 Found 315 hits with Last Name = 'ngo' and Initial = 'k'
Found 315 hits with Last Name = 'ngo' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000041

((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...)Show InChI InChI=1S/C19H24N2O/c1-22-18-12-6-5-10-16(18)14-21-17-11-7-13-20-19(17)15-8-3-2-4-9-15/h2-6,8-10,12,17,19-21H,7,11,13-14H2,1H3/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity for the Tachykinin receptor 1 in human IM-9 cell using [125I]-BH-SP of the compound. |
J Med Chem 35: 4911-3 (1993)
BindingDB Entry DOI: 10.7270/Q2GF0SG7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000041

((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...)Show InChI InChI=1S/C19H24N2O/c1-22-18-12-6-5-10-16(18)14-21-17-11-7-13-20-19(17)15-8-3-2-4-9-15/h2-6,8-10,12,17,19-21H,7,11,13-14H2,1H3/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity for the Tachykinin receptor 1 in human IM-9 cell using [125I]-BH-SP of the compound. |
J Med Chem 35: 4911-3 (1993)
BindingDB Entry DOI: 10.7270/Q2GF0SG7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000040

(((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1ccccc1CN[C@H]1C2CCN(CC2)[C@H]1C(c1ccccc1)c1ccccc1 |wD:10.10,17.20,(11.79,-2.71,;10.31,-3.13,;9.22,-2.04,;10.55,-1.27,;10.57,.27,;9.22,1.04,;7.89,.27,;7.89,-1.26,;6.56,-2.01,;6.56,-3.55,;5.21,-4.32,;3.92,-3.55,;3.16,-4.88,;4.65,-5.3,;3.88,-6.63,;2.57,-5.86,;2.57,-4.32,;5.21,-5.86,;6.56,-6.63,;6.54,-8.17,;5.21,-8.94,;5.21,-10.48,;6.54,-11.25,;7.89,-10.48,;7.87,-8.94,;7.89,-5.86,;9.22,-6.63,;10.55,-5.88,;10.55,-4.34,;9.22,-3.55,;7.89,-4.34,)| Show InChI InChI=1S/C28H32N2O/c1-31-25-15-9-8-14-24(25)20-29-27-23-16-18-30(19-17-23)28(27)26(21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,23,26-29H,16-20H2,1H3/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity for the Tachykinin receptor 1 in human IM-9 cell using [125I]-BH-SP of the compound. |
J Med Chem 35: 4911-3 (1993)
BindingDB Entry DOI: 10.7270/Q2GF0SG7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000040

(((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1ccccc1CN[C@H]1C2CCN(CC2)[C@H]1C(c1ccccc1)c1ccccc1 |wD:10.10,17.20,(11.79,-2.71,;10.31,-3.13,;9.22,-2.04,;10.55,-1.27,;10.57,.27,;9.22,1.04,;7.89,.27,;7.89,-1.26,;6.56,-2.01,;6.56,-3.55,;5.21,-4.32,;3.92,-3.55,;3.16,-4.88,;4.65,-5.3,;3.88,-6.63,;2.57,-5.86,;2.57,-4.32,;5.21,-5.86,;6.56,-6.63,;6.54,-8.17,;5.21,-8.94,;5.21,-10.48,;6.54,-11.25,;7.89,-10.48,;7.87,-8.94,;7.89,-5.86,;9.22,-6.63,;10.55,-5.88,;10.55,-4.34,;9.22,-3.55,;7.89,-4.34,)| Show InChI InChI=1S/C28H32N2O/c1-31-25-15-9-8-14-24(25)20-29-27-23-16-18-30(19-17-23)28(27)26(21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,23,26-29H,16-20H2,1H3/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity for the Tachykinin receptor 1 in human IM-9 cell using [125I]-BH-SP of the compound. |
J Med Chem 35: 4911-3 (1993)
BindingDB Entry DOI: 10.7270/Q2GF0SG7 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50511169

(CHEMBL4551377)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1cc(N)nc(N)c1 |r| Show InChI InChI=1S/C20H26N6O2.2C2HF3O2/c21-15(9-13-5-2-1-3-6-13)20(28)26-8-4-7-16(26)19(27)24-12-14-10-17(22)25-18(23)11-14;2*3-2(4,5)1(6)7/h1-3,5-6,10-11,15-16H,4,7-9,12,21H2,(H,24,27)(H4,22,23,25);2*(H,6,7)/t15-,16+;;/m1../s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin using varying levels of Tos-Gly-Pro-Arg-AMC-TFA as substrate by fluorescence based Michaelis-Menten equation analy... |
J Med Chem 63: 3274-3289 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02061
BindingDB Entry DOI: 10.7270/Q2WM1HRB |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000042
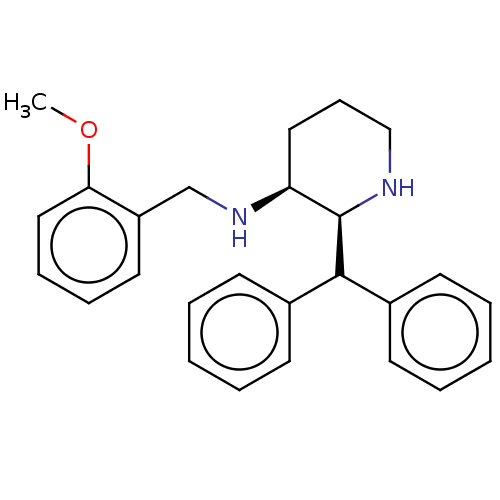
((2-Benzhydryl-piperidin-3-yl)-(2-methoxy-benzyl)-a...)Show SMILES COc1ccccc1CN[C@H]1CCCN[C@H]1C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C26H30N2O/c1-29-24-17-9-8-15-22(24)19-28-23-16-10-18-27-26(23)25(20-11-4-2-5-12-20)21-13-6-3-7-14-21/h2-9,11-15,17,23,25-28H,10,16,18-19H2,1H3/t23-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity for the Tachykinin receptor 1 in human IM-9 cell using [125I]-BH-SP of the compound. |
J Med Chem 35: 4911-3 (1993)
BindingDB Entry DOI: 10.7270/Q2GF0SG7 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50511166

(CHEMBL4513436)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1ccc(N)nc1 |r| Show InChI InChI=1S/C20H25N5O2.2C2HF3O2/c21-16(11-14-5-2-1-3-6-14)20(27)25-10-4-7-17(25)19(26)24-13-15-8-9-18(22)23-12-15;2*3-2(4,5)1(6)7/h1-3,5-6,8-9,12,16-17H,4,7,10-11,13,21H2,(H2,22,23)(H,24,26);2*(H,6,7)/t16-,17+;;/m1../s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin using varying levels of Tos-Gly-Pro-Arg-AMC-TFA as substrate by fluorescence based Michaelis-Menten equation analy... |
J Med Chem 63: 3274-3289 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02061
BindingDB Entry DOI: 10.7270/Q2WM1HRB |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50511167

(CHEMBL4563187)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1ccnc(N)c1 |r| Show InChI InChI=1S/C20H25N5O2.2C2HF3O2/c21-16(11-14-5-2-1-3-6-14)20(27)25-10-4-7-17(25)19(26)24-13-15-8-9-23-18(22)12-15;2*3-2(4,5)1(6)7/h1-3,5-6,8-9,12,16-17H,4,7,10-11,13,21H2,(H2,22,23)(H,24,26);2*(H,6,7)/t16-,17+;;/m1../s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 379 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin using varying levels of Tos-Gly-Pro-Arg-AMC-TFA as substrate by fluorescence based Michaelis-Menten equation analy... |
J Med Chem 63: 3274-3289 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02061
BindingDB Entry DOI: 10.7270/Q2WM1HRB |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50511168

