 Found 605 hits with Last Name = 'ness' and Initial = 'd'
Found 605 hits with Last Name = 'ness' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM82547
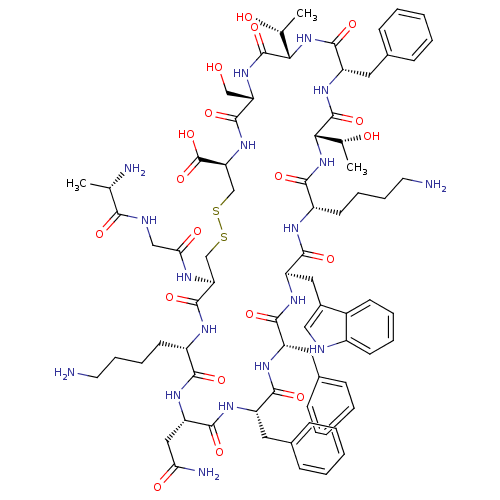
(SRIF-D-Trp8)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55+,56-,57-,58-,59-,62-,63-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
FEBS Lett 321: 279-84 (1993)
Article DOI: 10.1016/0014-5793(93)80124-d
BindingDB Entry DOI: 10.7270/Q2PR7THQ |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50258529

(CHEMBL449158 | bryostatin 1)Show SMILES CCC\C=C\C=C\C(=O)O[C@H]1\C(C[C@H]2C[C@@H](OC(=O)C[C@H](O)C[C@@H]3C[C@H](OC(C)=O)C(C)(C)[C@](O)(C[C@@H]4C\C(C[C@@H](O4)\C=C\C(C)(C)[C@]1(O)O2)=C\C(=O)OC)O3)[C@@H](C)O)=C\C(=O)OC |r,t:43| Show InChI InChI=1S/C47H68O17/c1-10-11-12-13-14-15-39(51)62-43-31(22-41(53)58-9)21-34-25-37(28(2)48)61-42(54)24-32(50)23-35-26-38(59-29(3)49)45(6,7)46(55,63-35)27-36-19-30(20-40(52)57-8)18-33(60-36)16-17-44(4,5)47(43,56)64-34/h12-17,20,22,28,32-38,43,48,50,55-56H,10-11,18-19,21,23-27H2,1-9H3/b13-12+,15-14+,17-16+,30-20+,31-22+/t28-,32-,33+,34+,35-,36+,37-,38+,43+,46+,47-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... |
J Nat Prod 79: 680-4 (2016)
Article DOI: 10.1021/acs.jnatprod.5b01017
BindingDB Entry DOI: 10.7270/Q2BK1GXV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50535777
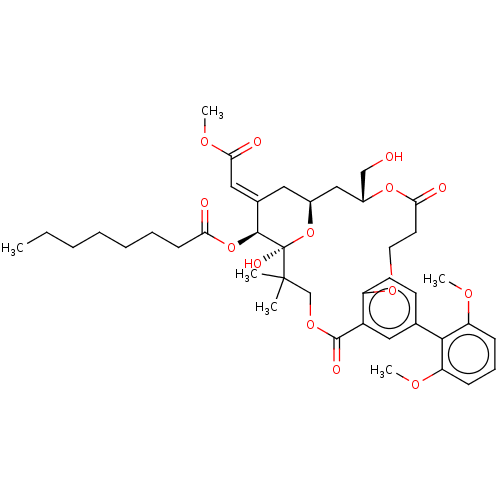
(CHEMBL4565484)Show SMILES CCCCCCCC(=O)O[C@H]1\C(C[C@H]2C[C@H](CO)OC(=O)CCOc3ccc(cc3C(=O)OCC(C)(C)[C@]1(O)O2)-c1c(OC)cccc1OC)=C\C(=O)OC |r| Show InChI InChI=1S/C41H54O14/c1-7-8-9-10-11-15-34(43)54-38-27(22-36(45)50-6)20-28-23-29(24-42)53-35(44)18-19-51-31-17-16-26(37-32(48-4)13-12-14-33(37)49-5)21-30(31)39(46)52-25-40(2,3)41(38,47)55-28/h12-14,16-17,21-22,28-29,38,42,47H,7-11,15,18-20,23-25H2,1-6H3/b27-22+/t28-,29+,38-,41+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... |
J Nat Prod 79: 680-4 (2016)
Article DOI: 10.1021/acs.jnatprod.5b01017
BindingDB Entry DOI: 10.7270/Q2BK1GXV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50535778
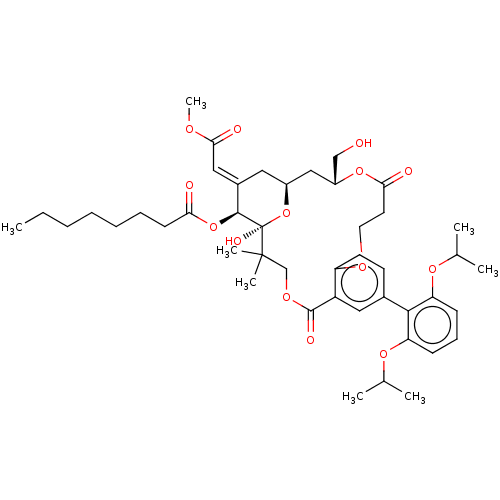
(CHEMBL4533216)Show SMILES CCCCCCCC(=O)O[C@H]1\C(C[C@H]2C[C@H](CO)OC(=O)CCOc3ccc(cc3C(=O)OCC(C)(C)[C@]1(O)O2)-c1c(OC(C)C)cccc1OC(C)C)=C\C(=O)OC |r| Show InChI InChI=1S/C45H62O14/c1-9-10-11-12-13-17-38(47)58-42-31(24-40(49)52-8)22-32-25-33(26-46)57-39(48)20-21-53-35-19-18-30(23-34(35)43(50)54-27-44(6,7)45(42,51)59-32)41-36(55-28(2)3)15-14-16-37(41)56-29(4)5/h14-16,18-19,23-24,28-29,32-33,42,46,51H,9-13,17,20-22,25-27H2,1-8H3/b31-24+/t32-,33+,42-,45+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... |
J Nat Prod 79: 680-4 (2016)
Article DOI: 10.1021/acs.jnatprod.5b01017
BindingDB Entry DOI: 10.7270/Q2BK1GXV |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50019568

(Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52+,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
FEBS Lett 321: 279-84 (1993)
Article DOI: 10.1016/0014-5793(93)80124-d
BindingDB Entry DOI: 10.7270/Q2PR7THQ |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50535789

