 Found 1314 hits with Last Name = 'nayak' and Initial = 'ak'
Found 1314 hits with Last Name = 'nayak' and Initial = 'ak' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50514163

(CHEMBL4568985)Show SMILES COc1cc(NC(=O)CSCC(=O)Nc2cc(nn2-c2ccccc2)-c2cc(C)c(C)cc2C)cc(OC)c1OC Show InChI InChI=1S/C31H34N4O5S/c1-19-12-21(3)24(13-20(19)2)25-16-28(35(34-25)23-10-8-7-9-11-23)33-30(37)18-41-17-29(36)32-22-14-26(38-4)31(40-6)27(15-22)39-5/h7-16H,17-18H2,1-6H3,(H,32,36)(H,33,37) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversal of P-gp mediated multidrug resistance in human DU145-TxR cells overexpressing P-gp assessed as potentiation of paclitaxel-induced cytotoxici... |
J Med Chem 62: 10645-10663 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00966
BindingDB Entry DOI: 10.7270/Q27084RB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM636722
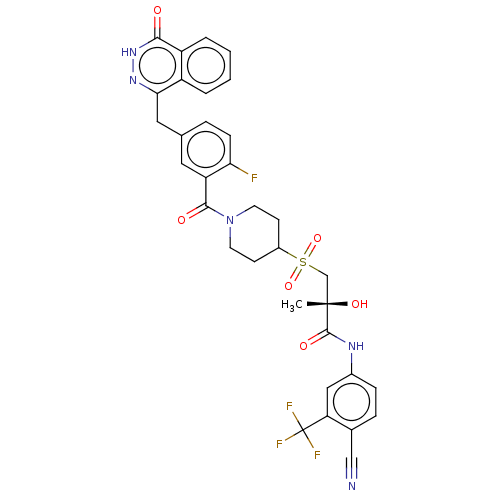
((R)óN-(4-cyano-3-(trifluoromethyl)phenyl)-3-(1-(2-...)Show SMILES C[C@](O)(CS(=O)(=O)C1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM636718
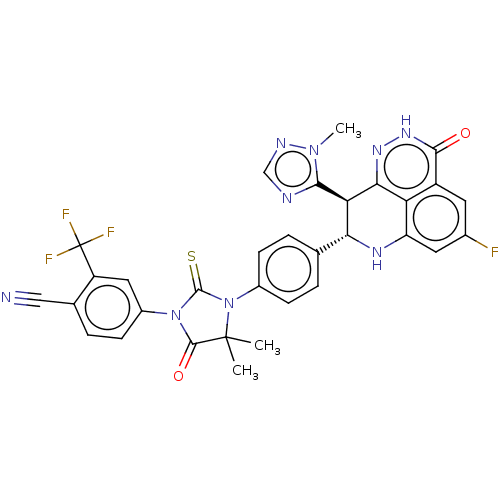
(US11826430, Compound 1.2a)Show SMILES Cn1ncnc1[C@@H]1[C@H](Nc2cc(F)cc3c2c1n[nH]c3=O)c1ccc(cc1)N1C(=S)N(C(=O)C1(C)C)c1ccc(C#N)c(c1)C(F)(F)F |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM636723
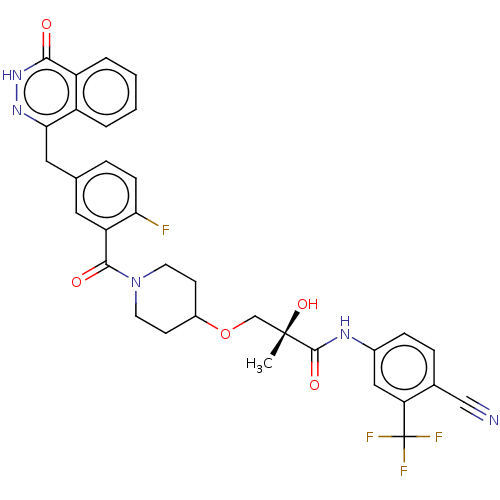
((S)óN-(4-cyano-3-(trifluoromethyl)phenyl)-3-(1-(2-...)Show SMILES C[C@](O)(COC1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50323728
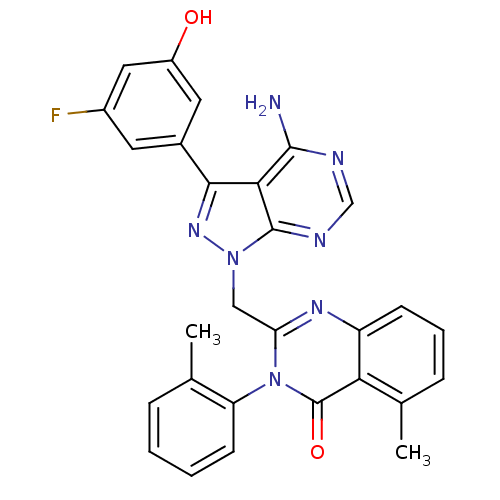
(2-((4-amino-3-(3-fluoro-5-hydroxyphenyl)-1H-pyrazo...)Show SMILES Cc1ccccc1-n1c(Cn2nc(-c3cc(O)cc(F)c3)c3c(N)ncnc23)nc2cccc(C)c2c1=O |(2.13,-14.38,;2.12,-12.84,;3.46,-12.06,;3.45,-10.52,;2.1,-9.75,;.77,-10.55,;.79,-12.08,;-.54,-12.85,;-.54,-14.41,;.81,-15.18,;.81,-16.72,;-.43,-17.63,;.05,-19.1,;-.74,-20.41,;-2.27,-20.38,;-3.06,-21.69,;-4.6,-21.66,;-2.32,-23.04,;-.78,-23.06,;-.03,-24.4,;.01,-21.74,;1.59,-19.09,;2.62,-20.22,;2.15,-21.69,;4.12,-19.9,;4.59,-18.43,;3.56,-17.3,;2.07,-17.63,;-1.87,-15.18,;-3.21,-14.41,;-4.55,-15.19,;-5.88,-14.42,;-5.87,-12.87,;-4.55,-12.1,;-4.56,-10.56,;-3.21,-12.87,;-1.88,-12.1,;-1.89,-10.56,)| Show InChI InChI=1S/C28H22FN7O2/c1-15-6-3-4-9-21(15)36-22(33-20-8-5-7-16(2)23(20)28(36)38)13-35-27-24(26(30)31-14-32-27)25(34-35)17-10-18(29)12-19(37)11-17/h3-12,14,37H,13H2,1-2H3,(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM636724
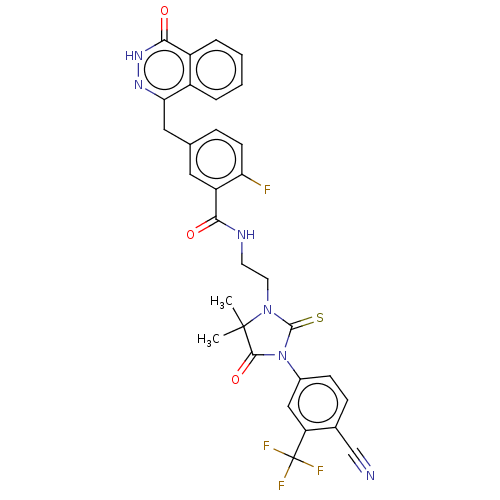
(N-(2-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-di...)Show SMILES CC1(C)N(CCNC(=O)c2cc(Cc3n[nH]c(=O)c4ccccc34)ccc2F)C(=S)N(C1=O)c1ccc(C#N)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM636721

