 Found 268 hits with Last Name = 'nicklin' and Initial = 'p'
Found 268 hits with Last Name = 'nicklin' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50207120

(3-isobutyl-8-((6-methoxyisoquinolin-4-yl)methyl)-1...)Show SMILES COc1ccc2cncc(Cc3nc4n(CC(C)C)c(=O)n(C)c(=O)c4[nH]3)c2c1 Show InChI InChI=1S/C21H23N5O3/c1-12(2)11-26-19-18(20(27)25(3)21(26)28)23-17(24-19)7-14-10-22-9-13-5-6-15(29-4)8-16(13)14/h5-6,8-10,12H,7,11H2,1-4H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of adenosine A1 receptor |
Bioorg Med Chem Lett 17: 2376-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.019
BindingDB Entry DOI: 10.7270/Q2X63NSC |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285776
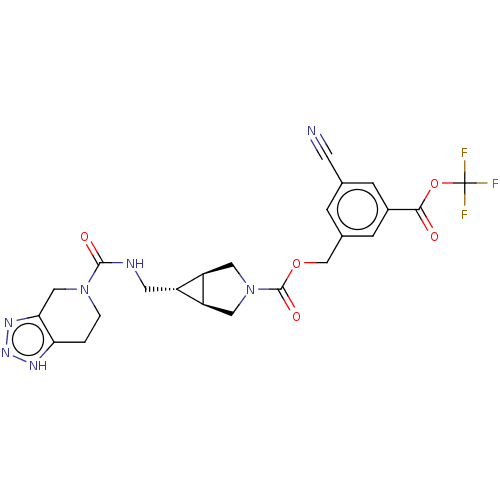
(CHEMBL4172309)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)N1CCc2[nH]nnc2C1)C(=O)OCc1cc(cc(c1)C(=O)OC(F)(F)F)C#N |r| Show InChI InChI=1S/C23H22F3N7O5/c24-23(25,26)38-20(34)14-4-12(6-27)3-13(5-14)11-37-22(36)33-8-16-15(17(16)9-33)7-28-21(35)32-2-1-18-19(10-32)30-31-29-18/h3-5,15-17H,1-2,7-11H2,(H,28,35)(H,29,30,31)/t15-,16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285750
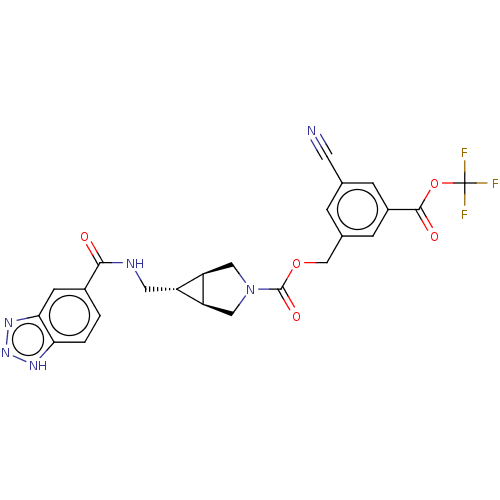
(CHEMBL4173049)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]nnc2c1)C(=O)OCc1cc(cc(c1)C(=O)OC(F)(F)F)C#N |r| Show InChI InChI=1S/C24H19F3N6O5/c25-24(26,27)38-22(35)15-4-12(7-28)3-13(5-15)11-37-23(36)33-9-17-16(18(17)10-33)8-29-21(34)14-1-2-19-20(6-14)31-32-30-19/h1-6,16-18H,8-11H2,(H,29,34)(H,30,31,32)/t16-,17-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285745
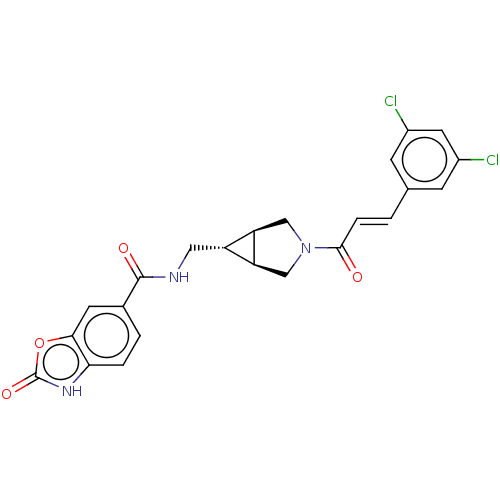
(CHEMBL4162641)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]c(=O)oc2c1)C(=O)\C=C\c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C23H19Cl2N3O4/c24-14-5-12(6-15(25)8-14)1-4-21(29)28-10-17-16(18(17)11-28)9-26-22(30)13-2-3-19-20(7-13)32-23(31)27-19/h1-8,16-18H,9-11H2,(H,26,30)(H,27,31)/b4-1+/t16-,17-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285744