(CHEMBL4465587)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@H](C(c1ccccc1)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1ccncc1 |r| Show InChI InChI=1S/C26H28N4O2.2C2HF3O2/c27-24(23(20-8-3-1-4-9-20)21-10-5-2-6-11-21)26(32)30-17-7-12-22(30)25(31)29-18-19-13-15-28-16-14-19;2*3-2(4,5)1(6)7/h1-6,8-11,13-16,22-24H,7,12,17-18,27H2,(H,29,31);2*(H,6,7)/t22-,24+;;/m0../s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin using varying levels of Tos-Gly-Pro-Arg-AMC-TFA as substrate by fluorescence based Michaelis-Menten equation analy... |
J Med Chem 63: 3274-3289 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02061
BindingDB Entry DOI: 10.7270/Q2WM1HRB |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000041

((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...)Show InChI InChI=1S/C19H24N2O/c1-22-18-12-6-5-10-16(18)14-21-17-11-7-13-20-19(17)15-8-3-2-4-9-15/h2-6,8-10,12,17,19-21H,7,11,13-14H2,1H3/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity for the Tachykinin receptor 1 in human IM-9 cell using [125I]BH-SP of the compound. |
J Med Chem 35: 4911-3 (1993)
BindingDB Entry DOI: 10.7270/Q2GF0SG7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50272889

(2-(isopropoxymethyl)-N-(4-(trifluoromethyl)phenyl)...)Show SMILES CC(C)OCc1nc(Nc2ccc(cc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C25H20F6N4O/c1-14(2)36-13-21-34-20-12-15(22-19(25(29,30)31)4-3-11-32-22)5-10-18(20)23(35-21)33-17-8-6-16(7-9-17)24(26,27)28/h3-12,14H,13H2,1-2H3,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50272976

(2-((2,6-dimethylmorpholino)methyl)-N-(4-(trifluoro...)Show SMILES CC1CN(Cc2nc(Nc3ccc(cc3)C(F)(F)F)c3ccc(cc3n2)-c2ncccc2C(F)(F)F)CC(C)O1 Show InChI InChI=1S/C28H25F6N5O/c1-16-13-39(14-17(2)40-16)15-24-37-23-12-18(25-22(28(32,33)34)4-3-11-35-25)5-10-21(23)26(38-24)36-20-8-6-19(7-9-20)27(29,30)31/h3-12,16-17H,13-15H2,1-2H3,(H,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50030232
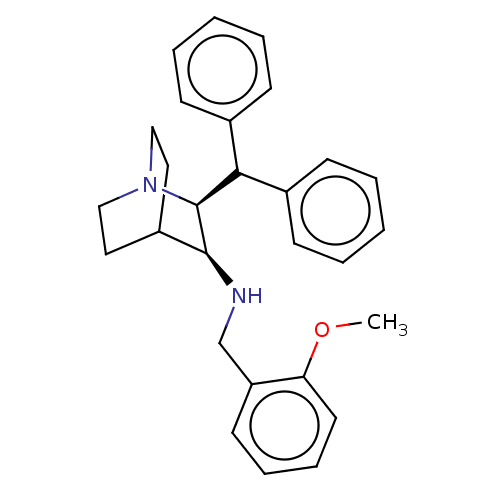
((S)-((S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1ccccc1CNC1C2CCN(CC2)[C@H]1C(c1ccccc1)c1ccccc1 |wD:17.20,(7.71,-5.53,;6.37,-4.76,;6.37,-3.22,;7.71,-2.45,;7.71,-.89,;6.37,-.12,;5.04,-.91,;5.04,-2.45,;3.69,-3.22,;3.69,-4.76,;2.36,-5.53,;1.03,-4.74,;-.3,-5.51,;-.3,-7.05,;1.07,-7.8,;.31,-6.47,;1.8,-6.07,;2.36,-7.07,;3.71,-7.82,;3.69,-9.38,;2.36,-10.13,;2.36,-11.67,;3.69,-12.44,;5.04,-11.67,;5.02,-10.13,;5.19,-7.43,;6.28,-8.52,;7.77,-8.1,;8.15,-6.63,;7.05,-5.54,;5.58,-5.95,)| Show InChI InChI=1S/C28H32N2O/c1-31-25-15-9-8-14-24(25)20-29-27-23-16-18-30(19-17-23)28(27)26(21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,23,26-29H,16-20H2,1H3/t27?,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc.
Curated by ChEMBL
| Assay Description
Tested in vitro for the binding affinity towards NK1 receptor in human IM-9 cells using [125I]-labeled bolton-hunter substance P as ligand |
J Med Chem 37: 2831-40 (1994)
BindingDB Entry DOI: 10.7270/Q2CZ365J |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50272850

(2-(methoxymethyl)-N-(4-(trifluoromethyl)phenyl)-7-...)Show SMILES COCc1nc(Nc2ccc(cc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C23H16F6N4O/c1-34-12-19-32-18-11-13(20-17(23(27,28)29)3-2-10-30-20)4-9-16(18)21(33-19)31-15-7-5-14(6-8-15)22(24,25)26/h2-11H,12H2,1H3,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV1 receptor assessed as inhibition of low pH-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50272848
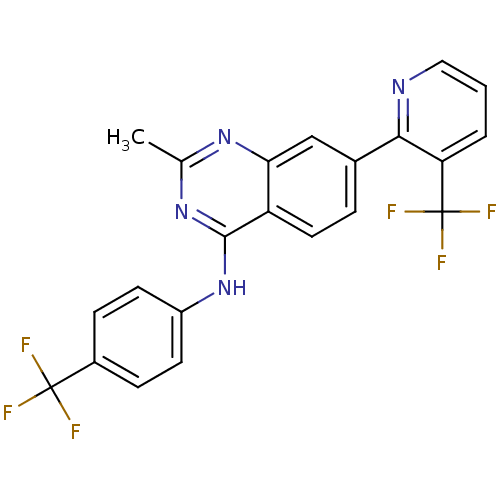
(2-methyl-N-(4-(trifluoromethyl)phenyl)-7-(3-(trifl...)Show SMILES Cc1nc(Nc2ccc(cc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C22H14F6N4/c1-12-30-18-11-13(19-17(22(26,27)28)3-2-10-29-19)4-9-16(18)20(31-12)32-15-7-5-14(6-8-15)21(23,24)25/h2-11H,1H3,(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50272890
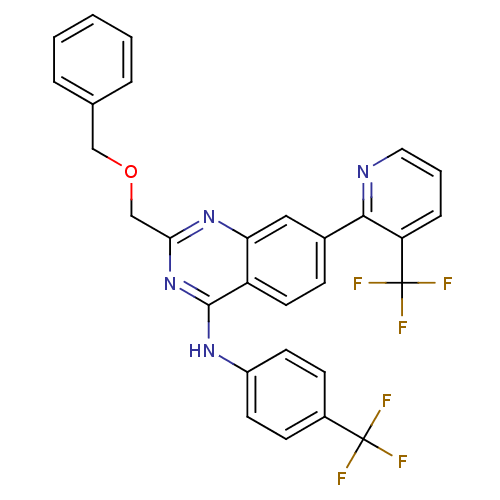
(2-(benzyloxymethyl)-N-(4-(trifluoromethyl)phenyl)-...)Show SMILES FC(F)(F)c1ccc(Nc2nc(COCc3ccccc3)nc3cc(ccc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C29H20F6N4O/c30-28(31,32)20-9-11-21(12-10-20)37-27-22-13-8-19(26-23(29(33,34)35)7-4-14-36-26)15-24(22)38-25(39-27)17-40-16-18-5-2-1-3-6-18/h1-15H,16-17H2,(H,37,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000040