(4-(3-(1-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-...)Show SMILES CC1(C)N(C2CCN(CC2)C(=O)c2cc(Cc3n[nH]c(=O)c4ccccc34)ccc2F)C(=S)N(C1=O)c1ccc(C#N)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM636722

((R)óN-(4-cyano-3-(trifluoromethyl)phenyl)-3-(1-(2-...)Show SMILES C[C@](O)(CS(=O)(=O)C1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50514163

(CHEMBL4568985)Show SMILES COc1cc(NC(=O)CSCC(=O)Nc2cc(nn2-c2ccccc2)-c2cc(C)c(C)cc2C)cc(OC)c1OC Show InChI InChI=1S/C31H34N4O5S/c1-19-12-21(3)24(13-20(19)2)25-16-28(35(34-25)23-10-8-7-9-11-23)33-30(37)18-41-17-29(36)32-22-14-26(38-4)31(40-6)27(15-22)39-5/h7-16H,17-18H2,1-6H3,(H,32,36)(H,33,37) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversal of P-gp mediated multidrug resistance in human DU145-TxR cells overexpressing P-gp assessed as potentiation of paclitaxel-induced cytotoxici... |
J Med Chem 62: 10645-10663 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00966
BindingDB Entry DOI: 10.7270/Q27084RB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50323728

(2-((4-amino-3-(3-fluoro-5-hydroxyphenyl)-1H-pyrazo...)Show SMILES Cc1ccccc1-n1c(Cn2nc(-c3cc(O)cc(F)c3)c3c(N)ncnc23)nc2cccc(C)c2c1=O |(2.13,-14.38,;2.12,-12.84,;3.46,-12.06,;3.45,-10.52,;2.1,-9.75,;.77,-10.55,;.79,-12.08,;-.54,-12.85,;-.54,-14.41,;.81,-15.18,;.81,-16.72,;-.43,-17.63,;.05,-19.1,;-.74,-20.41,;-2.27,-20.38,;-3.06,-21.69,;-4.6,-21.66,;-2.32,-23.04,;-.78,-23.06,;-.03,-24.4,;.01,-21.74,;1.59,-19.09,;2.62,-20.22,;2.15,-21.69,;4.12,-19.9,;4.59,-18.43,;3.56,-17.3,;2.07,-17.63,;-1.87,-15.18,;-3.21,-14.41,;-4.55,-15.19,;-5.88,-14.42,;-5.87,-12.87,;-4.55,-12.1,;-4.56,-10.56,;-3.21,-12.87,;-1.88,-12.1,;-1.89,-10.56,)| Show InChI InChI=1S/C28H22FN7O2/c1-15-6-3-4-9-21(15)36-22(33-20-8-5-7-16(2)23(20)28(36)38)13-35-27-24(26(30)31-14-32-27)25(34-35)17-10-18(29)12-19(37)11-17/h3-12,14,37H,13H2,1-2H3,(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) in presence of [gamma-32P]ATP by phosphorimaging assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50514158

(CHEMBL4526913)Show SMILES Cc1cc(C)c(cc1C)-c1cc(NC(=O)CSCC(=O)Nc2ccc3ncsc3c2)n(n1)-c1ccccc1 Show InChI InChI=1S/C29H27N5O2S2/c1-18-11-20(3)23(12-19(18)2)25-14-27(34(33-25)22-7-5-4-6-8-22)32-29(36)16-37-15-28(35)31-21-9-10-24-26(13-21)38-17-30-24/h4-14,17H,15-16H2,1-3H3,(H,31,35)(H,32,36) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversal of P-gp mediated multidrug resistance in human DU145-TxR cells overexpressing P-gp assessed as potentiation of paclitaxel-induced cytotoxici... |
J Med Chem 62: 10645-10663 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00966
BindingDB Entry DOI: 10.7270/Q27084RB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM636718