(CHEMBL4574406)Show SMILES CCCCCCCC(=O)O[C@H]1\C(C[C@H]2C[C@H](CO)OC(=O)CCOc3ccc(cc3C(=O)OCC(C)(C)[C@]1(O)O2)-c1ccc(OC(C)C)cc1)=C\C(=O)OC |r| Show InChI InChI=1S/C42H56O13/c1-7-8-9-10-11-12-36(44)54-39-30(23-38(46)49-6)21-32-24-33(25-43)53-37(45)19-20-50-35-18-15-29(28-13-16-31(17-14-28)52-27(2)3)22-34(35)40(47)51-26-41(4,5)42(39,48)55-32/h13-18,22-23,27,32-33,39,43,48H,7-12,19-21,24-26H2,1-6H3/b30-23+/t32-,33+,39-,42+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... |
J Nat Prod 79: 680-4 (2016)
Article DOI: 10.1021/acs.jnatprod.5b01017
BindingDB Entry DOI: 10.7270/Q2BK1GXV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50535791
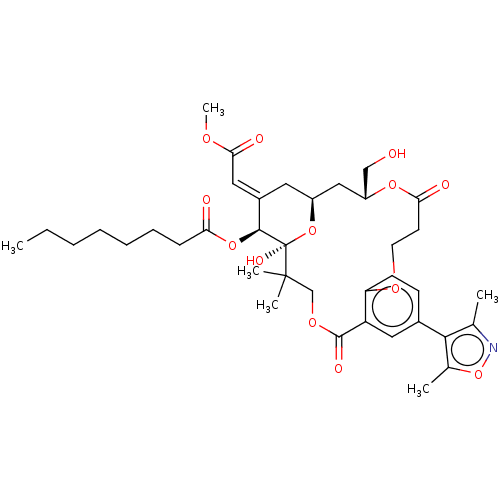
(CHEMBL4593581)Show SMILES CCCCCCCC(=O)O[C@H]1\C(C[C@H]2C[C@H](CO)OC(=O)CCOc3ccc(cc3C(=O)OCC(C)(C)[C@]1(O)O2)-c1c(C)noc1C)=C\C(=O)OC |r| Show InChI InChI=1S/C38H51NO13/c1-7-8-9-10-11-12-31(41)50-35-26(19-33(43)46-6)17-27-20-28(21-40)49-32(42)15-16-47-30-14-13-25(34-23(2)39-52-24(34)3)18-29(30)36(44)48-22-37(4,5)38(35,45)51-27/h13-14,18-19,27-28,35,40,45H,7-12,15-17,20-22H2,1-6H3/b26-19+/t27-,28+,35-,38+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... |
J Nat Prod 79: 680-4 (2016)
Article DOI: 10.1021/acs.jnatprod.5b01017
BindingDB Entry DOI: 10.7270/Q2BK1GXV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50535790
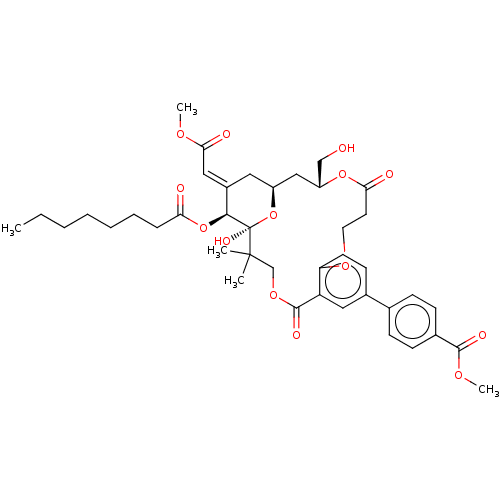
(CHEMBL4536091)Show SMILES CCCCCCCC(=O)O[C@H]1\C(C[C@H]2C[C@H](CO)OC(=O)CCOc3ccc(cc3C(=O)OCC(C)(C)[C@]1(O)O2)-c1ccc(cc1)C(=O)OC)=C\C(=O)OC |r| Show InChI InChI=1S/C41H52O14/c1-6-7-8-9-10-11-34(43)54-37-29(22-36(45)49-4)20-30-23-31(24-42)53-35(44)18-19-51-33-17-16-28(26-12-14-27(15-13-26)38(46)50-5)21-32(33)39(47)52-25-40(2,3)41(37,48)55-30/h12-17,21-22,30-31,37,42,48H,6-11,18-20,23-25H2,1-5H3/b29-22+/t30-,31+,37-,41+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... |
J Nat Prod 79: 680-4 (2016)
Article DOI: 10.1021/acs.jnatprod.5b01017
BindingDB Entry DOI: 10.7270/Q2BK1GXV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50535785
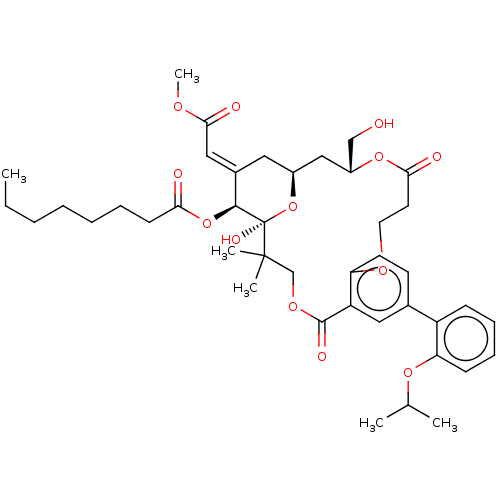
(CHEMBL4526756)Show SMILES CCCCCCCC(=O)O[C@H]1\C(C[C@H]2C[C@H](CO)OC(=O)CCOc3ccc(cc3C(=O)OCC(C)(C)[C@]1(O)O2)-c1ccccc1OC(C)C)=C\C(=O)OC |r| Show InChI InChI=1S/C42H56O13/c1-7-8-9-10-11-16-36(44)54-39-29(23-38(46)49-6)21-30-24-31(25-43)53-37(45)19-20-50-34-18-17-28(32-14-12-13-15-35(32)52-27(2)3)22-33(34)40(47)51-26-41(4,5)42(39,48)55-30/h12-15,17-18,22-23,27,30-31,39,43,48H,7-11,16,19-21,24-26H2,1-6H3/b29-23+/t30-,31+,39-,42+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... |
J Nat Prod 79: 680-4 (2016)
Article DOI: 10.1021/acs.jnatprod.5b01017
BindingDB Entry DOI: 10.7270/Q2BK1GXV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50535782
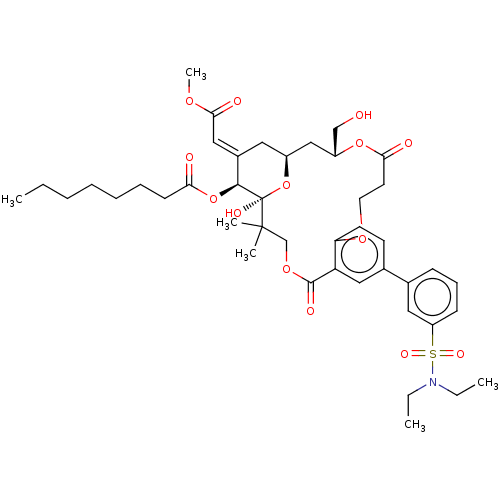
(CHEMBL4576842)Show SMILES CCCCCCCC(=O)O[C@H]1\C(C[C@H]2C[C@H](CO)OC(=O)CCOc3ccc(cc3C(=O)OCC(C)(C)[C@]1(O)O2)-c1cccc(c1)S(=O)(=O)N(CC)CC)=C\C(=O)OC |r| Show InChI InChI=1S/C43H59NO14S/c1-7-10-11-12-13-17-37(46)57-40-31(25-39(48)53-6)22-32-26-33(27-45)56-38(47)20-21-54-36-19-18-30(24-35(36)41(49)55-28-42(4,5)43(40,50)58-32)29-15-14-16-34(23-29)59(51,52)44(8-2)9-3/h14-16,18-19,23-25,32-33,40,45,50H,7-13,17,20-22,26-28H2,1-6H3/b31-25+/t32-,33+,40-,43+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... |
J Nat Prod 79: 680-4 (2016)
Article DOI: 10.1021/acs.jnatprod.5b01017
BindingDB Entry DOI: 10.7270/Q2BK1GXV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50535775

(CHEMBL4525186)Show SMILES CCCCCCCC(=O)O[C@H]1\C(C[C@H]2C[C@H](CO)OC(=O)CCOc3ccc(cc3C(=O)OCC(C)(C)[C@]1(O)O2)-c1ccc(OC)cc1)=C\C(=O)OC |r| Show InChI InChI=1S/C40H52O13/c1-6-7-8-9-10-11-34(42)52-37-28(22-36(44)48-5)20-30-23-31(24-41)51-35(43)18-19-49-33-17-14-27(26-12-15-29(47-4)16-13-26)21-32(33)38(45)50-25-39(2,3)40(37,46)53-30/h12-17,21-22,30-31,37,41,46H,6-11,18-20,23-25H2,1-5H3/b28-22+/t30-,31+,37-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... |
J Nat Prod 79: 680-4 (2016)
Article DOI: 10.1021/acs.jnatprod.5b01017
BindingDB Entry DOI: 10.7270/Q2BK1GXV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50535772