(((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1ccccc1CN[C@H]1C2CCN(CC2)[C@H]1C(c1ccccc1)c1ccccc1 |wD:10.10,17.20,(11.79,-2.71,;10.31,-3.13,;9.22,-2.04,;10.55,-1.27,;10.57,.27,;9.22,1.04,;7.89,.27,;7.89,-1.26,;6.56,-2.01,;6.56,-3.55,;5.21,-4.32,;3.92,-3.55,;3.16,-4.88,;4.65,-5.3,;3.88,-6.63,;2.57,-5.86,;2.57,-4.32,;5.21,-5.86,;6.56,-6.63,;6.54,-8.17,;5.21,-8.94,;5.21,-10.48,;6.54,-11.25,;7.89,-10.48,;7.87,-8.94,;7.89,-5.86,;9.22,-6.63,;10.55,-5.88,;10.55,-4.34,;9.22,-3.55,;7.89,-4.34,)| Show InChI InChI=1S/C28H32N2O/c1-31-25-15-9-8-14-24(25)20-29-27-23-16-18-30(19-17-23)28(27)26(21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,23,26-29H,16-20H2,1H3/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards Tachykinin receptor 1 in human IM-9 cells using [3H]-substance P as ligand |
J Med Chem 35: 2591-600 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1JJF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50191726
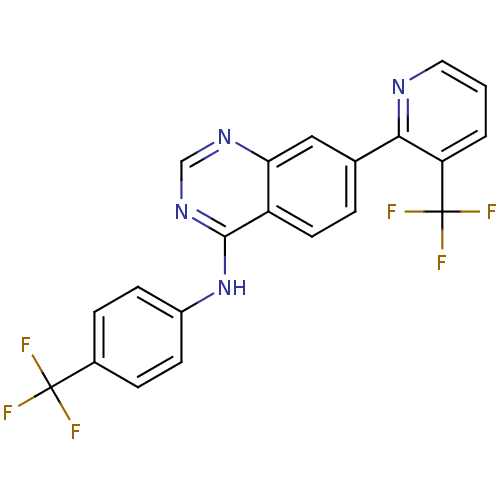
((4-Trifluoromethylphenyl)-[7-(3-trifluoromethylpyr...)Show SMILES FC(F)(F)c1ccc(Nc2ncnc3cc(ccc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C21H12F6N4/c22-20(23,24)13-4-6-14(7-5-13)31-19-15-8-3-12(10-17(15)29-11-30-19)18-16(21(25,26)27)2-1-9-28-18/h1-11H,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50272851
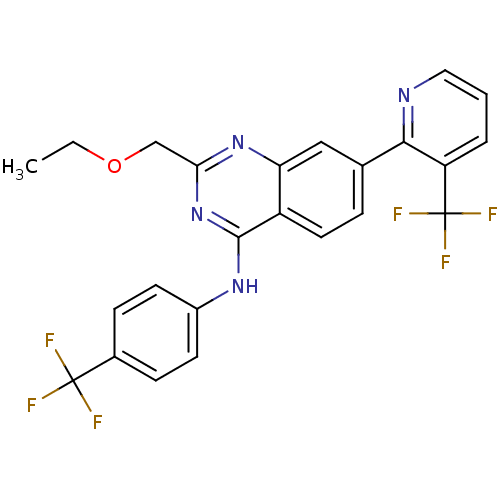
(2-(ethoxymethyl)-N-(4-(trifluoromethyl)phenyl)-7-(...)Show SMILES CCOCc1nc(Nc2ccc(cc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C24H18F6N4O/c1-2-35-13-20-33-19-12-14(21-18(24(28,29)30)4-3-11-31-21)5-10-17(19)22(34-20)32-16-8-6-15(7-9-16)23(25,26)27/h3-12H,2,13H2,1H3,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50030236

((S)-((S)-8-Benzhydryl-7-aza-tricyclo[4.3.1.0*3,7*]...)Show SMILES COc1ccccc1CNC1C2CC3CCC(C2)N3[C@H]1C(c1ccccc1)c1ccccc1 |TLB:14:13:19.10:16.17| Show InChI InChI=1S/C30H34N2O/c1-33-27-15-9-8-14-23(27)20-31-29-24-18-25-16-17-26(19-24)32(25)30(29)28(21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,24-26,28-31H,16-20H2,1H3/t24?,25?,26?,29?,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc.
Curated by ChEMBL
| Assay Description
Tested in vitro for the binding affinity towards NK1 receptor in human IM-9 cells using [125I]-labeled bolton-hunter substance P as ligand |
J Med Chem 37: 2831-40 (1994)
BindingDB Entry DOI: 10.7270/Q2CZ365J |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273251

(2-(isopropoxymethyl)-7-(3-(trifluoromethyl)pyridin...)Show SMILES CC(C)OCc1nc(Nc2ccc(nc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C24H19F6N5O/c1-13(2)36-12-20-34-18-10-14(21-17(23(25,26)27)4-3-9-31-21)5-7-16(18)22(35-20)33-15-6-8-19(32-11-15)24(28,29)30/h3-11,13H,12H2,1-2H3,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273213
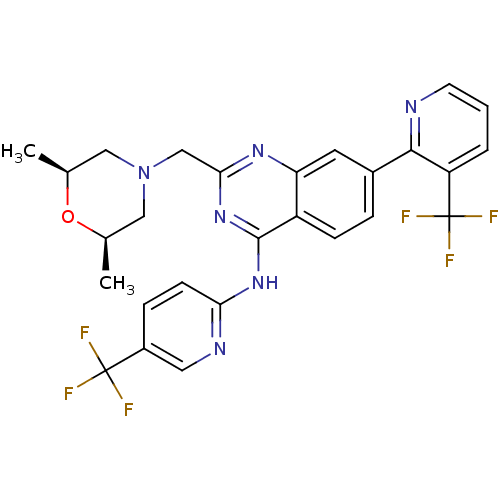
(2-(((2S,6R)-2,6-dimethylmorpholino)methyl)-7-(3-(t...)Show SMILES C[C@H]1CN(Cc2nc(Nc3ccc(cn3)C(F)(F)F)c3ccc(cc3n2)-c2ncccc2C(F)(F)F)C[C@@H](C)O1 |r| Show InChI InChI=1S/C27H24F6N6O/c1-15-12-39(13-16(2)40-15)14-23-36-21-10-17(24-20(27(31,32)33)4-3-9-34-24)5-7-19(21)25(38-23)37-22-8-6-18(11-35-22)26(28,29)30/h3-11,15-16H,12-14H2,1-2H3,(H,35,36,37,38)/t15-,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
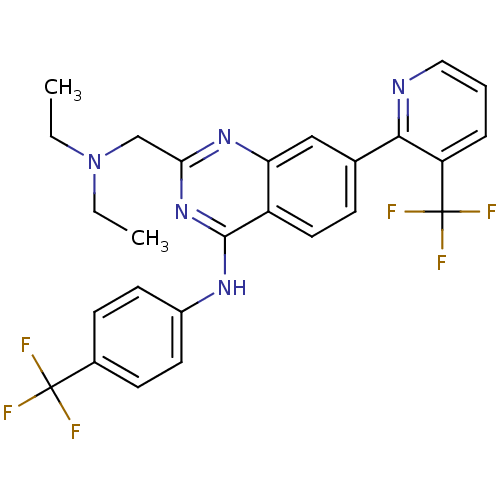
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273085
(2-((diethylamino)methyl)-N-(4-(trifluoromethyl)phe...)Show SMILES CCN(CC)Cc1nc(Nc2ccc(cc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C26H23F6N5/c1-3-37(4-2)15-22-35-21-14-16(23-20(26(30,31)32)6-5-13-33-23)7-12-19(21)24(36-22)34-18-10-8-17(9-11-18)25(27,28)29/h5-14H,3-4,15H2,1-2H3,(H,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50272850