(US11826430, Compound 1.2a)Show SMILES Cn1ncnc1[C@@H]1[C@H](Nc2cc(F)cc3c2c1n[nH]c3=O)c1ccc(cc1)N1C(=S)N(C(=O)C1(C)C)c1ccc(C#N)c(c1)C(F)(F)F |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM636725

(N-(2-(5,5-dimethyl-3-(4-nitro-3-(trifluoromethyl)p...)Show SMILES CC1(C)N(CCNC(=O)c2cc(Cc3n[nH]c(=O)c4ccccc34)ccc2F)C(=O)N(C1=O)c1ccc(c(c1)C(F)(F)F)[N+]([O-])=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM636726
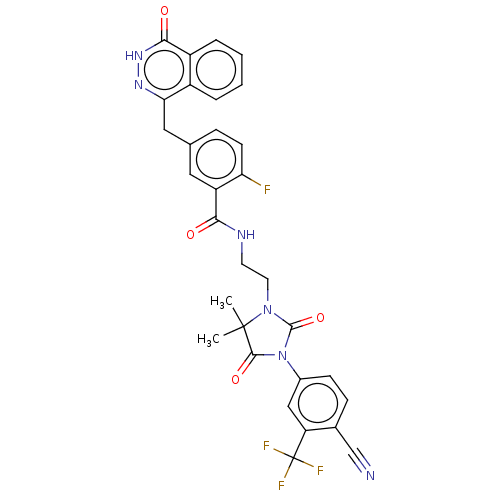
(US11826430, Compound 1.8 | imidazolidin-1-yl)ethyl...)Show SMILES CC1(C)N(CCNC(=O)c2cc(Cc3n[nH]c(=O)c4ccccc34)ccc2F)C(=O)N(C1=O)c1ccc(C#N)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM594883
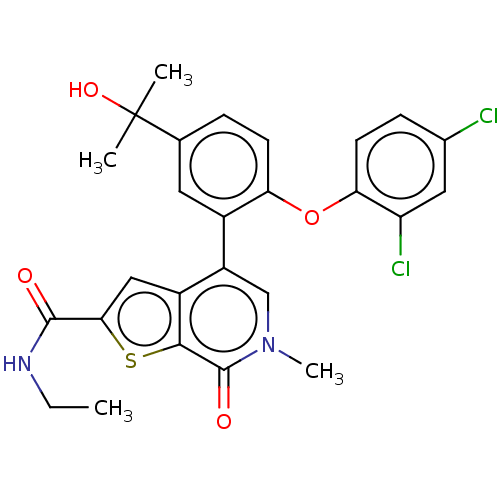
(US11584756, Compound 2 | US11584756, Example S-2)Show SMILES CCNC(=O)c1cc2c(cn(C)c(=O)c2s1)-c1cc(ccc1Oc1ccc(Cl)cc1Cl)C(C)(C)O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The bromodomain binding assays were performed by Reaction Biology Corp., Malvern, Pa., USA (www.reactionbiology.com). The BET binding assays were con... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K0786J |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [353-454]
(Homo sapiens (Human)) | BDBM528638

(US11192900, Compound 584 | US11192900, Example S-6...)Show SMILES COc1cc(=O)n(C)cc1-c1cc(NC(=O)C=C)ccc1Oc1c(C)cccc1C | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The bromodomain binding assays were performed by Reaction Biology Corp., Malvern, Pa., USA (www.reactionbiology.com). The BET binding assays were con... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2XS5ZJK |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM594901

(US11584756, Compound 150 | US11584756, Example S-2...)Show SMILES CNC(=O)c1cc2c(cn(C)c(=O)c2s1)-c1cc(ccc1Oc1c(C)cc(F)cc1C)C(C)(C)O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The bromodomain binding assays were performed by Reaction Biology Corp., Malvern, Pa., USA (www.reactionbiology.com). The BET binding assays were con... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K0786J |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM594911

(US11584756, Compound 159 | US11584756, Example S-3...)Show SMILES CCNC(=O)c1cc2c(s1)c(cn(C)c2=O)-c1cc(ccc1Oc1ccc(F)cc1F)C(C)(C)O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The bromodomain binding assays were performed by Reaction Biology Corp., Malvern, Pa., USA (www.reactionbiology.com). The BET binding assays were con... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K0786J |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM594896
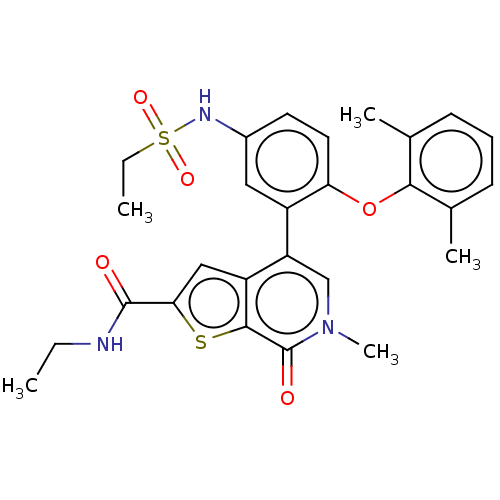
(US11584756, Compound 147 | US11584756, Example S-1...)Show SMILES CCNC(=O)c1cc2c(cn(C)c(=O)c2s1)-c1cc(NS(=O)(=O)CC)ccc1Oc1c(C)cccc1C | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The bromodomain binding assays were performed by Reaction Biology Corp., Malvern, Pa., USA (www.reactionbiology.com). The BET binding assays were con... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K0786J |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50514163

