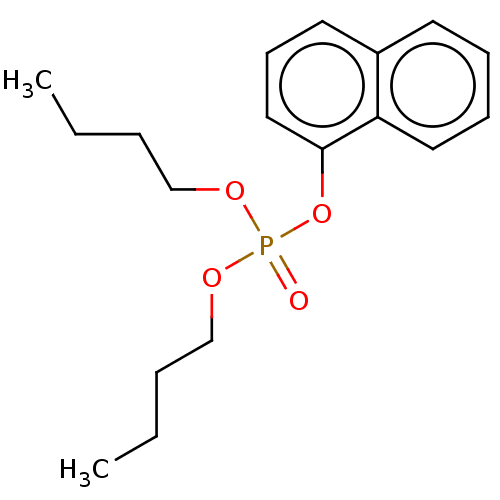 (CHEMBL4084914) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California State University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine as substrate by spectrophotometry | Bioorg Med Chem 25: 3171-3181 (2017) Article DOI: 10.1016/j.bmc.2017.04.002 BindingDB Entry DOI: 10.7270/Q2NV9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50251530 (CHEMBL4073516) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California State University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine as substrate by spectrophotometry | Bioorg Med Chem 25: 3171-3181 (2017) Article DOI: 10.1016/j.bmc.2017.04.002 BindingDB Entry DOI: 10.7270/Q2NV9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Mus musculus) | BDBM50121995 (CHEMBL3617316) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant DDO using D-Asp | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50251511 (CHEMBL4094240) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California State University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine as substrate by spectrophotometry | Bioorg Med Chem 25: 3171-3181 (2017) Article DOI: 10.1016/j.bmc.2017.04.002 BindingDB Entry DOI: 10.7270/Q2NV9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50251560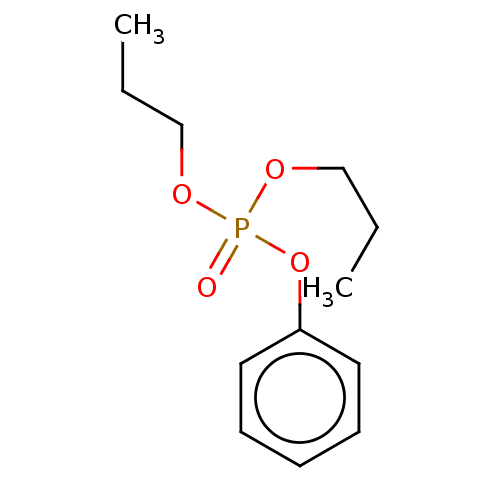 (CHEMBL4065237) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California State University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine as substrate by spectrophotometry | Bioorg Med Chem 25: 3171-3181 (2017) Article DOI: 10.1016/j.bmc.2017.04.002 BindingDB Entry DOI: 10.7270/Q2NV9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50251576 (CHEMBL4074962) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California State University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine as substrate by spectrophotometry | Bioorg Med Chem 25: 3171-3181 (2017) Article DOI: 10.1016/j.bmc.2017.04.002 BindingDB Entry DOI: 