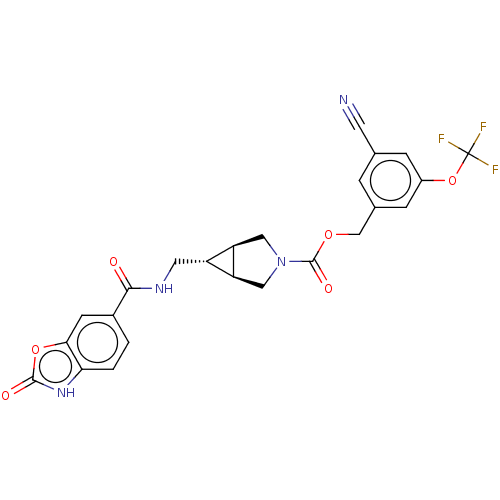
(CHEMBL4168498)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]c(=O)oc2c1)C(=O)OCc1cc(OC(F)(F)F)cc(c1)C#N |r| Show InChI InChI=1S/C24H19F3N4O6/c25-24(26,27)37-15-4-12(7-28)3-13(5-15)11-35-23(34)31-9-17-16(18(17)10-31)8-29-21(32)14-1-2-19-20(6-14)36-22(33)30-19/h1-6,16-18H,8-11H2,(H,29,32)(H,30,33)/t16-,17-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50207130

(8-((6,7-dimethoxy-1-methylisoquinolin-4-yl)methyl)...)Show SMILES COc1cc2c(C)ncc(Cc3nc4n(CC(C)C)c(=O)n(C)c(=O)c4[nH]3)c2cc1OC Show InChI InChI=1S/C23H27N5O4/c1-12(2)11-28-21-20(22(29)27(4)23(28)30)25-19(26-21)7-14-10-24-13(3)15-8-17(31-5)18(32-6)9-16(14)15/h8-10,12H,7,11H2,1-6H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human platelet PDE5 by [3H]cGMP scintillation proximity assay |
Bioorg Med Chem Lett 17: 2376-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.019
BindingDB Entry DOI: 10.7270/Q2X63NSC |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50207120

(3-isobutyl-8-((6-methoxyisoquinolin-4-yl)methyl)-1...)Show SMILES COc1ccc2cncc(Cc3nc4n(CC(C)C)c(=O)n(C)c(=O)c4[nH]3)c2c1 Show InChI InChI=1S/C21H23N5O3/c1-12(2)11-26-19-18(20(27)25(3)21(26)28)23-17(24-19)7-14-10-22-9-13-5-6-15(29-4)8-16(13)14/h5-6,8-10,12H,7,11H2,1-4H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human platelet PDE5 by [3H]cGMP scintillation proximity assay |
Bioorg Med Chem Lett 17: 2376-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.019
BindingDB Entry DOI: 10.7270/Q2X63NSC |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50285777
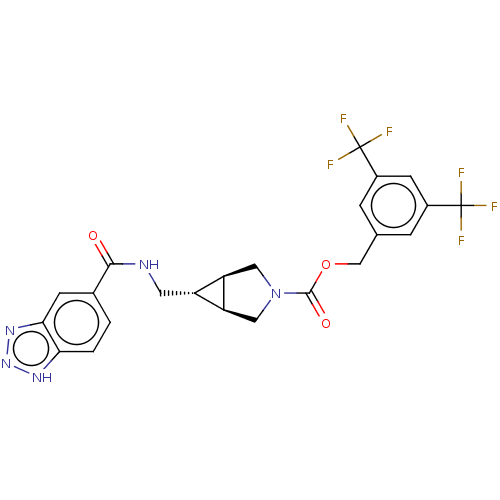