(CHEMBL4568985)Show SMILES COc1cc(NC(=O)CSCC(=O)Nc2cc(nn2-c2ccccc2)-c2cc(C)c(C)cc2C)cc(OC)c1OC Show InChI InChI=1S/C31H34N4O5S/c1-19-12-21(3)24(13-20(19)2)25-16-28(35(34-25)23-10-8-7-9-11-23)33-30(37)18-41-17-29(36)32-22-14-26(38-4)31(40-6)27(15-22)39-5/h7-16H,17-18H2,1-6H3,(H,32,36)(H,33,37) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversal of P-gp mediated multidrug resistance in human DU145-TxR cells overexpressing P-gp assessed as potentiation of paclitaxel-induced cytotoxici... |
J Med Chem 62: 10645-10663 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00966
BindingDB Entry DOI: 10.7270/Q27084RB |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM594892
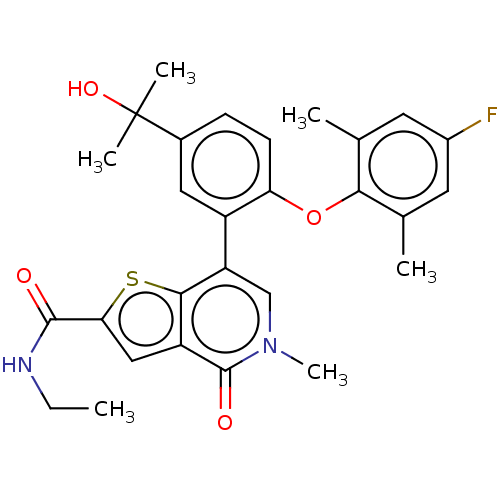
(US11584756, Compound 146 | US11584756, Example S-1...)Show SMILES CCNC(=O)c1cc2c(s1)c(cn(C)c2=O)-c1cc(ccc1Oc1c(C)cc(F)cc1C)C(C)(C)O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The bromodomain binding assays were performed by Reaction Biology Corp., Malvern, Pa., USA (www.reactionbiology.com). The BET binding assays were con... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K0786J |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50514161

(CHEMBL4469705)Show SMILES Cc1cc(C)c(cc1C)-c1cc(NC(=O)CSCC(=O)Nc2ccc3ccccc3c2)n(n1)-c1ccccc1 Show InChI InChI=1S/C32H30N4O2S/c1-21-15-23(3)28(16-22(21)2)29-18-30(36(35-29)27-11-5-4-6-12-27)34-32(38)20-39-19-31(37)33-26-14-13-24-9-7-8-10-25(24)17-26/h4-18H,19-20H2,1-3H3,(H,33,37)(H,34,38) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversal of P-gp mediated multidrug resistance in human DU145-TxR cells overexpressing P-gp assessed as potentiation of paclitaxel-induced cytotoxici... |
J Med Chem 62: 10645-10663 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00966
BindingDB Entry DOI: 10.7270/Q27084RB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50199118
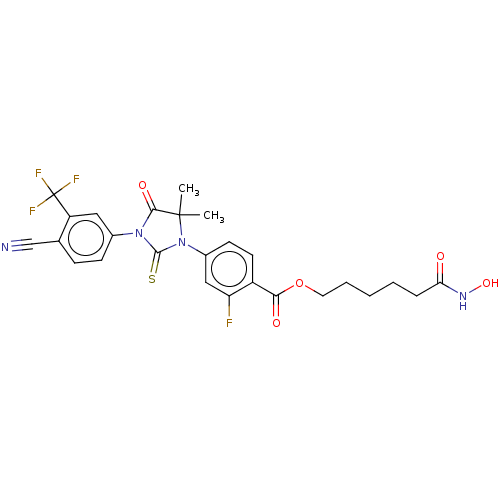
(CHEMBL3894825)Show SMILES CC1(C)N(C(=S)N(C1=O)c1ccc(C#N)c(c1)C(F)(F)F)c1ccc(C(=O)OCCCCCC(=O)NO)c(F)c1 Show InChI InChI=1S/C26H24F4N4O5S/c1-25(2)23(37)33(16-8-7-15(14-31)19(12-16)26(28,29)30)24(40)34(25)17-9-10-18(20(27)13-17)22(36)39-11-5-3-4-6-21(35)32-38/h7-10,12-13,38H,3-6,11H2,1-2H3,(H,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) assessed as inhibition of fluorogenic peptide deacetylation |
Bioorg Med Chem Lett 26: 5222-5228 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.058
BindingDB Entry DOI: 10.7270/Q29W0HFD |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM594884
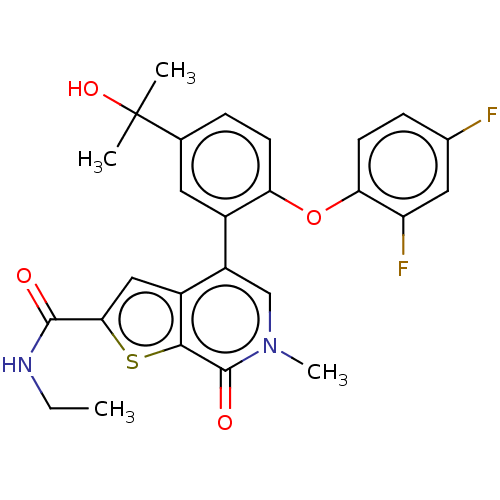
(US11584756, Compound 3 | US11584756, Example S-3)Show SMILES CCNC(=O)c1cc2c(cn(C)c(=O)c2s1)-c1cc(ccc1Oc1ccc(F)cc1F)C(C)(C)O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The bromodomain binding assays were performed by Reaction Biology Corp., Malvern, Pa., USA (www.reactionbiology.com). The BET binding assays were con... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K0786J |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM594882
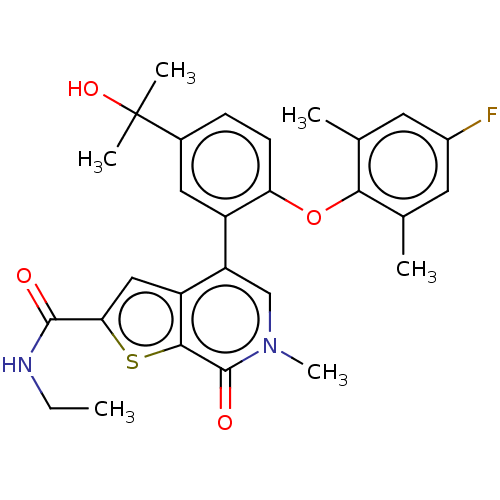
(US11584756, Compound 1 | US11584756, Example S-1)Show SMILES CCNC(=O)c1cc2c(cn(C)c(=O)c2s1)-c1cc(ccc1Oc1c(C)cc(F)cc1C)C(C)(C)O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The bromodomain binding assays were performed by Reaction Biology Corp., Malvern, Pa., USA (www.reactionbiology.com). The BET binding assays were con... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K0786J |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM636723