(CHEMBL4574986)Show SMILES CCCCCCCCc1ccc2OCCC(=O)O[C@@H](CO)C[C@@H]3C\C(=C/C(=O)OC)[C@H](OC(=O)CCCCCCC)[C@@](O)(O3)C(C)(C)COC(=O)c2c1 |r| Show InChI InChI=1S/C41H62O12/c1-6-8-10-12-14-15-17-29-19-20-34-33(23-29)39(46)50-28-40(3,4)41(47)38(52-35(43)18-16-13-11-9-7-2)30(25-37(45)48-5)24-31(53-41)26-32(27-42)51-36(44)21-22-49-34/h19-20,23,25,31-32,38,42,47H,6-18,21-22,24,26-28H2,1-5H3/b30-25+/t31-,32+,38-,41+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... |
J Nat Prod 79: 680-4 (2016)
Article DOI: 10.1021/acs.jnatprod.5b01017
BindingDB Entry DOI: 10.7270/Q2BK1GXV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50535776
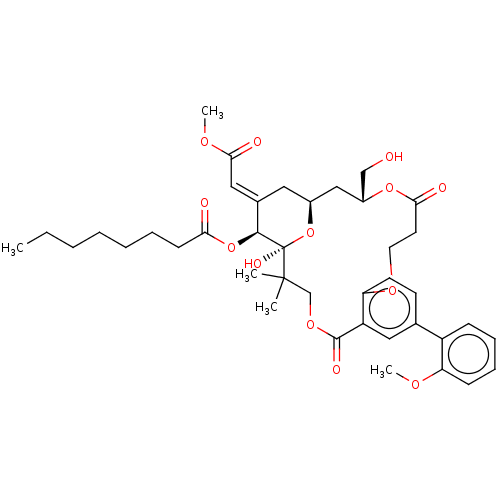
(CHEMBL4550131)Show SMILES CCCCCCCC(=O)O[C@H]1\C(C[C@H]2C[C@H](CO)OC(=O)CCOc3ccc(cc3C(=O)OCC(C)(C)[C@]1(O)O2)-c1ccccc1OC)=C\C(=O)OC |r| Show InChI InChI=1S/C40H52O13/c1-6-7-8-9-10-15-34(42)52-37-27(22-36(44)48-5)20-28-23-29(24-41)51-35(43)18-19-49-33-17-16-26(30-13-11-12-14-32(30)47-4)21-31(33)38(45)50-25-39(2,3)40(37,46)53-28/h11-14,16-17,21-22,28-29,37,41,46H,6-10,15,18-20,23-25H2,1-5H3/b27-22+/t28-,29+,37-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... |
J Nat Prod 79: 680-4 (2016)
Article DOI: 10.1021/acs.jnatprod.5b01017
BindingDB Entry DOI: 10.7270/Q2BK1GXV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50535786

(CHEMBL4542079)Show SMILES CCCCCCCC(=O)O[C@H]1\C(C[C@H]2C[C@H](CO)OC(=O)CCOc3ccc(cc3C(=O)OCC(C)(C)[C@]1(O)O2)-c1ccc(cc1)S(=O)(=O)N(CC)CC)=C\C(=O)OC |r| Show InChI InChI=1S/C43H59NO14S/c1-7-10-11-12-13-14-37(46)57-40-31(25-39(48)53-6)23-32-26-33(27-45)56-38(47)21-22-54-36-20-17-30(24-35(36)41(49)55-28-42(4,5)43(40,50)58-32)29-15-18-34(19-16-29)59(51,52)44(8-2)9-3/h15-20,24-25,32-33,40,45,50H,7-14,21-23,26-28H2,1-6H3/b31-25+/t32-,33+,40-,43+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... |
J Nat Prod 79: 680-4 (2016)
Article DOI: 10.1021/acs.jnatprod.5b01017
BindingDB Entry DOI: 10.7270/Q2BK1GXV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50535780
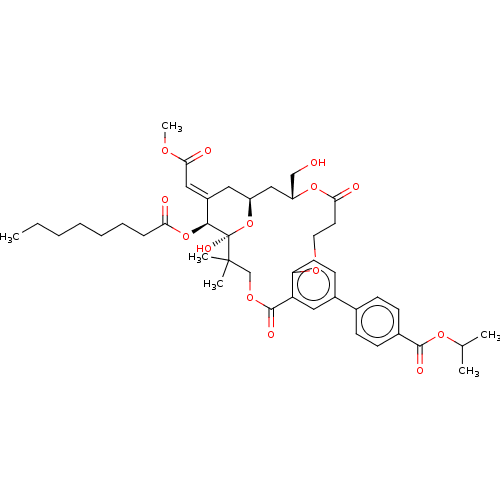
(CHEMBL4560593)Show SMILES CCCCCCCC(=O)O[C@H]1\C(C[C@H]2C[C@H](CO)OC(=O)CCOc3ccc(cc3C(=O)OCC(C)(C)[C@]1(O)O2)-c1ccc(cc1)C(=O)OC(C)C)=C\C(=O)OC |r| Show InChI InChI=1S/C43H56O14/c1-7-8-9-10-11-12-36(45)56-39-31(23-38(47)51-6)21-32-24-33(25-44)55-37(46)19-20-52-35-18-17-30(28-13-15-29(16-14-28)40(48)54-27(2)3)22-34(35)41(49)53-26-42(4,5)43(39,50)57-32/h13-18,22-23,27,32-33,39,44,50H,7-12,19-21,24-26H2,1-6H3/b31-23+/t32-,33+,39-,43+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... |
J Nat Prod 79: 680-4 (2016)
Article DOI: 10.1021/acs.jnatprod.5b01017
BindingDB Entry DOI: 10.7270/Q2BK1GXV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50535781