10.7270/Q2NV9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50251531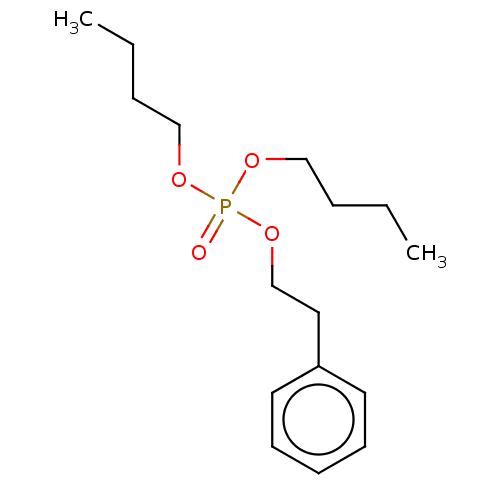 (CHEMBL4080456) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California State University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine as substrate by spectrophotometry | Bioorg Med Chem 25: 3171-3181 (2017) Article DOI: 10.1016/j.bmc.2017.04.002 BindingDB Entry DOI: 10.7270/Q2NV9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RCG38204, isoform CRA_d (Rattus norvegicus) | BDBM50121995 (CHEMBL3617316) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of rat recombinant DDO using D-Asp | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50251529 (CHEMBL4067296) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California State University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine as substrate by spectrophotometry | Bioorg Med Chem 25: 3171-3181 (2017) Article DOI: 10.1016/j.bmc.2017.04.002 BindingDB Entry DOI: 10.7270/Q2NV9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50251559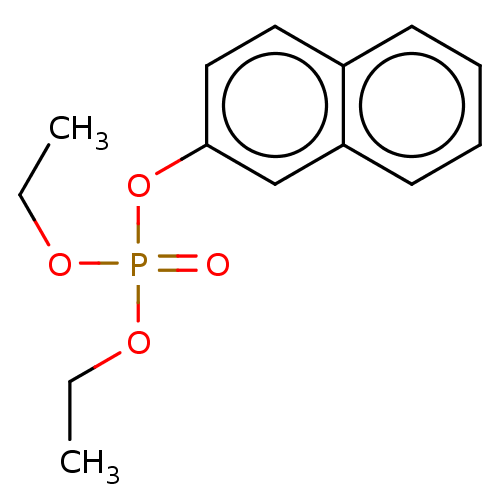 (CHEMBL4083188) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.47E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California State University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine as substrate by spectrophotometry | Bioorg Med Chem 25: 3171-3181 (2017) Article DOI: 10.1016/j.bmc.2017.04.002 BindingDB Entry DOI: 10.7270/Q2NV9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM14673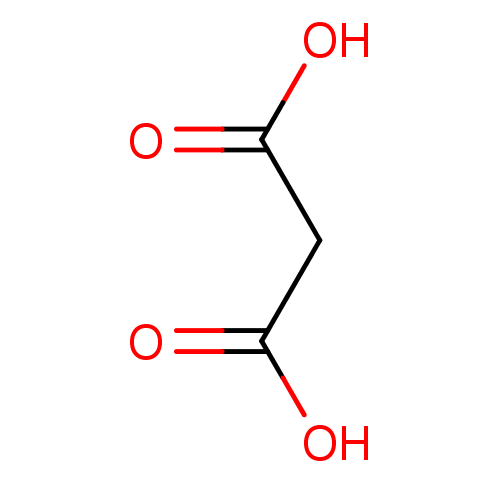 (Fragment 3 | Malonic Acid | propanedioic acid) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.53E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Binding affinity to human recombinant DDO | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50251544 (CHEMBL4105019) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5.83E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California State University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine as substrate by spectrophotometry | Bioorg Med Chem 