(2-(methoxymethyl)-N-(4-(trifluoromethyl)phenyl)-7-...)Show SMILES COCc1nc(Nc2ccc(cc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C23H16F6N4O/c1-34-12-19-32-18-11-13(20-17(23(27,28)29)3-2-10-30-20)4-9-16(18)21(33-19)31-15-7-5-14(6-8-15)22(24,25)26/h2-11H,12H2,1H3,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50030238
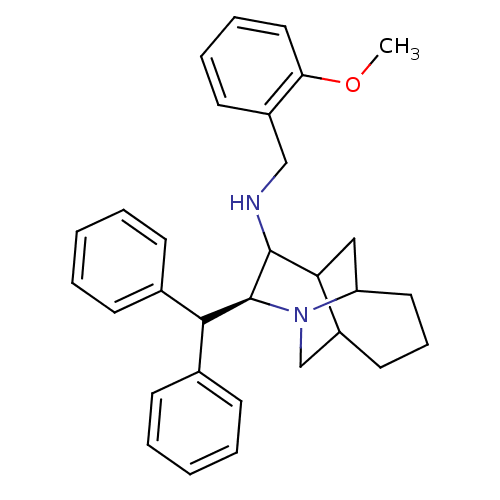
((S)-((S)-11-Benzhydryl-1-aza-tricyclo[5.4.0.0*3,9*...)Show SMILES COc1ccccc1CNC1C2CC3CCCC2CN3[C@H]1C(c1ccccc1)c1ccccc1 |TLB:14:13:20.10:18.17| Show InChI InChI=1S/C31H36N2O/c1-34-28-18-9-8-15-24(28)20-32-30-27-19-26-17-10-16-25(27)21-33(26)31(30)29(22-11-4-2-5-12-22)23-13-6-3-7-14-23/h2-9,11-15,18,25-27,29-32H,10,16-17,19-21H2,1H3/t25?,26?,27?,30?,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc.
Curated by ChEMBL
| Assay Description
Tested in vitro for the binding affinity towards NK1 receptor in human IM-9 cells using [125I]-labeled bolton-hunter substance P as ligand |
J Med Chem 37: 2831-40 (1994)
BindingDB Entry DOI: 10.7270/Q2CZ365J |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000040

(((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1ccccc1CN[C@H]1C2CCN(CC2)[C@H]1C(c1ccccc1)c1ccccc1 |wD:10.10,17.20,(11.79,-2.71,;10.31,-3.13,;9.22,-2.04,;10.55,-1.27,;10.57,.27,;9.22,1.04,;7.89,.27,;7.89,-1.26,;6.56,-2.01,;6.56,-3.55,;5.21,-4.32,;3.92,-3.55,;3.16,-4.88,;4.65,-5.3,;3.88,-6.63,;2.57,-5.86,;2.57,-4.32,;5.21,-5.86,;6.56,-6.63,;6.54,-8.17,;5.21,-8.94,;5.21,-10.48,;6.54,-11.25,;7.89,-10.48,;7.87,-8.94,;7.89,-5.86,;9.22,-6.63,;10.55,-5.88,;10.55,-4.34,;9.22,-3.55,;7.89,-4.34,)| Show InChI InChI=1S/C28H32N2O/c1-31-25-15-9-8-14-24(25)20-29-27-23-16-18-30(19-17-23)28(27)26(21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,23,26-29H,16-20H2,1H3/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration for displacement of [3H]-Substance P (SP) in human IM-9 cells |
Bioorg Med Chem Lett 4: 839-842 (1994)
Article DOI: 10.1016/S0960-894X(01)80859-3
BindingDB Entry DOI: 10.7270/Q2542NJB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50272974
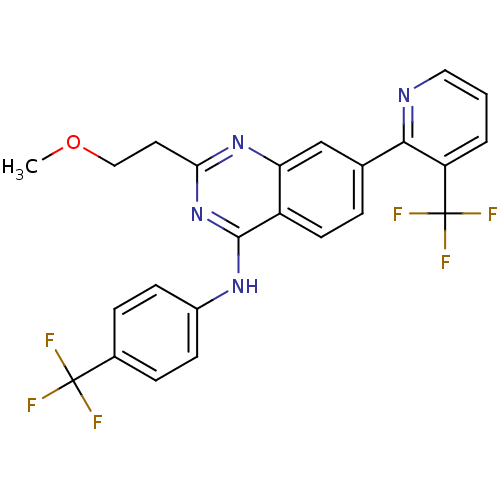
(2-(2-methoxyethyl)-N-(4-(trifluoromethyl)phenyl)-7...)Show SMILES COCCc1nc(Nc2ccc(cc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C24H18F6N4O/c1-35-12-10-20-33-19-13-14(21-18(24(28,29)30)3-2-11-31-21)4-9-17(19)22(34-20)32-16-7-5-15(6-8-16)23(25,26)27/h2-9,11,13H,10,12H2,1H3,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273166
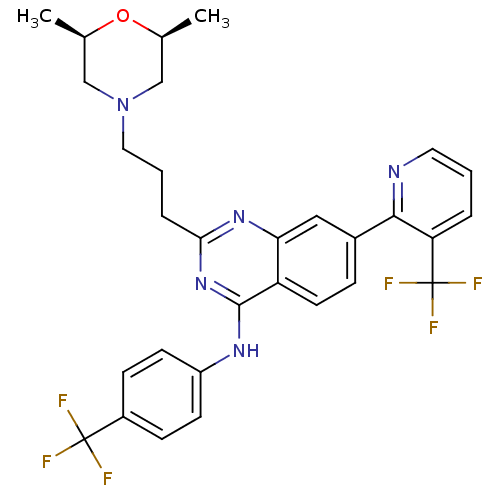
(2-(3-((2S,6R)-2,6-dimethylmorpholino)propyl)-N-(4-...)Show SMILES C[C@H]1CN(CCCc2nc(Nc3ccc(cc3)C(F)(F)F)c3ccc(cc3n2)-c2ncccc2C(F)(F)F)C[C@@H](C)O1 |r| Show InChI InChI=1S/C30H29F6N5O/c1-18-16-41(17-19(2)42-18)14-4-6-26-39-25-15-20(27-24(30(34,35)36)5-3-13-37-27)7-12-23(25)28(40-26)38-22-10-8-21(9-11-22)29(31,32)33/h3,5,7-13,15,18-19H,4,6,14,16-17H2,1-2H3,(H,38,39,40)/t18-,19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273285