(CHEMBL4165749)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]nnc2c1)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H19F6N5O3/c24-22(25,26)13-3-11(4-14(6-13)23(27,28)29)10-37-21(36)34-8-16-15(17(16)9-34)7-30-20(35)12-1-2-18-19(5-12)32-33-31-18/h1-6,15-17H,7-10H2,(H,30,35)(H,31,32,33)/t15-,16-,17+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human ATX |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285748
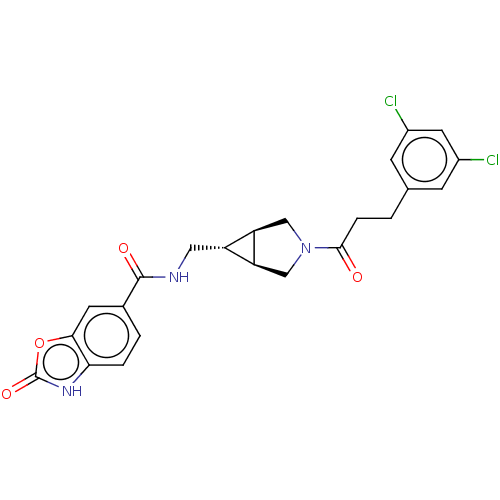
(CHEMBL4173341)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]c(=O)oc2c1)C(=O)CCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C23H21Cl2N3O4/c24-14-5-12(6-15(25)8-14)1-4-21(29)28-10-17-16(18(17)11-28)9-26-22(30)13-2-3-19-20(7-13)32-23(31)27-19/h2-3,5-8,16-18H,1,4,9-11H2,(H,26,30)(H,27,31)/t16-,17-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285774
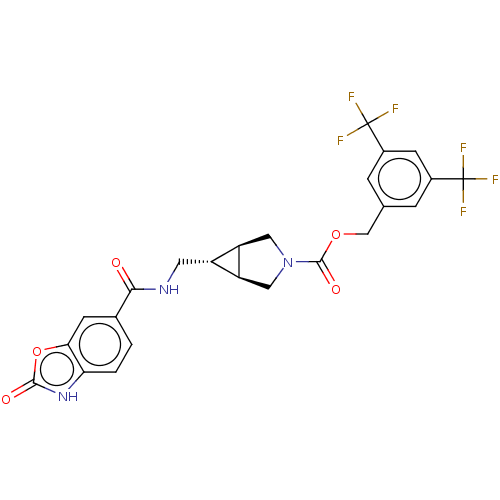
(CHEMBL4169550)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]c(=O)oc2c1)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C24H19F6N3O5/c25-23(26,27)13-3-11(4-14(6-13)24(28,29)30)10-37-22(36)33-8-16-15(17(16)9-33)7-31-20(34)12-1-2-18-19(5-12)38-21(35)32-18/h1-6,15-17H,7-10H2,(H,31,34)(H,32,35)/t15-,16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403068
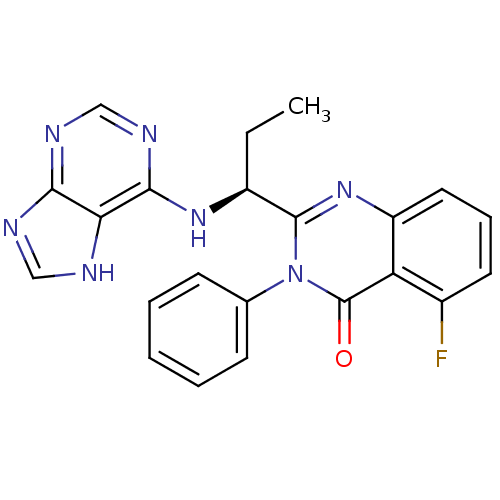
(CHEMBL2216870 | IDELALISIB | US9745321, CAL-101)Show SMILES CC[C@H](Nc1ncnc2nc[nH]c12)c1nc2cccc(F)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C22H18FN7O/c1-2-15(28-20-18-19(25-11-24-18)26-12-27-20)21-29-16-10-6-9-14(23)17(16)22(31)30(21)13-7-4-3-5-8-13/h3-12,15H,2H2,1H3,(H2,24,25,26,27,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) by Selectscreen kinase assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00986
BindingDB Entry DOI: 10.7270/Q27M0CRD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