((S)óN-(4-cyano-3-(trifluoromethyl)phenyl)-3-(1-(2-...)Show SMILES C[C@](O)(COC1CCN(CC1)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [353-454]
(Homo sapiens (Human)) | BDBM528643
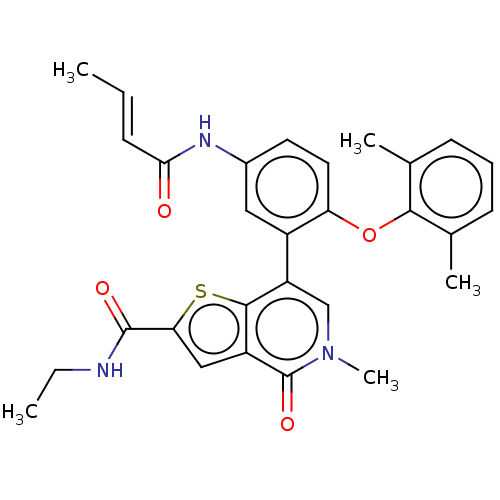
(US11192900, Compound 611 | US11192900, Example S-6...)Show SMILES CCNC(=O)c1cc2c(s1)c(cn(C)c2=O)-c1cc(NC(=O)\C=C\C)ccc1Oc1c(C)cccc1C | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The bromodomain binding assays were performed by Reaction Biology Corp., Malvern, Pa., USA (www.reactionbiology.com). The BET binding assays were con... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2XS5ZJK |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280366

(2-(5-chloro-2-fluorophenyl)-5-cyclopropyl-N-{1H-py...)Show SMILES Fc1ccc(Cl)cc1-c1cc(Nc2ccnc3[nH]ccc23)c(cn1)C1CC1 Show InChI InChI=1S/C21H16ClFN4/c22-13-3-4-17(23)15(9-13)19-10-20(16(11-26-19)12-1-2-12)27-18-6-8-25-21-14(18)5-7-24-21/h3-12H,1-2H2,(H2,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10030004 (2018)
BindingDB Entry DOI: 10.7270/Q2QC05HQ |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280366

(2-(5-chloro-2-fluorophenyl)-5-cyclopropyl-N-{1H-py...)Show SMILES Fc1ccc(Cl)cc1-c1cc(Nc2ccnc3[nH]ccc23)c(cn1)C1CC1 Show InChI InChI=1S/C21H16ClFN4/c22-13-3-4-17(23)15(9-13)19-10-20(16(11-26-19)12-1-2-12)27-18-6-8-25-21-14(18)5-7-24-21/h3-12H,1-2H2,(H2,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10501436 (2019)
BindingDB Entry DOI: 10.7270/Q20867PH |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280366

(2-(5-chloro-2-fluorophenyl)-5-cyclopropyl-N-{1H-py...)Show SMILES Fc1ccc(Cl)cc1-c1cc(Nc2ccnc3[nH]ccc23)c(cn1)C1CC1 Show InChI InChI=1S/C21H16ClFN4/c22-13-3-4-17(23)15(9-13)19-10-20(16(11-26-19)12-1-2-12)27-18-6-8-25-21-14(18)5-7-24-21/h3-12H,1-2H2,(H2,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2W380HG |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280366

(2-(5-chloro-2-fluorophenyl)-5-cyclopropyl-N-{1H-py...)Show SMILES Fc1ccc(Cl)cc1-c1cc(Nc2ccnc3[nH]ccc23)c(cn1)C1CC1 Show InChI InChI=1S/C21H16ClFN4/c22-13-3-4-17(23)15(9-13)19-10-20(16(11-26-19)12-1-2-12)27-18-6-8-25-21-14(18)5-7-24-21/h3-12H,1-2H2,(H2,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24M98N9 |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50514158