(2-(((2S,6R)-2,6-dimethylmorpholino)methyl)-7-(3-(t...)Show SMILES C[C@H]1CN(Cc2nc(Nc3ccc(nc3)C(F)(F)F)c3ccc(cc3n2)-c2ncccc2C(F)(F)F)C[C@@H](C)O1 |r| Show InChI InChI=1S/C27H24F6N6O/c1-15-12-39(13-16(2)40-15)14-23-37-21-10-17(24-20(26(28,29)30)4-3-9-34-24)5-7-19(21)25(38-23)36-18-6-8-22(35-11-18)27(31,32)33/h3-11,15-16H,12-14H2,1-2H3,(H,36,37,38)/t15-,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273087
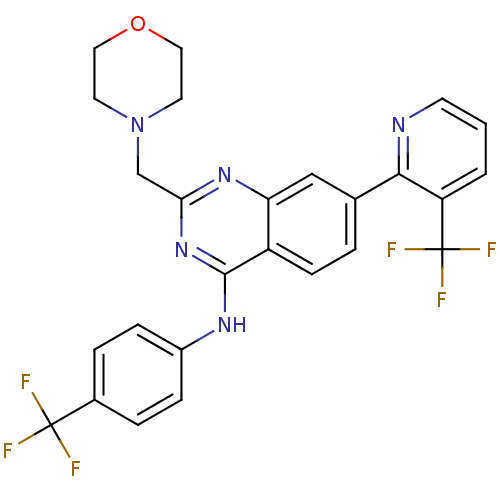
(2-(morpholinomethyl)-N-(4-(trifluoromethyl)phenyl)...)Show SMILES FC(F)(F)c1ccc(Nc2nc(CN3CCOCC3)nc3cc(ccc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C26H21F6N5O/c27-25(28,29)17-4-6-18(7-5-17)34-24-19-8-3-16(23-20(26(30,31)32)2-1-9-33-23)14-21(19)35-22(36-24)15-37-10-12-38-13-11-37/h1-9,14H,10-13,15H2,(H,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50280461

(((2R,3R)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1ccccc1CN[C@@H]1C2CCN(CC2)[C@@H]1C(c1ccccc1)c1ccccc1 |wU:10.10,17.20,(8.22,1.05,;9.53,1.84,;10.88,1.07,;12.19,1.84,;13.54,1.09,;13.54,-.45,;12.21,-1.24,;10.88,-.45,;9.55,-1.24,;9.57,-2.78,;8.24,-3.55,;6.89,-2.78,;5.56,-3.55,;5.56,-5.09,;6.82,-5.83,;7.61,-4.5,;6.12,-4.11,;8.24,-5.09,;9.55,-5.89,;11.04,-5.49,;12.13,-6.58,;13.61,-6.17,;14.01,-4.67,;12.91,-3.59,;11.42,-4,;9.53,-7.43,;8.17,-8.17,;8.15,-9.71,;9.48,-10.51,;10.83,-9.75,;10.85,-8.21,)| Show InChI InChI=1S/C28H32N2O/c1-31-25-15-9-8-14-24(25)20-29-27-23-16-18-30(19-17-23)28(27)26(21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,23,26-29H,16-20H2,1H3/t27-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards Tachykinin receptor 1 in human IM-9 cells using [3H]-substance P as ligand |
J Med Chem 35: 2591-600 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1JJF |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50030230
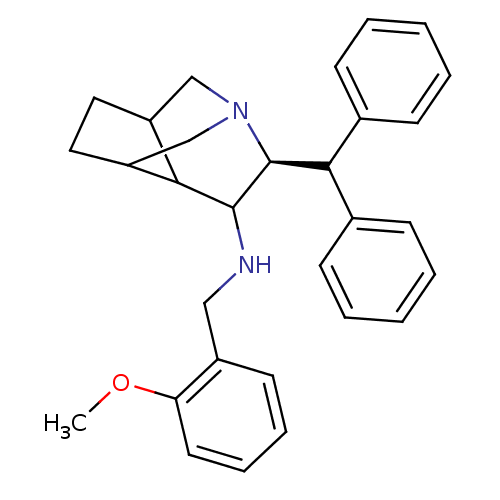
((S)-((S)-9-Benzhydryl-1-aza-tricyclo[4.3.1.0*3,7*]...)Show SMILES COc1ccccc1CNC1C2C3CCC2CN(C3)[C@H]1C(c1ccccc1)c1ccccc1 |wD:19.23,TLB:13:12:19.10:15.16,(7.71,-5.53,;6.37,-4.76,;6.37,-3.22,;7.71,-2.45,;7.71,-.89,;6.37,-.12,;5.04,-.91,;5.04,-2.45,;3.69,-3.22,;3.69,-4.76,;2.36,-5.53,;1.03,-4.74,;1.8,-6.07,;-1.63,-6.28,;-1.63,-4.74,;-.3,-5.51,;-.3,-7.05,;1.03,-7.82,;.37,-6.77,;2.36,-7.07,;3.71,-7.82,;3.69,-9.38,;5.02,-10.13,;5.04,-11.67,;3.69,-12.44,;2.36,-11.67,;2.36,-10.13,;5.19,-7.43,;5.58,-5.95,;7.05,-5.54,;8.15,-6.63,;7.77,-8.1,;6.28,-8.52,)| Show InChI InChI=1S/C30H34N2O/c1-33-26-15-9-8-14-23(26)18-31-29-27-24-16-17-25(27)20-32(19-24)30(29)28(21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,24-25,27-31H,16-20H2,1H3/t24?,25?,27?,29?,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc.
Curated by ChEMBL
| Assay Description
Tested in vitro for the binding affinity towards NK1 receptor in human IM-9 cells using [125I]-labeled bolton-hunter substance P as ligand |
J Med Chem 37: 2831-40 (1994)
BindingDB Entry DOI: 10.7270/Q2CZ365J |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273167
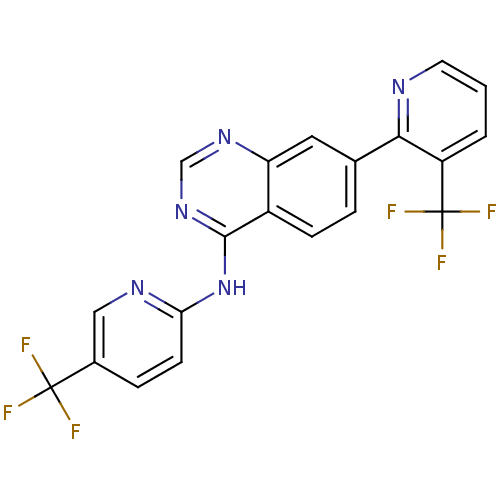
((5-Trifluoromethylpyridin-2-yl)-[7-(3-trifluoromet...)Show SMILES FC(F)(F)c1ccc(Nc2ncnc3cc(ccc23)-c2ncccc2C(F)(F)F)nc1 Show InChI InChI=1S/C20H11F6N5/c21-19(22,23)12-4-6-16(28-9-12)31-18-13-5-3-11(8-15(13)29-10-30-18)17-14(20(24,25)26)2-1-7-27-17/h1-10H,(H,28,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273165