(CHEMBL4535813)Show SMILES CCCCCCCC(=O)O[C@H]1\C(C[C@H]2C[C@H](CO)OC(=O)CCOc3ccc(cc3C(=O)OCC(C)(C)[C@]1(O)O2)-c1ccc(cc1)C(=O)N(CC)CC)=C\C(=O)OC |r| Show InChI InChI=1S/C44H59NO13/c1-7-10-11-12-13-14-37(47)57-40-32(25-39(49)53-6)23-33-26-34(27-46)56-38(48)21-22-54-36-20-19-31(29-15-17-30(18-16-29)41(50)45(8-2)9-3)24-35(36)42(51)55-28-43(4,5)44(40,52)58-33/h15-20,24-25,33-34,40,46,52H,7-14,21-23,26-28H2,1-6H3/b32-25+/t33-,34+,40-,44+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... |
J Nat Prod 79: 680-4 (2016)
Article DOI: 10.1021/acs.jnatprod.5b01017
BindingDB Entry DOI: 10.7270/Q2BK1GXV |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50254239
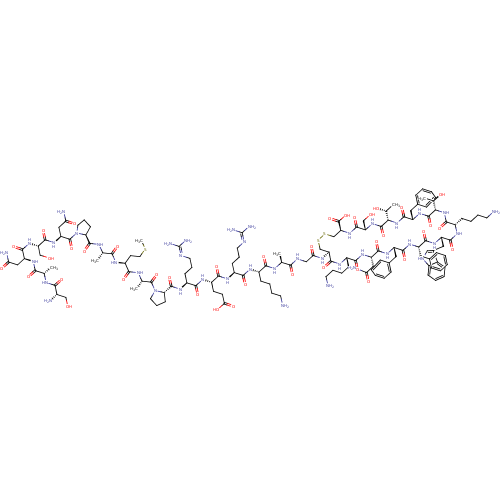
(somatostatin-28)Show SMILES CSCC[C@H](NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CO)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC1=O)[C@@H](C)O)[C@@H](C)O)C(O)=O |r,wU:42.43,28.31,13.12,4.4,66.67,86.87,106.108,115.116,194.201,172.177,149.152,134.136,124.126,120.224,wD:47.48,34.39,20.25,8.8,54.55,62.64,77.78,97.98,202.209,183.189,158.161,145.218,211.220,130.221,214.223,(-22.23,-7.83,;-23.64,-7.23,;-24.87,-8.16,;-26.29,-7.56,;-27.51,-8.49,;-28.93,-7.9,;-29.12,-6.37,;-27.9,-5.44,;-30.54,-5.78,;-31.77,-6.71,;-30.73,-4.25,;-32.15,-3.66,;-33.4,-4.59,;-32.34,-2.13,;-31.06,-1.26,;-31.8,.25,;-33.45,.03,;-33.78,-1.63,;-35.09,-2.39,;-35.19,-3.93,;-36.39,-1.55,;-37.77,-2.23,;-37.83,-3.76,;-39.2,-4.44,;-36.56,-4.61,;-36.28,-.01,;-37.58,.85,;-38.94,.16,;-37.46,2.37,;-36.09,3.05,;-34.83,2.21,;-38.75,3.22,;-38.65,4.76,;-37.27,5.45,;-39.93,5.6,;-41.31,4.92,;-41.42,3.4,;-40.12,2.54,;-42.8,2.71,;-39.84,7.14,;-41.12,8,;-42.5,7.31,;-41.02,9.53,;-39.65,10.21,;-42.31,10.39,;-42.21,11.93,;-40.83,12.61,;-43.49,12.76,;-43.38,14.3,;-44.87,12.08,;-44.96,10.56,;-27.32,-10.02,;-28.56,-10.95,;-25.9,-10.61,;-25.71,-12.14,;-26.93,-13.07,;-24.29,-12.75,;-23.09,-11.81,;-24.1,-14.26,;-25.25,-15.51,;-24.43,-16.97,;-22.76,-16.63,;-22.84,-15.08,;-21.53,-14.32,;-21.53,-12.78,;-20.18,-15.08,;-18.84,-14.32,;-18.84,-12.78,;-17.53,-12.02,;-17.53,-10.47,;-16.2,-9.71,;-16.2,-8.17,;-17.52,-7.41,;-14.86,-7.41,;-17.53,-15.08,;-17.53,-16.63,;-16.18,-14.32,;-14.85,-15.09,;-14.85,-16.63,;-13.54,-17.39,;-13.54,-18.94,;-14.87,-19.72,;-12.18,-19.71,;-13.54,-14.32,;-13.54,-12.78,;-12.19,-15.09,;-10.86,-14.33,;-10.86,-12.79,;-9.51,-12.02,;-9.51,-10.48,;-8.18,-9.72,;-8.18,-8.18,;-9.52,-7.42,;-6.84,-7.41,;-9.51,-15.11,;-9.51,-16.63,;-8.2,-14.33,;-6.85,-15.11,;-6.85,-16.64,;-5.52,-17.42,;-5.52,-18.94,;-4.2,-19.72,;-4.2,-21.27,;-5.52,-14.33,;-5.52,-12.79,;-4.19,-15.11,;-2.85,-14.33,;-2.85,-12.81,;-1.53,-15.11,;-1.53,-16.64,;-.2,-14.34,;1.14,-15.12,;2.46,-14.34,;2.46,-12.82,;3.81,-15.12,;5.13,-14.34,;5.14,-3.92,;6.49,-3.13,;50.46,-3.19,;50.41,-14.4,;49.07,-15.18,;47.74,-14.4,;46.4,-15.18,;46.4,-16.7,;45.07,-14.39,;45.07,-12.87,;46.4,-12.09,;43.75,-15.17,;42.41,-14.39,;42.41,-12.87,;41.08,-15.17,;39.75,-14.39,;38.42,-15.15,;38.42,-16.7,;37.09,-14.39,;37.09,-12.85,;38.42,-12.08,;39.76,-12.87,;41.08,-12.09,;41.09,-10.55,;39.76,-9.78,;38.42,-10.54,;35.75,-15.15,;34.42,-14.38,;34.42,-12.84,;33.1,-15.15,;31.76,-14.38,;30.44,-15.14,;30.44,-16.69,;29.09,-14.38,;29.09,-12.84,;30.44,-12.08,;30.44,-10.54,;31.76,-9.77,;31.76,-8.23,;27.77,-15.14,;26.44,-14.38,;26.44,-12.84,;25.1,-15.14,;25.1,-16.68,;26.44,-17.45,;27.84,-16.82,;28.87,-17.98,;28.09,-19.3,;28.57,-20.76,;27.53,-21.91,;26.03,-21.59,;25.57,-20.12,;26.59,-18.98,;23.78,-14.38,;22.44,-15.14,;22.44,-16.68,;21.11,-14.37,;21.11,-12.83,;22.44,-12.07,;23.78,-12.83,;25.1,-12.07,;25.1,-10.53,;23.78,-9.77,;22.44,-10.53,;19.79,-15.13,;18.44,-14.37,;18.44,-12.83,;17.12,-15.13,;17.12,-16.68,;18.44,-17.44,;19.76,-16.68,;21.1,-17.45,;21.09,-18.99,;19.75,-19.75,;18.44,-18.98,;15.78,-14.37,;14.45,-15.13,;14.45,-16.67,;13.11,-14.37,;13.11,-12.83,;14.45,-12.04,;15.78,-12.83,;14.45,-10.52,;11.79,-15.13,;10.46,-14.36,;10.46,-12.82,;9.12,-15.12,;9.12,-16.67,;10.46,-17.43,;10.46,-18.98,;11.78,-19.74,;11.78,-21.28,;7.79,-14.36,;6.47,-15.12,;6.47,-16.67,;33.1,-16.69,;31.76,-17.46,;34.42,-17.45,;41.08,-16.7,;39.75,-17.48,;42.41,-17.48,;49.07,-16.71,;47.74,-17.49,;50.41,-17.49,)| Show InChI InChI=1S/C137H207N41O39S3/c1-69(154-113(194)82(37-19-22-47-138)159-115(196)85(40-25-50-149-136(145)146)160-118(199)87(44-45-106(188)189)163-116(197)86(41-26-51-150-137(147)148)164-130(211)101-43-27-52-177(101)133(214)72(4)156-114(195)88(46-54-218-7)158-110(191)71(3)155-129(210)100-42-28-53-178(100)134(215)95(61-104(144)186)171-126(207)96(65-180)172-124(205)93(59-102(142)184)165-111(192)70(2)153-112(193)80(141)64-179)109(190)152-63-105(187)157-98-67-219-220-68-99(135(216)217)174-127(208)97(66-181)173-132(213)108(74(6)183)176-125(206)91(57-77-33-15-10-16-34-77)170-131(212)107(73(5)182)175-119(200)84(39-21-24-49-140)161-122(203)92(58-78-62-151-81-36-18-17-35-79(78)81)168-121(202)90(56-76-31-13-9-14-32-76)166-120(201)89(55-75-29-11-8-12-30-75)167-123(204)94(60-103(143)185)169-117(198)83(162-128(98)209)38-20-23-48-139/h8-18,29-36,62,69-74,80,82-101,107-108,151,179-183H,19-28,37-61,63-68,138-141H2,1-7H3,(H2,142,184)(H2,143,185)(H2,144,186)(H,152,190)(H,153,193)(H,154,194)(H,155,210)(H,156,195)(H,157,187)(H,158,191)(H,159,196)(H,160,199)(H,161,203)(H,162,209)(H,163,197)(H,164,211)(H,165,192)(H,166,201)(H,167,204)(H,168,202)(H,169,198)(H,170,212)(H,171,207)(H,172,205)(H,173,213)(H,174,208)(H,175,200)(H,176,206)(H,188,189)(H,216,217)(H4,145,146,149)(H4,147,148,150)/t69-,70-,71-,72-,73+,74+,80-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,107-,108-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
FEBS Lett 321: 279-84 (1993)
Article DOI: 10.1016/0014-5793(93)80124-d
BindingDB Entry DOI: 10.7270/Q2PR7THQ |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50535788
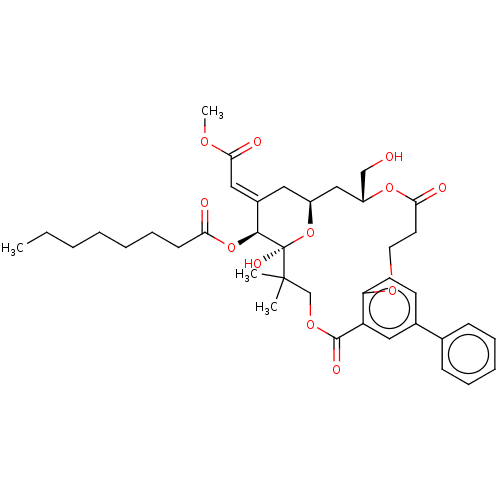
(CHEMBL4564420)Show SMILES CCCCCCCC(=O)O[C@H]1\C(C[C@H]2C[C@H](CO)OC(=O)CCOc3ccc(cc3C(=O)OCC(C)(C)[C@]1(O)O2)-c1ccccc1)=C\C(=O)OC |r| Show InChI InChI=1S/C39H50O12/c1-5-6-7-8-12-15-33(41)50-36-28(22-35(43)46-4)20-29-23-30(24-40)49-34(42)18-19-47-32-17-16-27(26-13-10-9-11-14-26)21-31(32)37(44)48-25-38(2,3)39(36,45)51-29/h9-11,13-14,16-17,21-22,29-30,36,40,45H,5-8,12,15,18-20,23-25H2,1-4H3/b28-22+/t29-,30+,36-,39+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... |
J Nat Prod 79: 680-4 (2016)
Article DOI: 10.1021/acs.jnatprod.5b01017
BindingDB Entry DOI: 10.7270/Q2BK1GXV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50535779
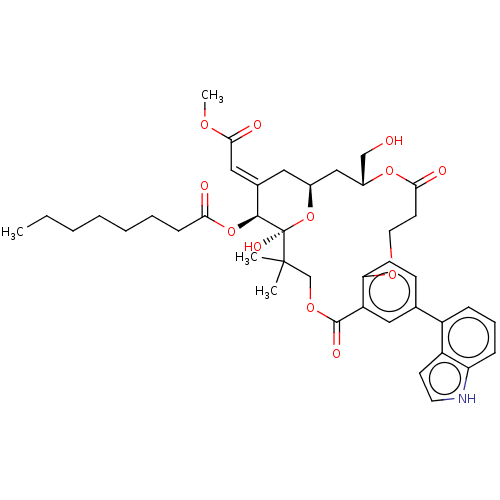
(CHEMBL4519769)Show SMILES CCCCCCCC(=O)O[C@H]1\C(C[C@H]2C[C@H](CO)OC(=O)CCOc3ccc(cc3C(=O)OCC(C)(C)[C@]1(O)O2)-c1cccc2[nH]ccc12)=C\C(=O)OC |r| Show InChI InChI=1S/C41H51NO12/c1-5-6-7-8-9-13-35(44)53-38-27(22-37(46)49-4)20-28-23-29(24-43)52-36(45)17-19-50-34-15-14-26(30-11-10-12-33-31(30)16-18-42-33)21-32(34)39(47)51-25-40(2,3)41(38,48)54-28/h10-12,14-16,18,21-22,28-29,38,42-43,48H,5-9,13,17,19-20,23-25H2,1-4H3/b27-22+/t28-,29+,38-,41+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... |
J Nat Prod 79: 680-4 (2016)
Article DOI: 10.1021/acs.jnatprod.5b01017
BindingDB Entry DOI: 10.7270/Q2BK1GXV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50535771
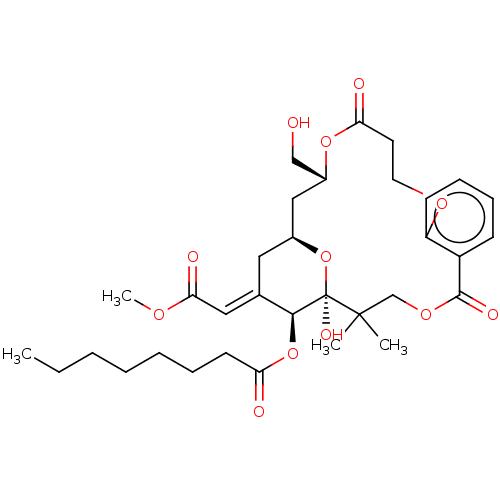
(CHEMBL4541641)Show SMILES CCCCCCCC(=O)O[C@H]1\C(C[C@H]2C[C@H](CO)OC(=O)CCOc3ccccc3C(=O)OCC(C)(C)[C@]1(O)O2)=C\C(=O)OC |r| Show InChI InChI=1S/C33H46O12/c1-5-6-7-8-9-14-27(35)44-30-22(18-29(37)40-4)17-23-19-24(20-34)43-28(36)15-16-41-26-13-11-10-12-25(26)31(38)42-21-32(2,3)33(30,39)45-23/h10-13,18,23-24,30,34,39H,5-9,14-17,19-21H2,1-4H3/b22-18+/t23-,24+,30-,33+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... |
J Nat Prod 79: 680-4 (2016)
Article DOI: 10.1021/acs.jnatprod.5b01017
BindingDB Entry DOI: 10.7270/Q2BK1GXV |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Rattus norvegicus) | BDBM50318112