P04972/P11541/P16586/P22571/P23439/Q95142
(Bos taurus-Bos taurus (Bovine)) | BDBM50117721
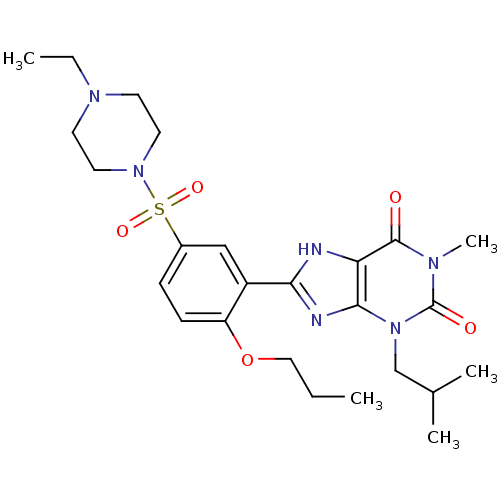
(3-Isobutyl-1-methyl-8-[5-(piperazin-1-ylmethanesul...)Show SMILES CCCOc1ccc(cc1-c1nc2n(CC(C)C)c(=O)n(C)c(=O)c2[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C25H36N6O5S/c1-6-14-36-20-9-8-18(37(34,35)30-12-10-29(7-2)11-13-30)15-19(20)22-26-21-23(27-22)31(16-17(3)4)25(33)28(5)24(21)32/h8-9,15,17H,6-7,10-14,16H2,1-5H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory concentration against bovine retina phosphodiesterase 6 activity |
Bioorg Med Chem Lett 12: 2587-90 (2002)
BindingDB Entry DOI: 10.7270/Q2KW5FC2 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50117734
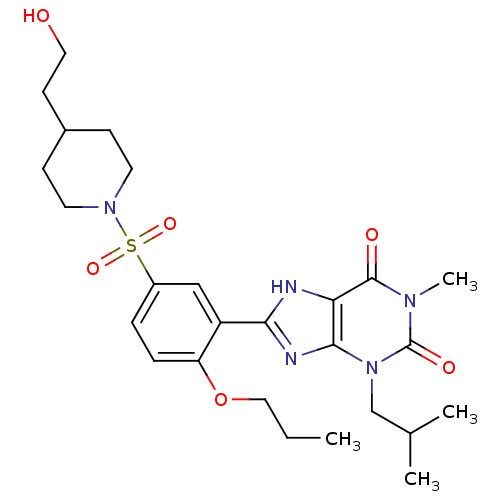
(8-{5-[4-(2-Hydroxy-ethyl)-piperidine-1-sulfonyl]-2...)Show SMILES CCCOc1ccc(cc1-c1nc2n(CC(C)C)c(=O)n(C)c(=O)c2[nH]1)S(=O)(=O)N1CCC(CCO)CC1 Show InChI InChI=1S/C26H37N5O6S/c1-5-14-37-21-7-6-19(38(35,36)30-11-8-18(9-12-30)10-13-32)15-20(21)23-27-22-24(28-23)31(16-17(2)3)26(34)29(4)25(22)33/h6-7,15,17-18,32H,5,8-14,16H2,1-4H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against human platelet Phosphodiesterase 5 (PDE5) |
Bioorg Med Chem Lett 12: 2587-90 (2002)
BindingDB Entry DOI: 10.7270/Q2KW5FC2 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187693

(CHEMBL3186509)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCN(CCC(=O)c3ccc4[nH]c(=O)oc4c3)CC2)c1 Show InChI InChI=1S/C22H21Cl2N3O5/c23-16-9-14(10-17(24)12-16)13-31-22(30)27-7-5-26(6-8-27)4-3-19(28)15-1-2-18-20(11-15)32-21(29)25-18/h1-2,9-12H,3-8,13H2,(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human full length ATX expressed in HEK cells using FS-3 as substrate incubated for 15 mins followed by substrate addition measured afte... |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285751