(2-(3-morpholinopropyl)-N-(4-(trifluoromethyl)pheny...)Show SMILES FC(F)(F)c1ccc(Nc2nc(CCCN3CCOCC3)nc3cc(ccc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C28H25F6N5O/c29-27(30,31)19-6-8-20(9-7-19)36-26-21-10-5-18(25-22(28(32,33)34)3-1-11-35-25)17-23(21)37-24(38-26)4-2-12-39-13-15-40-16-14-39/h1,3,5-11,17H,2,4,12-16H2,(H,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50030228
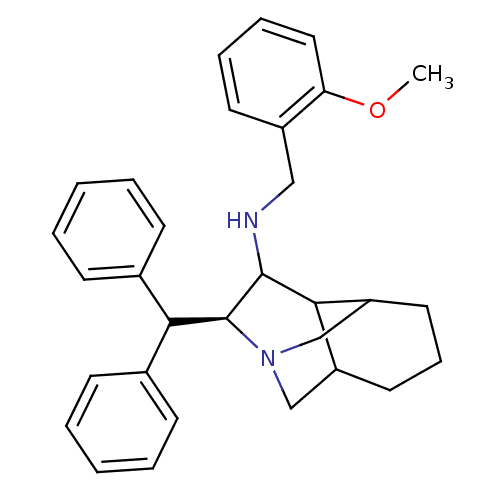
((S)-((S)-3-Benzhydryl-octahydro-2,5-methano-isoqui...)Show SMILES COc1ccccc1CNC1C2C3CCCC2CN(C3)[C@H]1C(c1ccccc1)c1ccccc1 |wD:20.24,TLB:13:12:20.10:16.17,(7.71,-5.53,;6.37,-4.76,;6.37,-3.22,;7.71,-2.45,;7.71,-.89,;6.37,-.12,;5.04,-.91,;5.04,-2.45,;3.69,-3.22,;3.69,-4.76,;2.36,-5.53,;1.03,-4.74,;1.8,-6.07,;-1.63,-6.28,;-2.63,-5.48,;-1.63,-4.74,;-.3,-5.51,;-.3,-7.05,;1.03,-7.82,;.37,-6.77,;2.36,-7.07,;3.71,-7.82,;3.69,-9.38,;5.02,-10.13,;5.04,-11.67,;3.69,-12.44,;2.36,-11.67,;2.36,-10.13,;5.19,-7.43,;5.58,-5.95,;7.05,-5.54,;8.15,-6.63,;7.77,-8.1,;6.28,-8.52,)| Show InChI InChI=1S/C31H36N2O/c1-34-27-18-9-8-15-24(27)19-32-30-28-25-16-10-17-26(28)21-33(20-25)31(30)29(22-11-4-2-5-12-22)23-13-6-3-7-14-23/h2-9,11-15,18,25-26,28-32H,10,16-17,19-21H2,1H3/t25?,26?,28?,30?,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc.
Curated by ChEMBL
| Assay Description
Tested in vitro for the binding affinity towards NK1 receptor in human IM-9 cells using [125I]-labeled bolton-hunter substance P as ligand |
J Med Chem 37: 2831-40 (1994)
BindingDB Entry DOI: 10.7270/Q2CZ365J |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000040

(((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1ccccc1CN[C@H]1C2CCN(CC2)[C@H]1C(c1ccccc1)c1ccccc1 |wD:10.10,17.20,(11.79,-2.71,;10.31,-3.13,;9.22,-2.04,;10.55,-1.27,;10.57,.27,;9.22,1.04,;7.89,.27,;7.89,-1.26,;6.56,-2.01,;6.56,-3.55,;5.21,-4.32,;3.92,-3.55,;3.16,-4.88,;4.65,-5.3,;3.88,-6.63,;2.57,-5.86,;2.57,-4.32,;5.21,-5.86,;6.56,-6.63,;6.54,-8.17,;5.21,-8.94,;5.21,-10.48,;6.54,-11.25,;7.89,-10.48,;7.87,-8.94,;7.89,-5.86,;9.22,-6.63,;10.55,-5.88,;10.55,-4.34,;9.22,-3.55,;7.89,-4.34,)| Show InChI InChI=1S/C28H32N2O/c1-31-25-15-9-8-14-24(25)20-29-27-23-16-18-30(19-17-23)28(27)26(21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,23,26-29H,16-20H2,1H3/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding potency of the compound against SP receptor (from ref. 1) |
Bioorg Med Chem Lett 1: 129-132 (1991)
Article DOI: 10.1016/S0960-894X(00)80246-2
BindingDB Entry DOI: 10.7270/Q28P60DN |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000040

(((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1ccccc1CN[C@H]1C2CCN(CC2)[C@H]1C(c1ccccc1)c1ccccc1 |wD:10.10,17.20,(11.79,-2.71,;10.31,-3.13,;9.22,-2.04,;10.55,-1.27,;10.57,.27,;9.22,1.04,;7.89,.27,;7.89,-1.26,;6.56,-2.01,;6.56,-3.55,;5.21,-4.32,;3.92,-3.55,;3.16,-4.88,;4.65,-5.3,;3.88,-6.63,;2.57,-5.86,;2.57,-4.32,;5.21,-5.86,;6.56,-6.63,;6.54,-8.17,;5.21,-8.94,;5.21,-10.48,;6.54,-11.25,;7.89,-10.48,;7.87,-8.94,;7.89,-5.86,;9.22,-6.63,;10.55,-5.88,;10.55,-4.34,;9.22,-3.55,;7.89,-4.34,)| Show InChI InChI=1S/C28H32N2O/c1-31-25-15-9-8-14-24(25)20-29-27-23-16-18-30(19-17-23)28(27)26(21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,23,26-29H,16-20H2,1H3/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding potency of the compound against SP receptor (from ref. 1) |
Bioorg Med Chem Lett 1: 129-132 (1991)
Article DOI: 10.1016/S0960-894X(00)80246-2
BindingDB Entry DOI: 10.7270/Q28P60DN |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000040

(((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1ccccc1CN[C@H]1C2CCN(CC2)[C@H]1C(c1ccccc1)c1ccccc1 |wD:10.10,17.20,(11.79,-2.71,;10.31,-3.13,;9.22,-2.04,;10.55,-1.27,;10.57,.27,;9.22,1.04,;7.89,.27,;7.89,-1.26,;6.56,-2.01,;6.56,-3.55,;5.21,-4.32,;3.92,-3.55,;3.16,-4.88,;4.65,-5.3,;3.88,-6.63,;2.57,-5.86,;2.57,-4.32,;5.21,-5.86,;6.56,-6.63,;6.54,-8.17,;5.21,-8.94,;5.21,-10.48,;6.54,-11.25,;7.89,-10.48,;7.87,-8.94,;7.89,-5.86,;9.22,-6.63,;10.55,-5.88,;10.55,-4.34,;9.22,-3.55,;7.89,-4.34,)| Show InChI InChI=1S/C28H32N2O/c1-31-25-15-9-8-14-24(25)20-29-27-23-16-18-30(19-17-23)28(27)26(21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,23,26-29H,16-20H2,1H3/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding potency of the compound against SP receptor in bovine caudate using [3H]- as radioligand |
Bioorg Med Chem Lett 1: 129-132 (1991)
Article DOI: 10.1016/S0960-894X(00)80246-2
BindingDB Entry DOI: 10.7270/Q28P60DN |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50272849
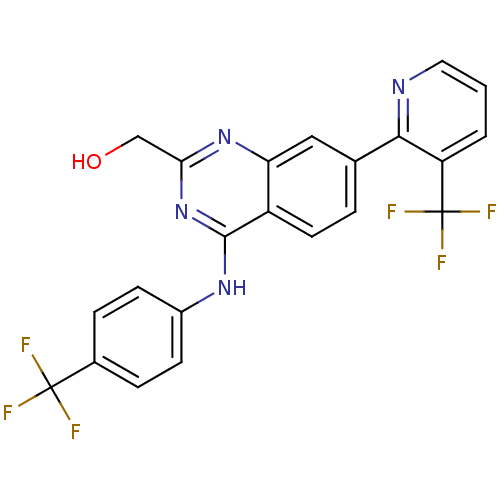
((4-(4-(trifluoromethyl)phenylamino)-7-(3-(trifluor...)Show SMILES OCc1nc(Nc2ccc(cc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C22H14F6N4O/c23-21(24,25)13-4-6-14(7-5-13)30-20-15-8-3-12(10-17(15)31-18(11-33)32-20)19-16(22(26,27)28)2-1-9-29-19/h1-10,33H,11H2,(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50030241
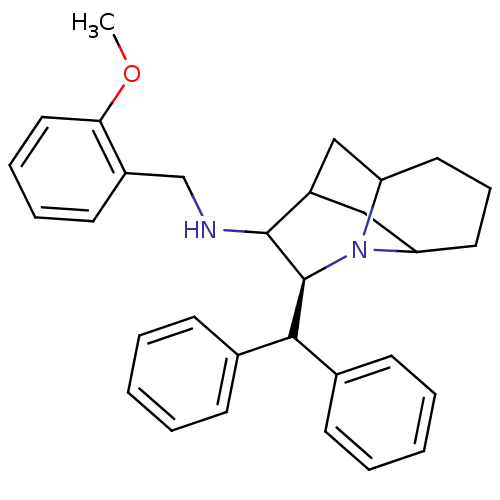
((S)-((S)-9-Benzhydryl-8-aza-tricyclo[5.3.1.0*3,8*]...)Show SMILES COc1ccccc1CNC1C2CC3CCCC(C2)N3[C@H]1C(c1ccccc1)c1ccccc1 |TLB:14:13:20.10:17.18| Show InChI InChI=1S/C31H36N2O/c1-34-28-18-9-8-15-24(28)21-32-30-25-19-26-16-10-17-27(20-25)33(26)31(30)29(22-11-4-2-5-12-22)23-13-6-3-7-14-23/h2-9,11-15,18,25-27,29-32H,10,16-17,19-21H2,1H3/t25?,26?,27?,30?,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc.
Curated by ChEMBL
| Assay Description
Tested in vitro for the binding affinity towards NK1 receptor in human IM-9 cells using [125I]-labeled bolton-hunter substance P as ligand |
J Med Chem 37: 2831-40 (1994)
BindingDB Entry DOI: 10.7270/Q2CZ365J |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000040