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES COCCN1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C28H33ClN6O3/c1-18-14-21(35-10-8-34(9-11-35)12-13-38-2)16-23-26(18)33-27(32-23)25-22(6-7-30-28(25)37)31-17-24(36)19-4-3-5-20(29)15-19/h3-7,14-16,24,36H,8-13,17H2,1-2H3,(H,32,33)(H2,30,31,37)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of rat IGF1R |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50535774

(CHEMBL4559327)Show SMILES CCCCCCCC(=O)O[C@H]1\C(C[C@H]2C[C@H](CO)OC(=O)CCOc3ccc(Br)cc3C(=O)OCC(C)(C)[C@]1(O)O2)=C\C(=O)OC |r| Show InChI InChI=1S/C33H45BrO12/c1-5-6-7-8-9-10-27(36)45-30-21(16-29(38)41-4)15-23-18-24(19-35)44-28(37)13-14-42-26-12-11-22(34)17-25(26)31(39)43-20-32(2,3)33(30,40)46-23/h11-12,16-17,23-24,30,35,40H,5-10,13-15,18-20H2,1-4H3/b21-16+/t23-,24+,30-,33+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... |
J Nat Prod 79: 680-4 (2016)
Article DOI: 10.1021/acs.jnatprod.5b01017
BindingDB Entry DOI: 10.7270/Q2BK1GXV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50535787
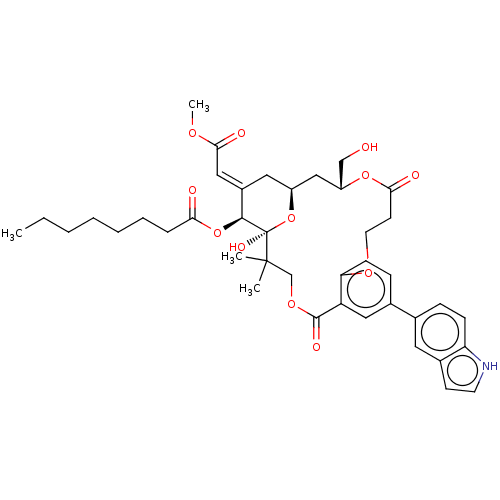
(CHEMBL4550478)Show SMILES CCCCCCCC(=O)O[C@H]1\C(C[C@H]2C[C@H](CO)OC(=O)CCOc3ccc(cc3C(=O)OCC(C)(C)[C@]1(O)O2)-c1ccc2[nH]ccc2c1)=C\C(=O)OC |r| Show InChI InChI=1S/C41H51NO12/c1-5-6-7-8-9-10-35(44)53-38-29(22-37(46)49-4)20-30-23-31(24-43)52-36(45)16-18-50-34-14-12-27(26-11-13-33-28(19-26)15-17-42-33)21-32(34)39(47)51-25-40(2,3)41(38,48)54-30/h11-15,17,19,21-22,30-31,38,42-43,48H,5-10,16,18,20,23-25H2,1-4H3/b29-22+/t30-,31+,38-,41+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... |
J Nat Prod 79: 680-4 (2016)
Article DOI: 10.1021/acs.jnatprod.5b01017
BindingDB Entry DOI: 10.7270/Q2BK1GXV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50535773
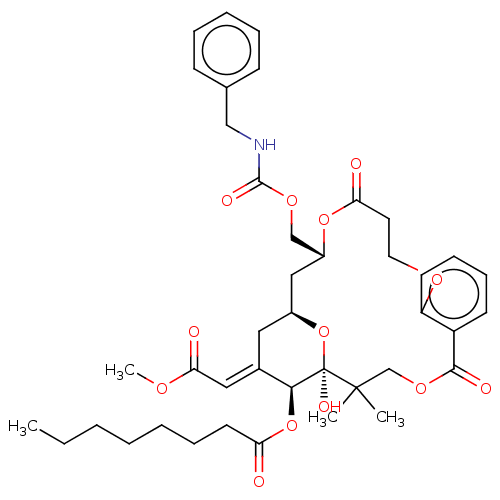
(CHEMBL4575057)Show SMILES CCCCCCCC(=O)O[C@H]1\C(C[C@H]2C[C@H](COC(=O)NCc3ccccc3)OC(=O)CCOc3ccccc3C(=O)OCC(C)(C)[C@]1(O)O2)=C\C(=O)OC |r| Show InChI InChI=1S/C41H53NO13/c1-5-6-7-8-12-19-34(43)54-37-29(23-36(45)49-4)22-30-24-31(26-51-39(47)42-25-28-15-10-9-11-16-28)53-35(44)20-21-50-33-18-14-13-17-32(33)38(46)52-27-40(2,3)41(37,48)55-30/h9-11,13-18,23,30-31,37,48H,5-8,12,19-22,24-27H2,1-4H3,(H,42,47)/b29-23+/t30-,31+,37-,41+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... |
J Nat Prod 79: 680-4 (2016)
Article DOI: 10.1021/acs.jnatprod.5b01017
BindingDB Entry DOI: 10.7270/Q2BK1GXV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50535784