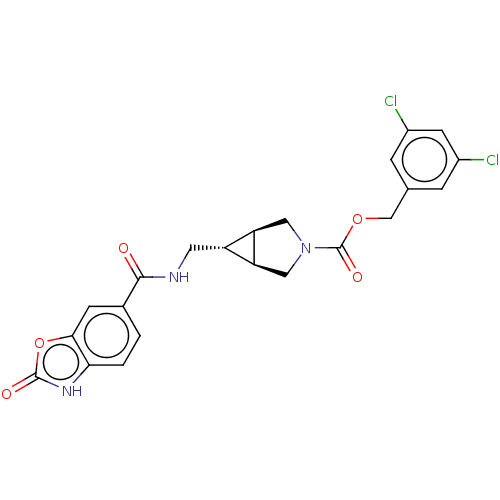
(CHEMBL4169912)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]c(=O)oc2c1)C(=O)OCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C22H19Cl2N3O5/c23-13-3-11(4-14(24)6-13)10-31-22(30)27-8-16-15(17(16)9-27)7-25-20(28)12-1-2-18-19(5-12)32-21(29)26-18/h1-6,15-17H,7-10H2,(H,25,28)(H,26,29)/t15-,16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285777

(CHEMBL4165749)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]nnc2c1)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H19F6N5O3/c24-22(25,26)13-3-11(4-14(6-13)23(27,28)29)10-37-21(36)34-8-16-15(17(16)9-34)7-30-20(35)12-1-2-18-19(5-12)32-33-31-18/h1-6,15-17H,7-10H2,(H,30,35)(H,31,32,33)/t15-,16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50117719

(4-[3-(3-Isobutyl-1-methyl-2,6-dioxo-2,3,6,7-tetrah...)Show SMILES CCCOc1ccc(cc1-c1nc2n(CC(C)C)c(=O)n(C)c(=O)c2[nH]1)S(=O)(=O)N1CCN(CC1)C(=O)N(C)C Show InChI InChI=1S/C26H37N7O6S/c1-7-14-39-20-9-8-18(40(37,38)32-12-10-31(11-13-32)25(35)29(4)5)15-19(20)22-27-21-23(28-22)33(16-17(2)3)26(36)30(6)24(21)34/h8-9,15,17H,7,10-14,16H2,1-6H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against human platelet Phosphodiesterase 5 (PDE5) |
Bioorg Med Chem Lett 12: 2587-90 (2002)
BindingDB Entry DOI: 10.7270/Q2KW5FC2 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
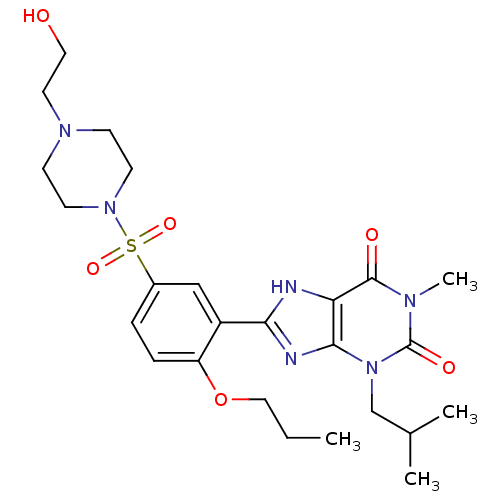
(Homo sapiens (Human)) | BDBM50117711
(8-{5-[4-(2-Hydroxy-ethyl)-piperazine-1-sulfonyl]-2...)Show SMILES CCCOc1ccc(cc1-c1nc2n(CC(C)C)c(=O)n(C)c(=O)c2[nH]1)S(=O)(=O)N1CCN(CCO)CC1 Show InChI InChI=1S/C25H36N6O6S/c1-5-14-37-20-7-6-18(38(35,36)30-10-8-29(9-11-30)12-13-32)15-19(20)22-26-21-23(27-22)31(16-17(2)3)25(34)28(4)24(21)33/h6-7,15,17,32H,5,8-14,16H2,1-4H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against human platelet Phosphodiesterase 5 (PDE5) |