(((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1ccccc1CN[C@H]1C2CCN(CC2)[C@H]1C(c1ccccc1)c1ccccc1 |wD:10.10,17.20,(11.79,-2.71,;10.31,-3.13,;9.22,-2.04,;10.55,-1.27,;10.57,.27,;9.22,1.04,;7.89,.27,;7.89,-1.26,;6.56,-2.01,;6.56,-3.55,;5.21,-4.32,;3.92,-3.55,;3.16,-4.88,;4.65,-5.3,;3.88,-6.63,;2.57,-5.86,;2.57,-4.32,;5.21,-5.86,;6.56,-6.63,;6.54,-8.17,;5.21,-8.94,;5.21,-10.48,;6.54,-11.25,;7.89,-10.48,;7.87,-8.94,;7.89,-5.86,;9.22,-6.63,;10.55,-5.88,;10.55,-4.34,;9.22,-3.55,;7.89,-4.34,)| Show InChI InChI=1S/C28H32N2O/c1-31-25-15-9-8-14-24(25)20-29-27-23-16-18-30(19-17-23)28(27)26(21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,23,26-29H,16-20H2,1H3/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of binding of [3H]-SP in human IM-9 cells |
Bioorg Med Chem Lett 3: 921-924 (1993)
Article DOI: 10.1016/S0960-894X(00)80693-9
BindingDB Entry DOI: 10.7270/Q2RV0NNF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273250
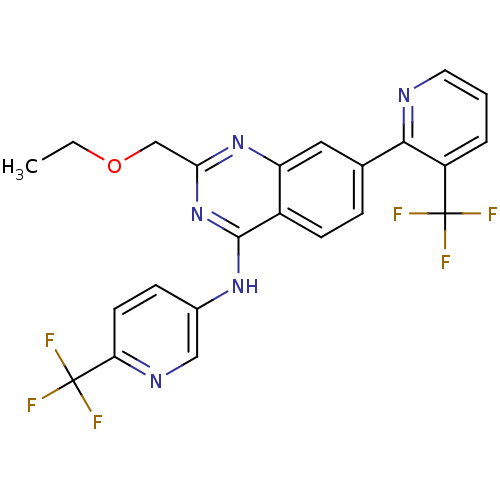
(2-(ethoxymethyl)-7-(3-(trifluoromethyl)pyridin-2-y...)Show SMILES CCOCc1nc(Nc2ccc(nc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C23H17F6N5O/c1-2-35-12-19-33-17-10-13(20-16(22(24,25)26)4-3-9-30-20)5-7-15(17)21(34-19)32-14-6-8-18(31-11-14)23(27,28)29/h3-11H,2,12H2,1H3,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273053
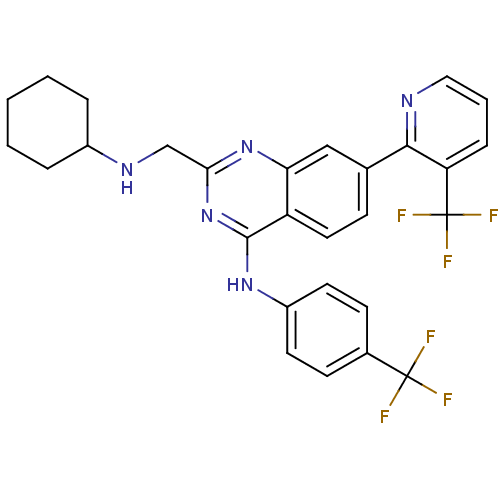
(2-((cyclohexylamino)methyl)-N-(4-(trifluoromethyl)...)Show SMILES FC(F)(F)c1ccc(Nc2nc(CNC3CCCCC3)nc3cc(ccc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C28H25F6N5/c29-27(30,31)18-9-11-20(12-10-18)37-26-21-13-8-17(25-22(28(32,33)34)7-4-14-35-25)15-23(21)38-24(39-26)16-36-19-5-2-1-3-6-19/h4,7-15,19,36H,1-3,5-6,16H2,(H,37,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273214
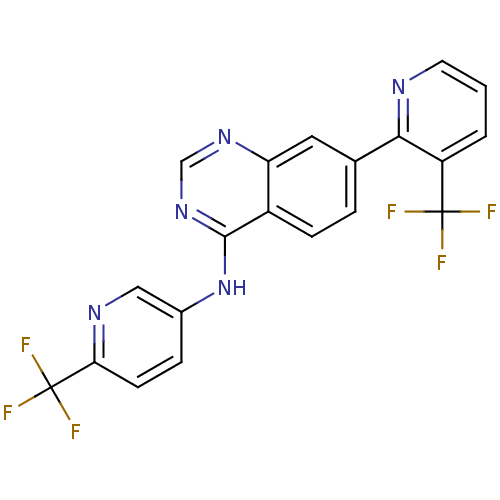
(7-(3-(trifluoromethyl)pyridin-2-yl)-N-(6-(trifluor...)Show SMILES FC(F)(F)c1ccc(Nc2ncnc3cc(ccc23)-c2ncccc2C(F)(F)F)cn1 Show InChI InChI=1S/C20H11F6N5/c21-19(22,23)14-2-1-7-27-17(14)11-3-5-13-15(8-11)29-10-30-18(13)31-12-4-6-16(28-9-12)20(24,25)26/h1-10H,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50030233
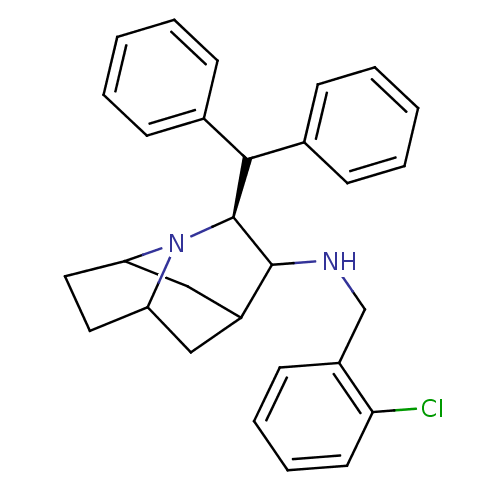
((S)-((S)-8-Benzhydryl-7-aza-tricyclo[4.3.1.0*3,7*]...)Show SMILES Clc1ccccc1CNC1C2CC3CCC(C2)N3[C@H]1C(c1ccccc1)c1ccccc1 |TLB:13:12:18.9:15.16| Show InChI InChI=1S/C29H31ClN2/c30-26-14-8-7-13-22(26)19-31-28-23-17-24-15-16-25(18-23)32(24)29(28)27(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-14,23-25,27-29,31H,15-19H2/t23?,24?,25?,28?,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc.
Curated by ChEMBL
| Assay Description
Tested in vitro for the binding affinity towards NK1 receptor in human IM-9 cells using [125I]-labeled bolton-hunter substance P as ligand |
J Med Chem 37: 2831-40 (1994)
BindingDB Entry DOI: 10.7270/Q2CZ365J |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50030237
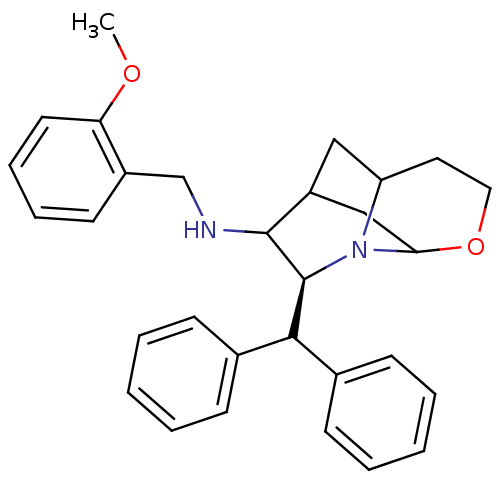
((S)-((S)-9-Benzhydryl-4-oxa-8-aza-tricyclo[5.3.1.0...)Show SMILES COc1ccccc1CNC1C2CC3CCOC(C2)N3[C@H]1C(c1ccccc1)c1ccccc1 |TLB:14:13:20.10:17.18| Show InChI InChI=1S/C30H34N2O2/c1-33-26-15-9-8-14-23(26)20-31-29-24-18-25-16-17-34-27(19-24)32(25)30(29)28(21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,24-25,27-31H,16-20H2,1H3/t24?,25?,27?,29?,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc.
Curated by ChEMBL
| Assay Description
Tested in vitro for the binding affinity towards NK1 receptor in human IM-9 cells using [125I]-labeled bolton-hunter substance P as ligand |
J Med Chem 37: 2831-40 (1994)
BindingDB Entry DOI: 10.7270/Q2CZ365J |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273088
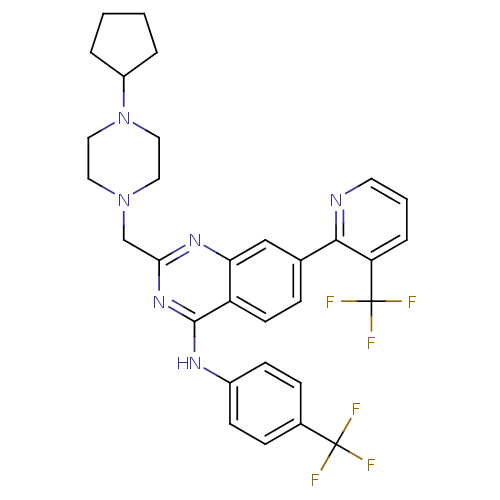
(2-((4-cyclopentylpiperazin-1-yl)methyl)-N-(4-(trif...)Show SMILES FC(F)(F)c1ccc(Nc2nc(CN3CCN(CC3)C3CCCC3)nc3cc(ccc23)-c2ncccc2C(F)(F)F)cc1 Show InChI InChI=1S/C31H30F6N6/c32-30(33,34)21-8-10-22(11-9-21)39-29-24-12-7-20(28-25(31(35,36)37)6-3-13-38-28)18-26(24)40-27(41-29)19-42-14-16-43(17-15-42)23-4-1-2-5-23/h3,6-13,18,23H,1-2,4-5,14-17,19H2,(H,39,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273052
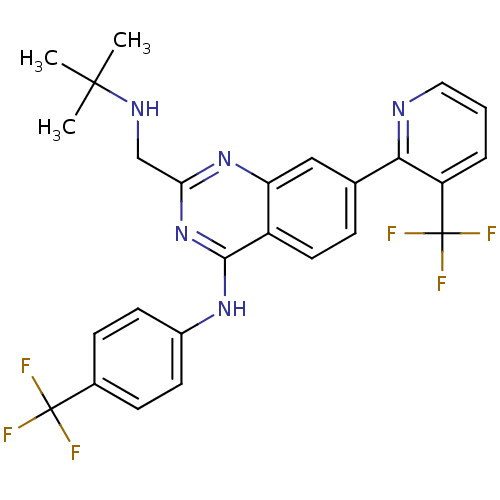
(2-((tert-butylamino)methyl)-N-(4-(trifluoromethyl)...)Show SMILES CC(C)(C)NCc1nc(Nc2ccc(cc2)C(F)(F)F)c2ccc(cc2n1)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C26H23F6N5/c1-24(2,3)34-14-21-36-20-13-15(22-19(26(30,31)32)5-4-12-33-22)6-11-18(20)23(37-21)35-17-9-7-16(8-10-17)25(27,28)29/h4-13,34H,14H2,1-3H3,(H,35,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50273212