(CHEMBL4212128)Show SMILES [H][C@@]12C\C(=C/C(=O)OC)[C@H](OC(=O)CCCCCCC)[C@@](O)(O1)C(C)(C)COC(=O)c1ccccc1OCCC(=O)O[C@@H](COC)C2 |r| Show InChI InChI=1S/C34H48O12/c1-6-7-8-9-10-15-28(35)45-31-23(19-30(37)41-5)18-24-20-25(21-40-4)44-29(36)16-17-42-27-14-12-11-13-26(27)32(38)43-22-33(2,3)34(31,39)46-24/h11-14,19,24-25,31,39H,6-10,15-18,20-22H2,1-5H3/b23-19+/t24-,25+,31-,34+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... |
J Nat Prod 79: 680-4 (2016)
Article DOI: 10.1021/acs.jnatprod.5b01017
BindingDB Entry DOI: 10.7270/Q2BK1GXV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50535783

(CHEMBL4482943)Show SMILES CCCCCCCC(=O)O[C@H]1\C(C[C@H]2C[C@H](COC(C)=O)OC(=O)CCOc3ccccc3C(=O)OCC(C)(C)[C@]1(O)O2)=C\C(=O)OC |r| Show InChI InChI=1S/C35H48O13/c1-6-7-8-9-10-15-29(37)47-32-24(19-31(39)42-5)18-25-20-26(21-44-23(2)36)46-30(38)16-17-43-28-14-12-11-13-27(28)33(40)45-22-34(3,4)35(32,41)48-25/h11-14,19,25-26,32,41H,6-10,15-18,20-22H2,1-5H3/b24-19+/t25-,26+,32-,35+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... |
J Nat Prod 79: 680-4 (2016)
Article DOI: 10.1021/acs.jnatprod.5b01017
BindingDB Entry DOI: 10.7270/Q2BK1GXV |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50458169

(Avagacestat | BMS 708163 | BMS-708163 | BMS-708163...)Show SMILES NC(=O)[C@@H](CCC(F)(F)F)N(Cc1ccc(cc1F)-c1ncon1)S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C20H17ClF4N4O4S/c21-14-3-5-15(6-4-14)34(31,32)29(17(18(26)30)7-8-20(23,24)25)10-13-2-1-12(9-16(13)22)19-27-11-33-28-19/h1-6,9,11,17H,7-8,10H2,(H2,26,30)/t17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISA |
Bioorg Med Chem Lett 23: 996-1000 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.039
BindingDB Entry DOI: 10.7270/Q2Q81H1X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Presenilin-1
(Homo sapiens (Human)) | BDBM50492082

(CHEMBL2396959)Show SMILES [H][C@]12Cc3n[nH]cc3[C@]([H])(N1S(=O)(=O)c1cccc(c1)C(F)(F)F)c1c2cccc1Cl |r,THB:11:10:25.24:2.7.3| Show InChI InChI=1S/C19H13ClF3N3O2S/c20-14-6-2-5-12-16-8-15-13(9-24-25-15)18(17(12)14)26(16)29(27,28)11-4-1-3-10(7-11)19(21,22)23/h1-7,9,16,18H,8H2,(H,24,25)/t16-,18+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISA |
Bioorg Med Chem Lett 23: 996-1000 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.039
BindingDB Entry DOI: 10.7270/Q2Q81H1X |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50492094
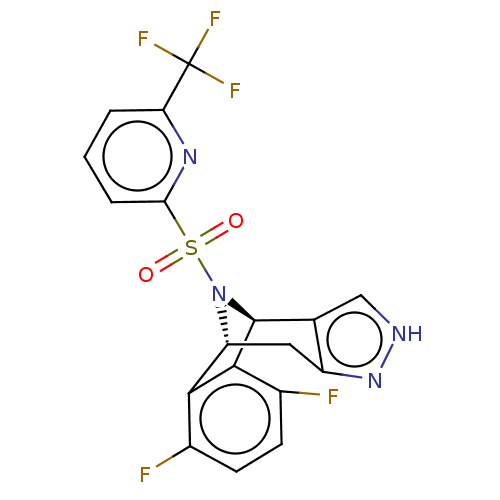
(CHEMBL2396964)Show SMILES [H][C@]12Cc3n[nH]cc3[C@]([H])(N1S(=O)(=O)c1cccc(n1)C(F)(F)F)c1c2c(F)ccc1F |r,THB:11:10:25.24:2.7.3| Show InChI InChI=1S/C18H11F5N4O2S/c19-9-4-5-10(20)16-15(9)12-6-11-8(7-24-26-11)17(16)27(12)30(28,29)14-3-1-2-13(25-14)18(21,22)23/h1-5,7,12,17H,6H2,(H,24,26)/t12-,17+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISA |
Bioorg Med Chem Lett 23: 996-1000 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.039
BindingDB Entry DOI: 10.7270/Q2Q81H1X |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50492093
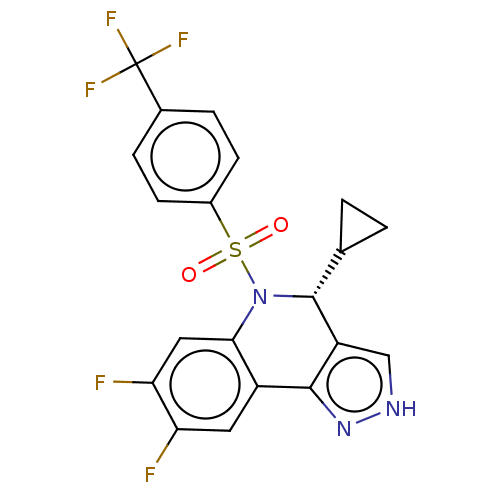
(CHEMBL2396778)Show SMILES Fc1cc2N([C@H](C3CC3)c3c[nH]nc3-c2cc1F)S(=O)(=O)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C20H14F5N3O2S/c21-15-7-13-17(8-16(15)22)28(19(10-1-2-10)14-9-26-27-18(13)14)31(29,30)12-5-3-11(4-6-12)20(23,24)25/h3-10,19H,1-2H2,(H,26,27)/t19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISA |
Bioorg Med Chem Lett 23: 996-1000 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.039
BindingDB Entry DOI: 10.7270/Q2Q81H1X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252094
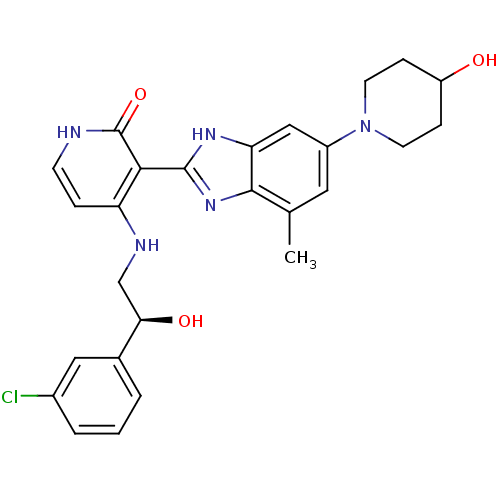
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(O)CC1 |r| Show InChI InChI=1S/C26H28ClN5O3/c1-15-11-18(32-9-6-19(33)7-10-32)13-21-24(15)31-25(30-21)23-20(5-8-28-26(23)35)29-14-22(34)16-3-2-4-17(27)12-16/h2-5,8,11-13,19,22,33-34H,6-7,9-10,14H2,1H3,(H,30,31)(H2,28,29,35)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50492085
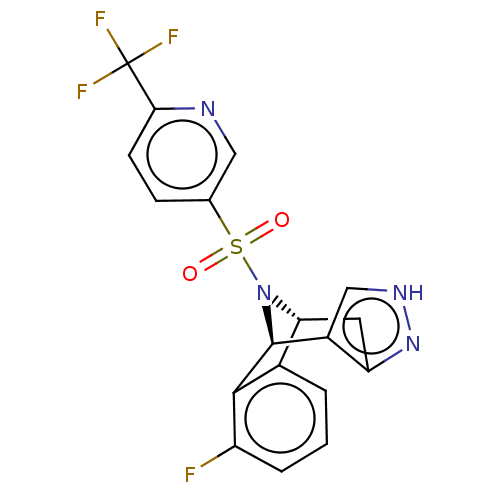
(CHEMBL2396953)Show SMILES [H][C@]12Cc3n[nH]cc3[C@]([H])(N1S(=O)(=O)c1ccc(nc1)C(F)(F)F)c1c2cccc1F |r,THB:11:10:25.24:2.7.3| Show InChI InChI=1S/C18H12F4N4O2S/c19-12-3-1-2-10-14-6-13-11(8-24-25-13)17(16(10)12)26(14)29(27,28)9-4-5-15(23-7-9)18(20,21)22/h1-5,7-8,14,17H,6H2,(H,24,25)/t14-,17+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISA |
Bioorg Med Chem Lett 23: 996-1000 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.039
BindingDB Entry DOI: 10.7270/Q2Q81H1X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252237