25: 3171-3181 (2017) Article DOI: 10.1016/j.bmc.2017.04.002 BindingDB Entry DOI: 10.7270/Q2NV9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Mus musculus) | BDBM14673 (Fragment 3 | Malonic Acid | propanedioic acid) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.22E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant DDO using D-Asp | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RCG38204, isoform CRA_d (Rattus norvegicus) | BDBM14673 (Fragment 3 | Malonic Acid | propanedioic acid) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.56E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of rat recombinant DDO using D-Asp | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50127647 (CHEMBL296745 | Phosphoric acid diethyl ester pheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents | Article PubMed | 1.59E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
California State University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine as substrate by spectrophotometry | Bioorg Med Chem 25: 3171-3181 (2017) Article DOI: 10.1016/j.bmc.2017.04.002 BindingDB Entry DOI: 10.7270/Q2NV9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269429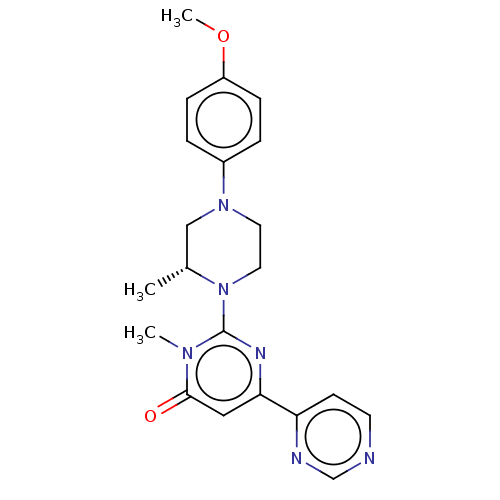 (CHEMBL4084855) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269428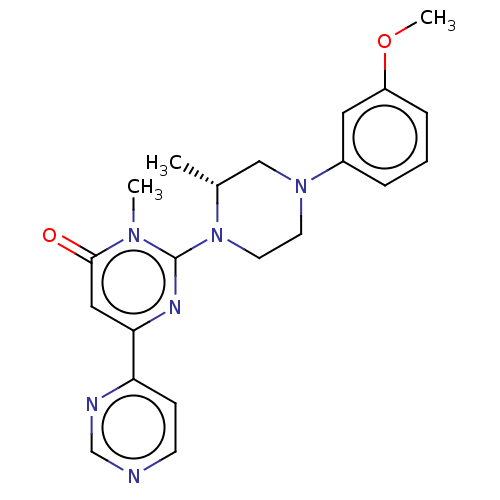 (CHEMBL4063206) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269437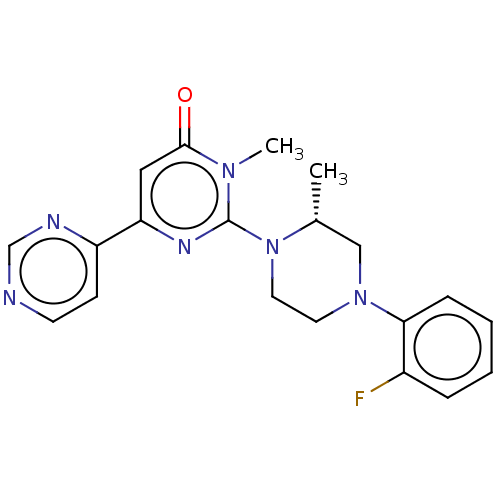 (CHEMBL4077376) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [C481S] (Homo sapiens (Human)) | BDBM499813 ((2-chloro-4-phenoxyphenyl)(4- (((3R,6R)-6-(hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [C481S] (Homo sapiens (Human)) | BDBM499811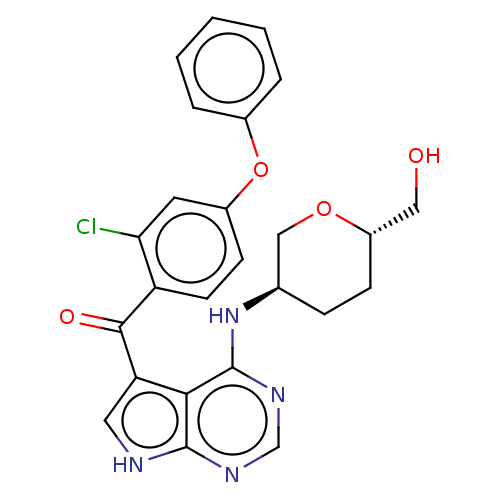 (US11020398, Compound I-123 | US20230364079, Exampl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269438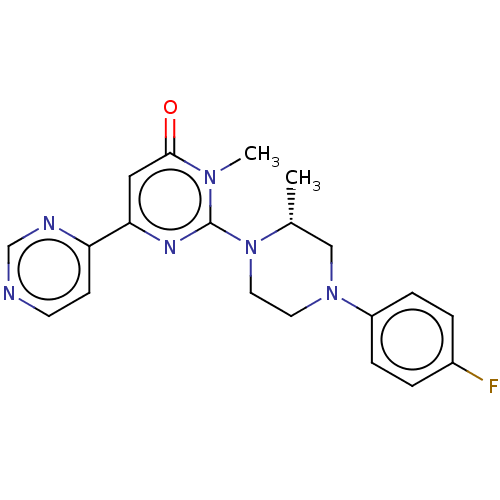 (CHEMBL4076060) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fer (Homo sapiens (Human)) | BDBM50501949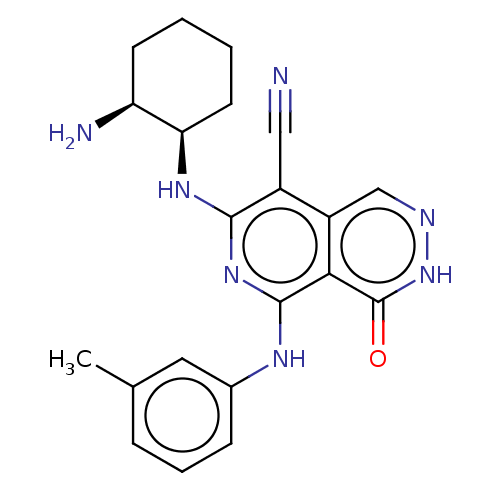 (CHEMBL4461851) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human FER (SH2 domain to C-terminal) expressed in Escherichia coli assessed as decrease in FL-Peptide... | ACS Med Chem Lett 10: 737-742 (2019) Article DOI: 10.1021/acsmedchemlett.8b00631 BindingDB Entry DOI: 10.7270/Q2WS8XG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269443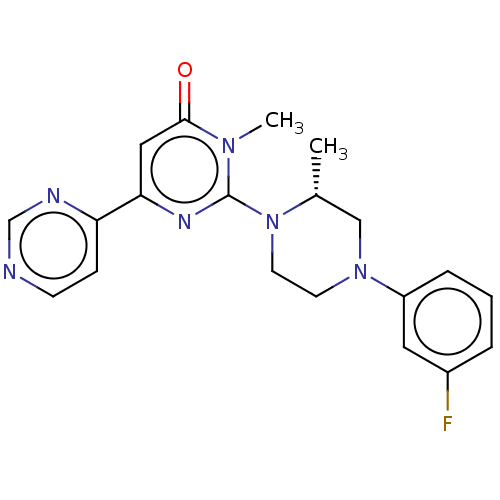 (CHEMBL4095848) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499813 ((2-chloro-4-phenoxyphenyl)(4- (((3R,6R)-6-(hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499813 ((2-chloro-4-phenoxyphenyl)(4- (((3R,6R)-6-(hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499810 (N-((S)-1-((2S,5R)-5-((5-(2-chloro-4- phenoxybenzoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Full length unphosphorylated form of BTK expressed in Sf9 cells was employed to test inhibitory activity in the inactive BTK assay. The assay was mea... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [C481S] (Homo sapiens (Human)) | BDBM499814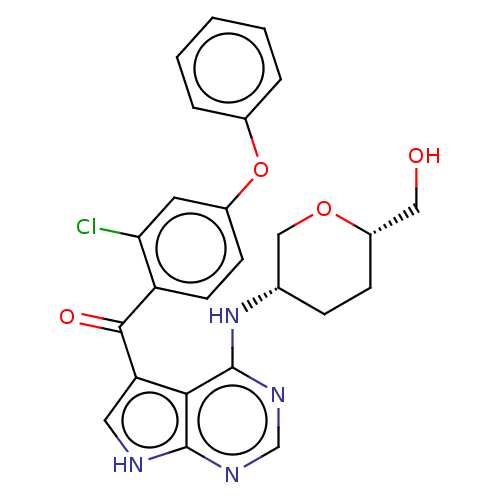 ((2-chloro-4-phenoxyphenyl)(4- (((3S,6S)-6-(hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50438462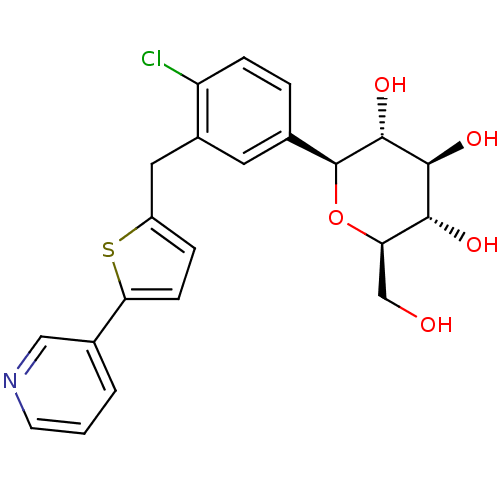 (CHEMBL2414623) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHOK cells assessed as reduction of [14C]alpha-methyl-D-glucopyranoside uptake after 120 mins by liquid scinti... | Bioorg Med Chem 21: 5561-72 (2013) Article DOI: 10.1016/j.bmc.2013.05.048 BindingDB Entry DOI: 10.7270/Q2RX9DHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269440 (CHEMBL4065818) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499811 (US11020398, Compound I-123 | US20230364079, Exampl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499811 (US11020398, Compound I-123 | US20230364079, Exampl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Enzyme assay using full length recombinant active form of wild-type BTK and BTK-C481S was measured as described previously (Anastassiadis T, et al., ... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499838 ((2-chloro-4-phenoxyphenyl)(4- (((3R,6S)-6-((R)-1- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Full length unphosphorylated form of BTK expressed in Sf9 cells was employed to test inhibitory activity in the inactive BTK assay. The assay was mea... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499974 ((racemic)-1-(2-(((5-(2-chloro-4- phenoxybenzoyl)-7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Purified full-length inactive BTK (wild type and C481 mutant, N-terminal 6XHIS tagged BTK, Mwt=78.2 kDa) were activated using soluble inositol hexaki... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499924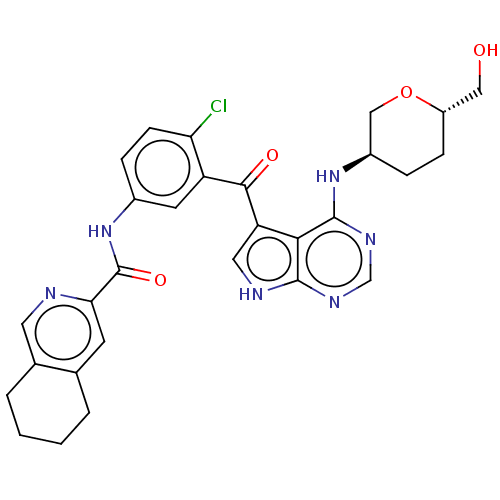 (N-(4-chloro-3-(4-(((3R,6S)-6- (hydroxymethyl)tetra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Purified full-length inactive BTK (wild type and C481 mutant, N-terminal 6XHIS tagged BTK, Mwt=78.2 kDa) were activated using soluble inositol hexaki... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499869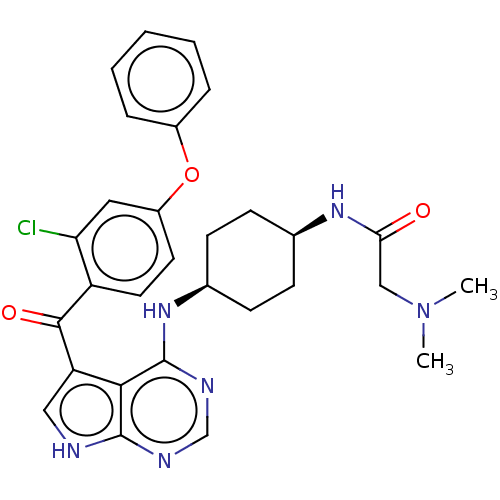 (N-(cis-4-((5-(2-chloro-4- phenoxybenzoyl)-7H-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499976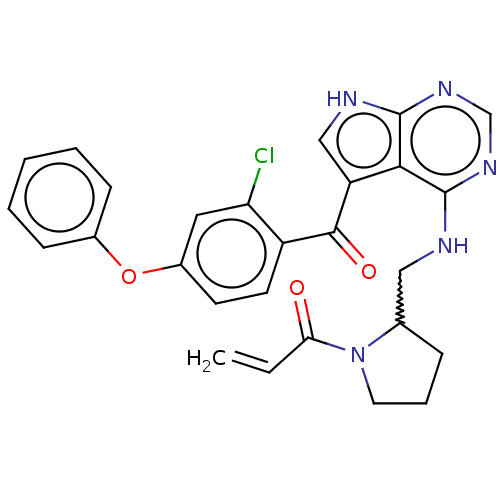 ((racemic)-1-(2-(((5-(2-chloro-4- phenoxybenzoyl)-7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Purified full-length inactive BTK (wild type and C481 mutant, N-terminal 6XHIS tagged BTK, Mwt=78.2 kDa) were activated using soluble inositol hexaki... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM499890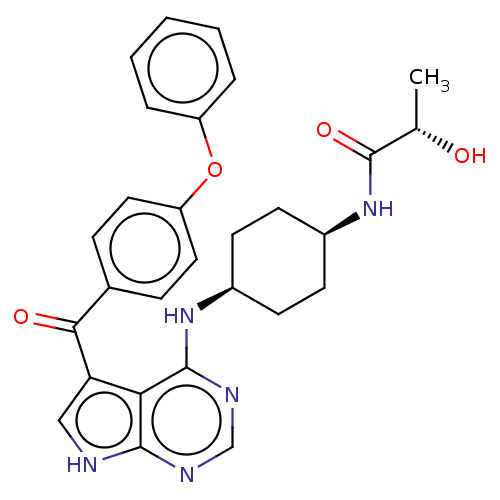 ((S)-2-hydroxy-N-(cis-4-((5-(4- phenoxybenzoyl)-7H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description The active BTK assay consisted of phosphorylated form of full length BTK. The assay was performed in a buffer solution utilized in the inactive BTK a... | US Patent US11020398 (2021) BindingDB Entry DOI: 10.7270/Q2DZ0CFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1147 total ) | Next | Last >> |