Bioorg Med Chem Lett 12: 2587-90 (2002)
BindingDB Entry DOI: 10.7270/Q2KW5FC2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50574856
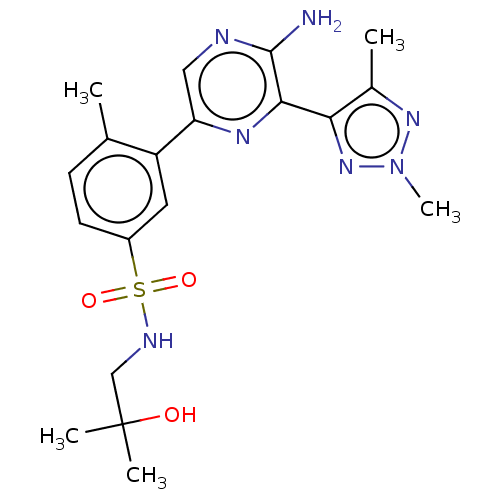
(CHEMBL4864109)Show SMILES Cc1nn(C)nc1-c1nc(cnc1N)-c1cc(ccc1C)S(=O)(=O)NCC(C)(C)O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00986
BindingDB Entry DOI: 10.7270/Q27M0CRD |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285774

(CHEMBL4169550)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]c(=O)oc2c1)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C24H19F6N3O5/c25-23(26,27)13-3-11(4-14(6-13)24(28,29)30)10-37-22(36)33-8-16-15(17(16)9-33)7-31-20(34)12-1-2-18-19(5-12)38-21(35)32-18/h1-6,15-17H,7-10H2,(H,31,34)(H,32,35)/t15-,16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of ATX in rat whole blood using LPA 17:0 as substrate after 1 hr by LC-MS/MS analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285773
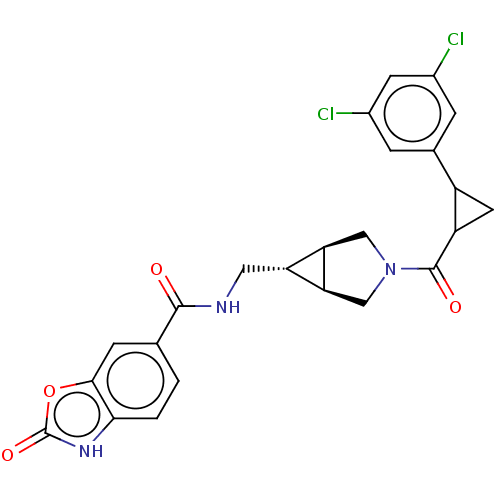
(CHEMBL4170966)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]c(=O)oc2c1)C(=O)C1CC1c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C24H21Cl2N3O4/c25-13-3-12(4-14(26)6-13)15-7-16(15)23(31)29-9-18-17(19(18)10-29)8-27-22(30)11-1-2-20-21(5-11)33-24(32)28-20/h1-6,15-19H,7-10H2,(H,27,30)(H,28,32)/t15?,16?,17-,18-,19+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50117724
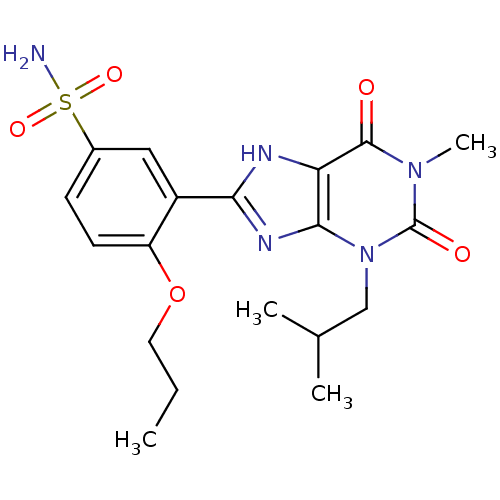
(3-(3-Isobutyl-1-methyl-2,6-dioxo-2,3,6,7-tetrahydr...)Show SMILES CCCOc1ccc(cc1-c1nc2n(CC(C)C)c(=O)n(C)c(=O)c2[nH]1)S(N)(=O)=O Show InChI InChI=1S/C19H25N5O5S/c1-5-8-29-14-7-6-12(30(20,27)28)9-13(14)16-21-15-17(22-16)24(10-11(2)3)19(26)23(4)18(15)25/h6-7,9,11H,5,8,10H2,1-4H3,(H,21,22)(H2,20,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against human platelet Phosphodiesterase 5 (PDE5) |