((S)-methyl 1-(2-(4-(2-(3-chlorophenyl)-2-hydroxyet...)Show SMILES COC(=O)NC1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C28H31ClN6O4/c1-16-12-20(35-10-7-19(8-11-35)32-28(38)39-2)14-22-25(16)34-26(33-22)24-21(6-9-30-27(24)37)31-15-23(36)17-4-3-5-18(29)13-17/h3-6,9,12-14,19,23,36H,7-8,10-11,15H2,1-2H3,(H,32,38)(H,33,34)(H2,30,31,37)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM27879

(4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCOCC1 |r| Show InChI InChI=1S/C25H26ClN5O3/c1-15-11-18(31-7-9-34-10-8-31)13-20-23(15)30-24(29-20)22-19(5-6-27-25(22)33)28-14-21(32)16-3-2-4-17(26)12-16/h2-6,11-13,21,32H,7-10,14H2,1H3,(H,29,30)(H2,27,28,33)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252295

(3-(6-(4-((1R,4S)-5-oxa-2-aza-bicyclo[2.2.1]heptan-...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(CC1)N1C[C@@H]2C[C@@H]1CO2 |r,THB:31:34:39.40:37| Show InChI InChI=1S/C31H35ClN6O3/c1-18-11-22(37-9-6-21(7-10-37)38-16-24-13-23(38)17-41-24)14-26-29(18)36-30(35-26)28-25(5-8-33-31(28)40)34-15-27(39)19-3-2-4-20(32)12-19/h2-5,8,11-12,14,21,23-24,27,39H,6-7,9-10,13,15-17H2,1H3,(H,35,36)(H2,33,34,40)/t23-,24+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50492083

(CHEMBL2396960)Show SMILES [H][C@]12Cc3n[nH]cc3[C@]([H])(N1S(=O)(=O)c1cccc(c1)C(F)(F)F)c1cc(Cl)ccc21 |r,THB:11:10:30.24:2.7.3| Show InChI InChI=1S/C19H13ClF3N3O2S/c20-11-4-5-13-14(7-11)18-15-9-24-25-16(15)8-17(13)26(18)29(27,28)12-3-1-2-10(6-12)19(21,22)23/h1-7,9,17-18H,8H2,(H,24,25)/t17-,18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISA |
Bioorg Med Chem Lett 23: 996-1000 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.039
BindingDB Entry DOI: 10.7270/Q2Q81H1X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252193
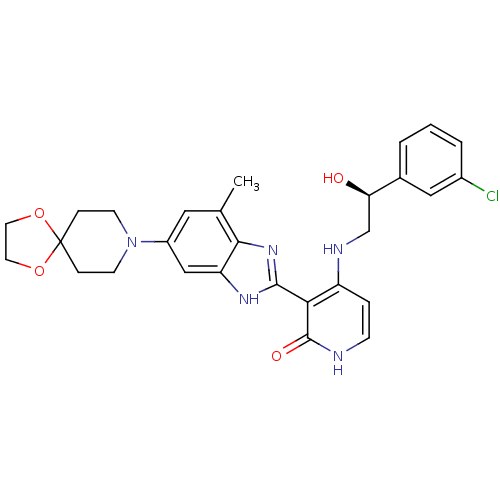
(4-((S)-2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC2(CC1)OCCO2 |r| Show InChI InChI=1S/C28H30ClN5O4/c1-17-13-20(34-9-6-28(7-10-34)37-11-12-38-28)15-22-25(17)33-26(32-22)24-21(5-8-30-27(24)36)31-16-23(35)18-3-2-4-19(29)14-18/h2-5,8,13-15,23,35H,6-7,9-12,16H2,1H3,(H,32,33)(H2,30,31,36)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252236
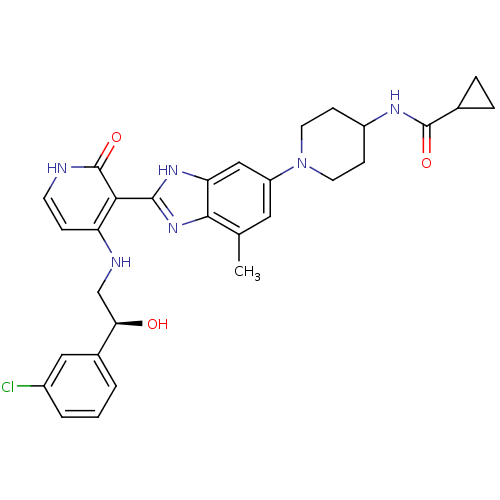
((S)-N-(1-(2-(4-(2-(3-chlorophenyl)-2-hydroxyethyla...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(CC1)NC(=O)C1CC1 |r| Show InChI InChI=1S/C30H33ClN6O3/c1-17-13-22(37-11-8-21(9-12-37)34-29(39)18-5-6-18)15-24-27(17)36-28(35-24)26-23(7-10-32-30(26)40)33-16-25(38)19-3-2-4-20(31)14-19/h2-4,7,10,13-15,18,21,25,38H,5-6,8-9,11-12,16H2,1H3,(H,34,39)(H,35,36)(H2,32,33,40)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252297
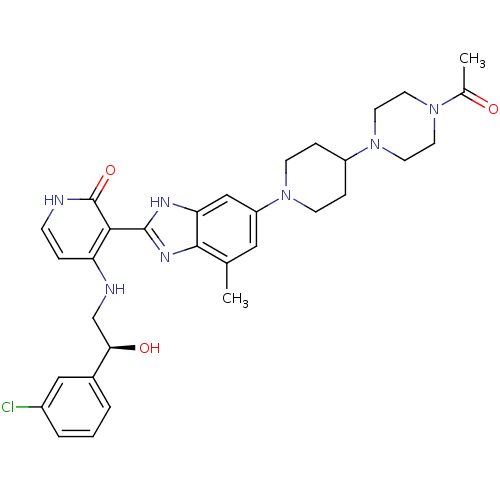
((S)-3-(6-(4-(4-acetylpiperazin-1-yl)piperidin-1-yl...)Show SMILES CC(=O)N1CCN(CC1)C1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C32H38ClN7O3/c1-20-16-25(39-10-7-24(8-11-39)40-14-12-38(13-15-40)21(2)41)18-27-30(20)37-31(36-27)29-26(6-9-34-32(29)43)35-19-28(42)22-4-3-5-23(33)17-22/h3-6,9,16-18,24,28,42H,7-8,10-15,19H2,1-2H3,(H,36,37)(H2,34,35,43)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252143
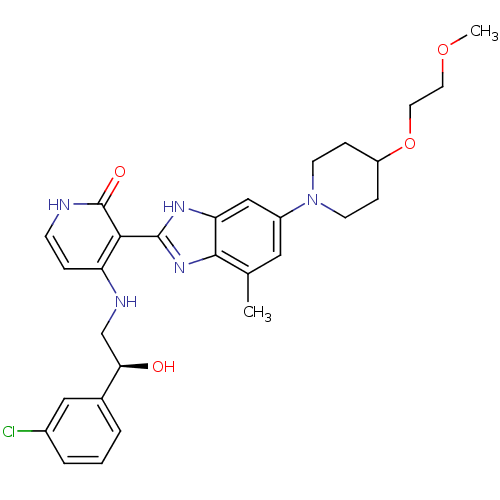
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES COCCOC1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C29H34ClN5O4/c1-18-14-21(35-10-7-22(8-11-35)39-13-12-38-2)16-24-27(18)34-28(33-24)26-23(6-9-31-29(26)37)32-17-25(36)19-4-3-5-20(30)15-19/h3-6,9,14-16,22,25,36H,7-8,10-13,17H2,1-2H3,(H,33,34)(H2,31,32,37)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107013