(CHEMBL4526913)Show SMILES Cc1cc(C)c(cc1C)-c1cc(NC(=O)CSCC(=O)Nc2ccc3ncsc3c2)n(n1)-c1ccccc1 Show InChI InChI=1S/C29H27N5O2S2/c1-18-11-20(3)23(12-19(18)2)25-14-27(34(33-25)22-7-5-4-6-8-22)32-29(36)16-37-15-28(35)31-21-9-10-24-26(13-21)38-17-30-24/h4-14,17H,15-16H2,1-3H3,(H,31,35)(H,32,36) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversal of P-gp mediated multidrug resistance in human DU145-TxR cells overexpressing P-gp assessed as potentiation of paclitaxel-induced cytotoxici... |
J Med Chem 62: 10645-10663 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00966
BindingDB Entry DOI: 10.7270/Q27084RB |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [353-454]
(Homo sapiens (Human)) | BDBM528646
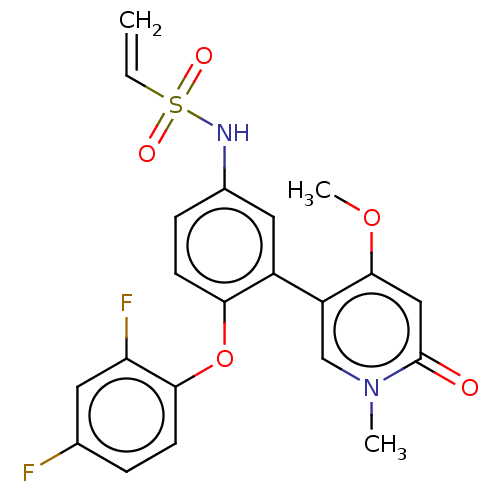
(US11192900, Compound 406 | US11192900, Example S-7...)Show SMILES COc1cc(=O)n(C)cc1-c1cc(NS(=O)(=O)C=C)ccc1Oc1ccc(F)cc1F | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The bromodomain binding assays were performed by Reaction Biology Corp., Malvern, Pa., USA (www.reactionbiology.com). The BET binding assays were con... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2XS5ZJK |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405402
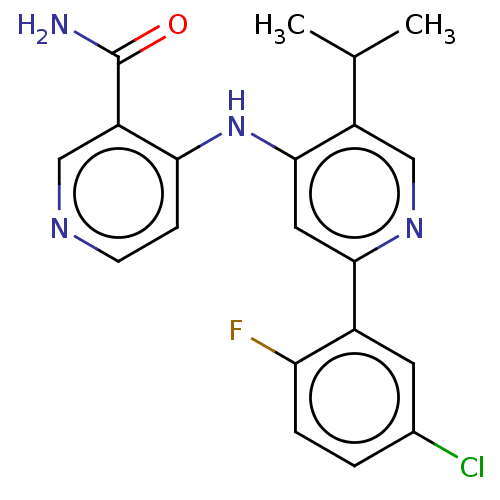
(CHEMBL5267945)Show SMILES COc1ccc(CCNCC(O)COc2ccc(COCc3ncc[nH]3)cc2)cc1OC Show InChI InChI=1S/C24H31N3O5/c1-29-22-8-5-18(13-23(22)30-2)9-10-25-14-20(28)16-32-21-6-3-19(4-7-21)15-31-17-24-26-11-12-27-24/h3-8,11-13,20,25,28H,9-10,14-17H2,1-2H3,(H,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405407

(CHEMBL5285361)Show SMILES COc1ccc(CCNC[C@H](O)COc2ccc(Cc3nc(c[nH]3)C(C)=O)cc2)cc1OC Show InChI InChI=1S/C25H31N3O5/c1-17(29)22-15-27-25(28-22)13-18-4-7-21(8-5-18)33-16-20(30)14-26-11-10-19-6-9-23(31-2)24(12-19)32-3/h4-9,12,15,20,26,30H,10-11,13-14,16H2,1-3H3,(H,27,28)/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50201717
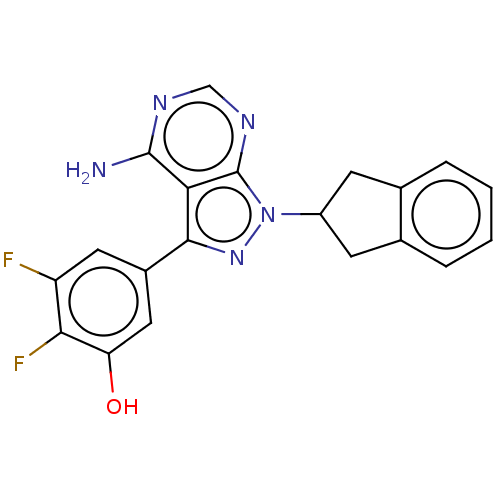
(CHEMBL3907591)Show SMILES Nc1ncnc2n(nc(-c3cc(O)c(F)c(F)c3)c12)C1Cc2ccccc2C1 Show InChI InChI=1S/C20H15F2N5O/c21-14-7-12(8-15(28)17(14)22)18-16-19(23)24-9-25-20(16)27(26-18)13-5-10-3-1-2-4-11(10)6-13/h1-4,7-9,13,28H,5-6H2,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate in presence of streptavidin-APC by FRET assay |
ACS Med Chem Lett 7: 1161-1166 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00356
BindingDB Entry DOI: 10.7270/Q29K4D6F |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM636721