(2-(morpholinomethyl)-7-(3-(trifluoromethyl)pyridin...)Show SMILES FC(F)(F)c1ccc(Nc2nc(CN3CCOCC3)nc3cc(ccc23)-c2ncccc2C(F)(F)F)nc1 Show InChI InChI=1S/C25H20F6N6O/c26-24(27,28)16-4-6-20(33-13-16)35-23-17-5-3-15(22-18(25(29,30)31)2-1-7-32-22)12-19(17)34-21(36-23)14-37-8-10-38-11-9-37/h1-7,12-13H,8-11,14H2,(H,33,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activation |
Bioorg Med Chem Lett 18: 4573-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.036
BindingDB Entry DOI: 10.7270/Q28P6099 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50284130
(CHEMBL122580 | [(2S,3S)-2-(3-Chloro-phenyl)-1-aza-...)Show SMILES COc1ccc(CN[C@H]2C3CCN(CC3)[C@H]2c2cccc(Cl)c2)c(OC)c1 |wD:15.17,8.7,(17.43,-7.33,;17.43,-8.87,;16.11,-9.64,;16.11,-11.18,;14.78,-11.95,;13.45,-11.18,;12.11,-11.95,;10.78,-11.18,;9.43,-11.95,;8.1,-11.18,;7.31,-12.51,;8.8,-12.91,;8.1,-14.29,;6.77,-13.52,;6.77,-11.95,;9.43,-13.52,;10.78,-14.29,;10.76,-15.83,;12.09,-16.6,;13.42,-15.83,;13.42,-14.28,;14.75,-13.51,;12.09,-13.52,;13.44,-9.64,;12.09,-8.89,;12.09,-7.35,;14.75,-8.87,)| Show InChI InChI=1S/C22H27ClN2O2/c1-26-19-7-6-17(20(13-19)27-2)14-24-21-15-8-10-25(11-9-15)22(21)16-4-3-5-18(23)12-16/h3-7,12-13,15,21-22,24H,8-11,14H2,1-2H3/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration for displacement of [3H]-Substance P (SP) in human IM-9 cells |
Bioorg Med Chem Lett 4: 839-842 (1994)
Article DOI: 10.1016/S0960-894X(01)80859-3
BindingDB Entry DOI: 10.7270/Q2542NJB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data