(US8592579, 211)Show SMILES O[C@H]1C[C@H](N(C1)c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1cccnc1 |r| Show InChI InChI=1S/C22H23N9O2/c32-15-9-18(21(33)24-14-3-1-7-23-11-14)30(12-15)22-26-20(17-4-2-8-31(17)29-22)25-19-10-16(27-28-19)13-5-6-13/h1-4,7-8,10-11,13,15,18,32H,5-6,9,12H2,(H,24,33)(H2,25,26,27,28,29)/t15-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM144614
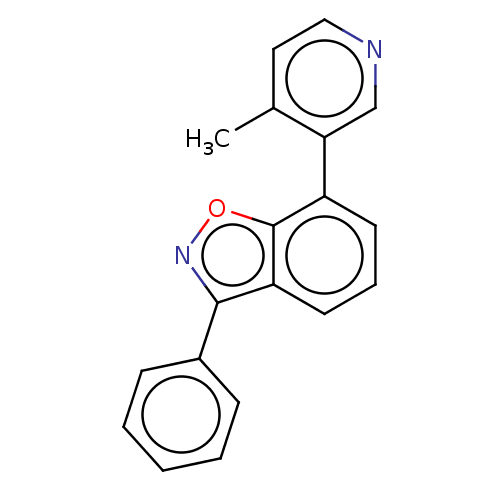
(US8969586, 1 | US9598436, 1)Show InChI InChI=1S/C19H14N2O/c1-13-10-11-20-12-17(13)15-8-5-9-16-18(21-22-19(15)16)14-6-3-2-4-7-14/h2-12H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against Melatonin receptor using ovine pars tuberalis membranes of the pituitary. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107018

(US8592579, 254)Show SMILES CO[C@H]1C[C@H](N(C1)c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1ccc(F)nc1 |r| Show InChI InChI=1S/C23H24FN9O2/c1-35-15-9-18(22(34)26-14-6-7-19(24)25-11-14)32(12-15)23-28-21(17-3-2-8-33(17)31-23)27-20-10-16(29-30-20)13-4-5-13/h2-3,6-8,10-11,13,15,18H,4-5,9,12H2,1H3,(H,26,34)(H2,27,28,29,30,31)/t15-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107023

(US8592579, 288)Show SMILES CC1(CC1)c1cc(Nc2nc(nn3cccc23)N2C[C@@H](O)C[C@H]2C(=O)Nc2ncns2)[nH]n1 |r| Show InChI InChI=1S/C20H22N10O2S/c1-20(4-5-20)14-8-15(27-26-14)23-16-12-3-2-6-30(12)28-18(24-16)29-9-11(31)7-13(29)17(32)25-19-21-10-22-33-19/h2-3,6,8,10-11,13,31H,4-5,7,9H2,1H3,(H,21,22,25,32)(H2,23,24,26,27,28)/t11-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107016
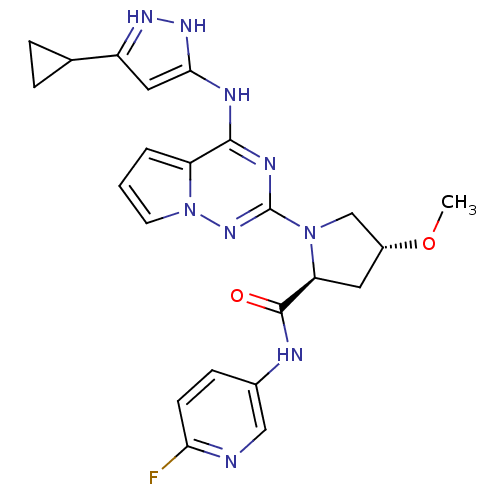
(US8592579, 219)Show SMILES CO[C@@H]1C[C@H](N(C1)c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1ccc(F)nc1 |r| Show InChI InChI=1S/C23H24FN9O2/c1-35-15-9-18(22(34)26-14-6-7-19(24)25-11-14)32(12-15)23-28-21(17-3-2-8-33(17)31-23)27-20-10-16(29-30-20)13-4-5-13/h2-3,6-8,10-11,13,15,18H,4-5,9,12H2,1H3,(H,26,34)(H2,27,28,29,30,31)/t15-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252142

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES COC1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C27H30ClN5O3/c1-16-12-19(33-10-7-20(36-2)8-11-33)14-22-25(16)32-26(31-22)24-21(6-9-29-27(24)35)30-15-23(34)17-4-3-5-18(28)13-17/h3-6,9,12-14,20,23,34H,7-8,10-11,15H2,1-2H3,(H,31,32)(H2,29,30,35)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252238

((S)-methyl 1-(2-(4-(2-(3-chlorophenyl)-2-hydroxyet...)Show SMILES COCCN(CCOC)C1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C32H41ClN6O4/c1-21-17-25(38-11-8-24(9-12-38)39(13-15-42-2)14-16-43-3)19-27-30(21)37-31(36-27)29-26(7-10-34-32(29)41)35-20-28(40)22-5-4-6-23(33)18-22/h4-7,10,17-19,24,28,40H,8-9,11-16,20H2,1-3H3,(H,36,37)(H2,34,35,41)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50492098
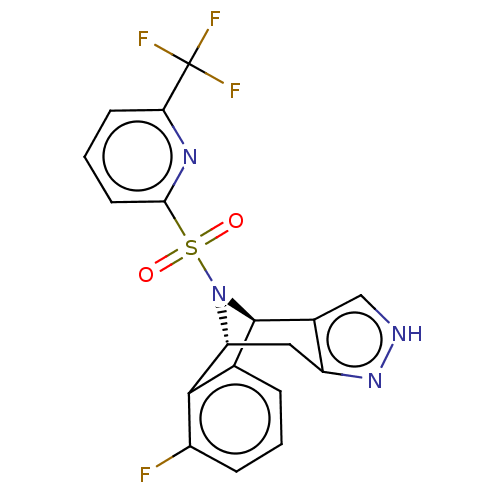
(CHEMBL2396963)Show SMILES [H][C@]12Cc3n[nH]cc3[C@]([H])(N1S(=O)(=O)c1cccc(n1)C(F)(F)F)c1cccc(F)c21 |r,THB:11:10:30.24:2.7.3| Show InChI InChI=1S/C18H12F4N4O2S/c19-11-4-1-3-9-16(11)13-7-12-10(8-23-25-12)17(9)26(13)29(27,28)15-6-2-5-14(24-15)18(20,21)22/h1-6,8,13,17H,7H2,(H,23,25)/t13-,17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISA |
Bioorg Med Chem Lett 23: 996-1000 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.039
BindingDB Entry DOI: 10.7270/Q2Q81H1X |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50492097
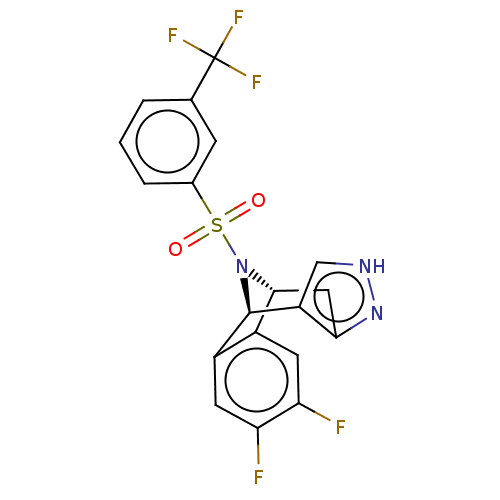
(CHEMBL2396965)Show SMILES [H][C@]12Cc3n[nH]cc3[C@]([H])(N1S(=O)(=O)c1cccc(c1)C(F)(F)F)c1cc(F)c(F)cc21 |r,THB:11:10:31.24:2.7.3| Show InChI InChI=1S/C19H12F5N3O2S/c20-14-5-11-12(6-15(14)21)18-13-8-25-26-16(13)7-17(11)27(18)30(28,29)10-3-1-2-9(4-10)19(22,23)24/h1-6,8,17-18H,7H2,(H,25,26)/t17-,18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISA |
Bioorg Med Chem Lett 23: 996-1000 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.039
BindingDB Entry DOI: 10.7270/Q2Q81H1X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252144

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(CC1)OCCO |r| Show InChI InChI=1S/C28H32ClN5O4/c1-17-13-20(34-9-6-21(7-10-34)38-12-11-35)15-23-26(17)33-27(32-23)25-22(5-8-30-28(25)37)31-16-24(36)18-3-2-4-19(29)14-18/h2-5,8,13-15,21,24,35-36H,6-7,9-12,16H2,1H3,(H,32,33)(H2,30,31,37)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data