(4-(3-(1-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-...)Show SMILES CC1(C)N(C2CCN(CC2)C(=O)c2cc(Cc3n[nH]c(=O)c4ccccc34)ccc2F)C(=S)N(C1=O)c1ccc(C#N)c(c1)C(F)(F)F | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM636728
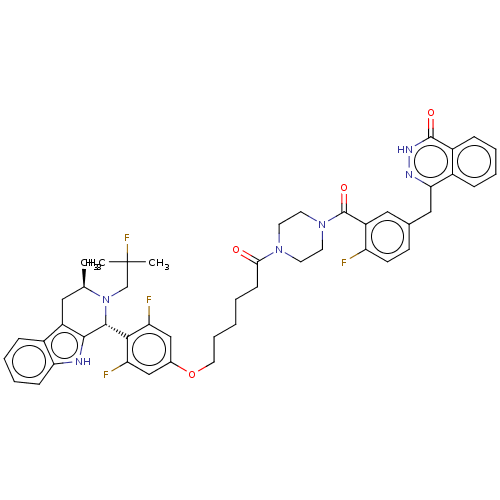
(4-(3-(4-(6-(3,5-difluoro-4-((1R,3R)-2-(2-fluoro-2-...)Show SMILES C[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1CC(C)(C)F)c1c(F)cc(OCCCCCC(=O)N2CCN(CC2)C(=O)c2cc(Cc3n[nH]c(=O)c4ccccc34)ccc2F)cc1F |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM594902
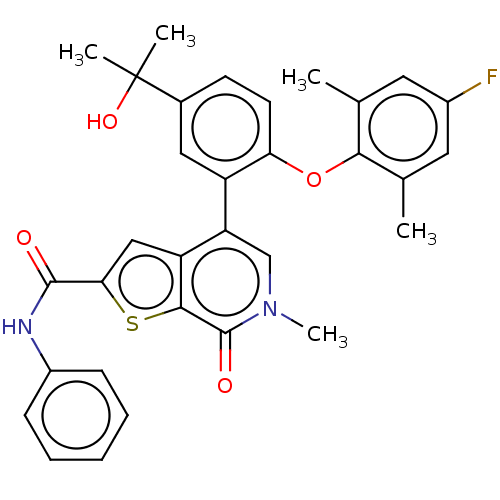
(US11584756, Compound 151 | US11584756, Example S-2...)Show SMILES Cc1cc(F)cc(C)c1Oc1ccc(cc1-c1cn(C)c(=O)c2sc(cc12)C(=O)Nc1ccccc1)C(C)(C)O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The bromodomain binding assays were performed by Reaction Biology Corp., Malvern, Pa., USA (www.reactionbiology.com). The BET binding assays were con... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K0786J |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM636731
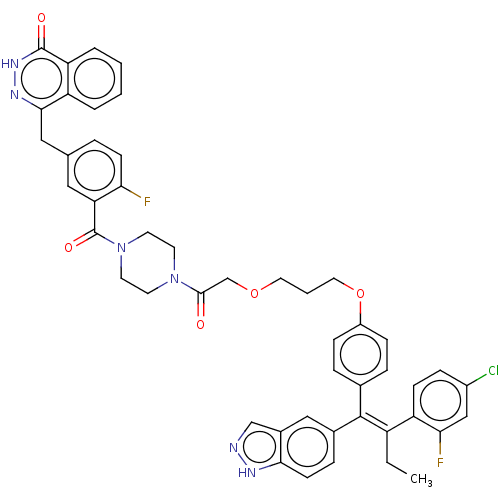
((E)-4-(3-(4-(2-(3-(4-(2-(4-chloro-2-fluorophenyl)-...)Show SMILES CC\C(=C(\c1ccc(OCCCOCC(=O)N2CCN(CC2)C(=O)c2cc(Cc3n[nH]c(=O)c4ccccc34)ccc2F)cc1)c1ccc2[nH]ncc2c1)c1ccc(Cl)cc1F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM594895
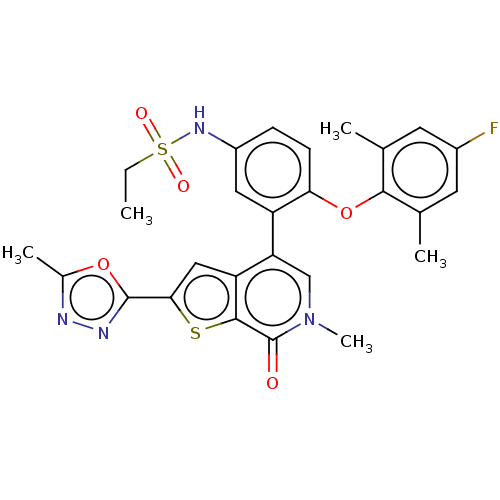
(US11584756, Compound 10 | US11584756, Example S-14)Show SMILES CCS(=O)(=O)Nc1ccc(Oc2c(C)cc(F)cc2C)c(c1)-c1cn(C)c(=O)c2sc(cc12)-c1nnc(C)o1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The bromodomain binding assays were performed by Reaction Biology Corp., Malvern, Pa., USA (www.reactionbiology.com). The BET binding assays were con... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K0786J |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280369
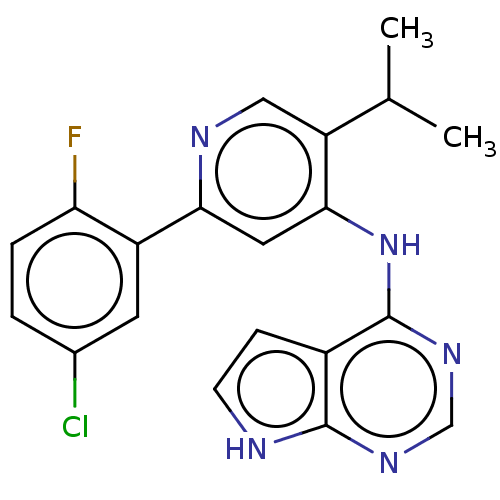
(2-(5-chloro-2-fluorophenyl)-5-(propan-2-yl)-N-{7H-...)Show SMILES COc1ccc(Cn2ccc3c(Nc4cc(ncc4C(C)C)-c4cc(Cl)ccc4F)ncnc23)cc1 Show InChI InChI=1S/C28H25ClFN5O/c1-17(2)23-14-31-25(22-12-19(29)6-9-24(22)30)13-26(23)34-27-21-10-11-35(28(21)33-16-32-27)15-18-4-7-20(36-3)8-5-18/h4-14,16-17H,15H2,1-3H3,(H,31,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24M98N9 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280369

(2-(5-chloro-2-fluorophenyl)-5-(propan-2-yl)-N-{7H-...)Show SMILES COc1ccc(Cn2ccc3c(Nc4cc(ncc4C(C)C)-c4cc(Cl)ccc4F)ncnc23)cc1 Show InChI InChI=1S/C28H25ClFN5O/c1-17(2)23-14-31-25(22-12-19(29)6-9-24(22)30)13-26(23)34-27-21-10-11-35(28(21)33-16-32-27)15-18-4-7-20(36-3)8-5-18/h4-14,16-17H,15H2,1-3H3,(H,31,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2W380HG |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280370

(2-(5-chloro-2-fluorophenyl)-5-(propan-2-yl)-N-{1H-...)Show SMILES COc1ccc(Cn2ncc3c(Nc4cc(ncc4C(C)C)-c4cc(Cl)ccc4F)ncnc23)cc1 Show InChI InChI=1S/C27H24ClFN6O/c1-16(2)21-12-30-24(20-10-18(28)6-9-23(20)29)11-25(21)34-26-22-13-33-35(27(22)32-15-31-26)14-17-4-7-19(36-3)8-5-17/h4-13,15-16H,14H2,1-3H3,(H,30,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2W380HG |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280369

(2-(5-chloro-2-fluorophenyl)-5-(propan-2-yl)-N-{7H-...)Show SMILES COc1ccc(Cn2ccc3c(Nc4cc(ncc4C(C)C)-c4cc(Cl)ccc4F)ncnc23)cc1 Show InChI InChI=1S/C28H25ClFN5O/c1-17(2)23-14-31-25(22-12-19(29)6-9-24(22)30)13-26(23)34-27-21-10-11-35(28(21)33-16-32-27)15-18-4-7-20(36-3)8-5-18/h4-14,16-17H,15H2,1-3H3,(H,31,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10501436 (2019)
BindingDB Entry DOI: 10.7270/Q20867PH |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280370

(2-(5-chloro-2-fluorophenyl)-5-(propan-2-yl)-N-{1H-...)Show SMILES COc1ccc(Cn2ncc3c(Nc4cc(ncc4C(C)C)-c4cc(Cl)ccc4F)ncnc23)cc1 Show InChI InChI=1S/C27H24ClFN6O/c1-16(2)21-12-30-24(20-10-18(28)6-9-23(20)29)11-25(21)34-26-22-13-33-35(27(22)32-15-31-26)14-17-4-7-19(36-3)8-5-17/h4-13,15-16H,14H2,1-3H3,(H,30,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10501436 (2019)
BindingDB Entry DOI: 10.7270/Q20867PH |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280369

(2-(5-chloro-2-fluorophenyl)-5-(propan-2-yl)-N-{7H-...)Show SMILES COc1ccc(Cn2ccc3c(Nc4cc(ncc4C(C)C)-c4cc(Cl)ccc4F)ncnc23)cc1 Show InChI InChI=1S/C28H25ClFN5O/c1-17(2)23-14-31-25(22-12-19(29)6-9-24(22)30)13-26(23)34-27-21-10-11-35(28(21)33-16-32-27)15-18-4-7-20(36-3)8-5-18/h4-14,16-17H,15H2,1-3H3,(H,31,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10030004 (2018)
BindingDB Entry DOI: 10.7270/Q2QC05HQ |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280370

(2-(5-chloro-2-fluorophenyl)-5-(propan-2-yl)-N-{1H-...)Show SMILES COc1ccc(Cn2ncc3c(Nc4cc(ncc4C(C)C)-c4cc(Cl)ccc4F)ncnc23)cc1 Show InChI InChI=1S/C27H24ClFN6O/c1-16(2)21-12-30-24(20-10-18(28)6-9-23(20)29)11-25(21)34-26-22-13-33-35(27(22)32-15-31-26)14-17-4-7-19(36-3)8-5-17/h4-13,15-16H,14H2,1-3H3,(H,30,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivation Technologies LLC
US Patent
| Assay Description
Compound of the invention were screened in an in vitro kinase assay against several members of the TGFβ family of Ser/Thr kinases. The kinases t... |
US Patent US10030004 (2018)
BindingDB Entry DOI: 10.7270/Q2QC05HQ |
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280370

(2-(5-chloro-2-fluorophenyl)-5-(propan-2-yl)-N-{1H-...)Show SMILES COc1ccc(Cn2ncc3c(Nc4cc(ncc4C(C)C)-c4cc(Cl)ccc4F)ncnc23)cc1 Show InChI InChI=1S/C27H24ClFN6O/c1-16(2)21-12-30-24(20-10-18(28)6-9-23(20)29)11-25(21)34-26-22-13-33-35(27(22)32-15-31-26)14-17-4-7-19(36-3)8-5-17/h4-13,15-16H,14H2,1-3H3,(H,30,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24M98N9 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [353-454]
(Homo sapiens (Human)) | BDBM528627
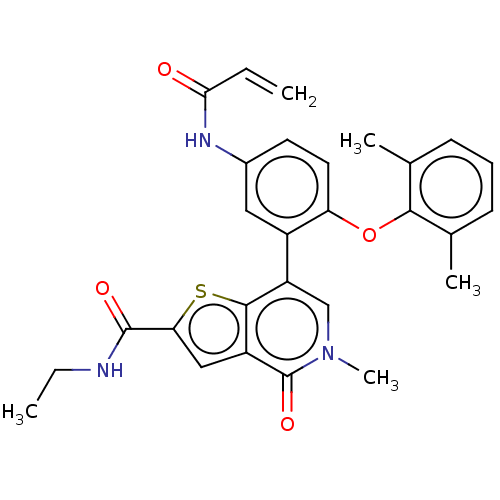
(US11192900, Compound 412 | US11192900, Example S-5...)Show SMILES CCNC(=O)c1cc2c(s1)c(cn(C)c2=O)-c1cc(NC(=O)C=C)ccc1Oc1c(C)cccc1C | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The bromodomain binding assays were performed by Reaction Biology Corp., Malvern, Pa., USA (www.reactionbiology.com). The BET binding assays were con... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2XS5ZJK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data