Bioorg Med Chem Lett 12: 2587-90 (2002)
BindingDB Entry DOI: 10.7270/Q2KW5FC2 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50207121
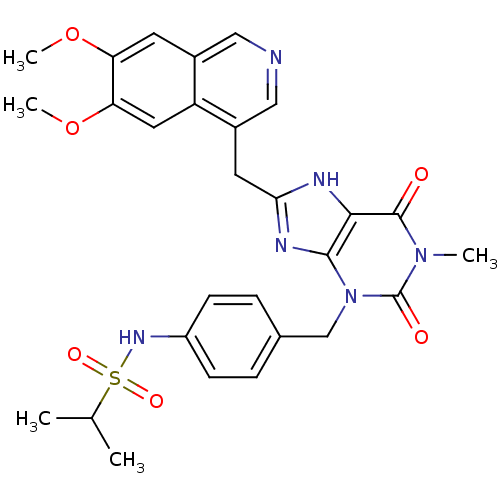
(CHEMBL245648 | N-(4-((8-((6,7-dimethoxyisoquinolin...)Show SMILES COc1cc2cncc(Cc3nc4n(Cc5ccc(NS(=O)(=O)C(C)C)cc5)c(=O)n(C)c(=O)c4[nH]3)c2cc1OC Show InChI InChI=1S/C28H30N6O6S/c1-16(2)41(37,38)32-20-8-6-17(7-9-20)15-34-26-25(27(35)33(3)28(34)36)30-24(31-26)11-19-14-29-13-18-10-22(39-4)23(40-5)12-21(18)19/h6-10,12-14,16,32H,11,15H2,1-5H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human platelet PDE5 by [3H]cGMP scintillation proximity assay |
Bioorg Med Chem Lett 17: 2376-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.019
BindingDB Entry DOI: 10.7270/Q2X63NSC |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50117721

(3-Isobutyl-1-methyl-8-[5-(piperazin-1-ylmethanesul...)Show SMILES CCCOc1ccc(cc1-c1nc2n(CC(C)C)c(=O)n(C)c(=O)c2[nH]1)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C25H36N6O5S/c1-6-14-36-20-9-8-18(37(34,35)30-12-10-29(7-2)11-13-30)15-19(20)22-26-21-23(27-22)31(16-17(3)4)25(33)28(5)24(21)32/h8-9,15,17H,6-7,10-14,16H2,1-5H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against human platelet Phosphodiesterase 5 (PDE5) |
Bioorg Med Chem Lett 12: 2587-90 (2002)
BindingDB Entry DOI: 10.7270/Q2KW5FC2 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285743
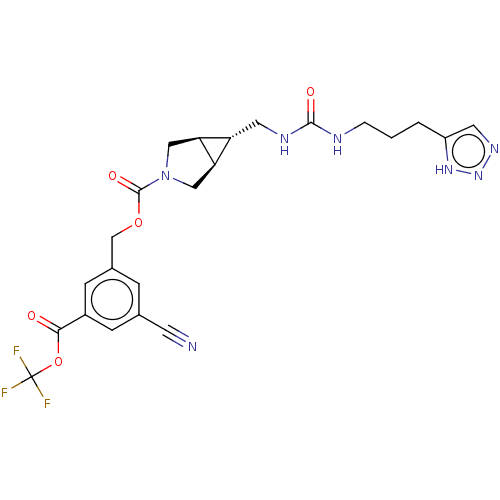
(CHEMBL4169136)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)NCCCc1cnn[nH]1)C(=O)OCc1cc(cc(c1)C(=O)OC(F)(F)F)C#N |r| Show InChI InChI=1S/C23H24F3N7O5/c24-23(25,26)38-20(34)15-5-13(7-27)4-14(6-15)12-37-22(36)33-10-18-17(19(18)11-33)9-29-21(35)28-3-1-2-16-8-30-32-31-16/h4-6,8,17-19H,1-3,9-12H2,(H2,28,29,35)(H,30,31,32)/t17-,18-,19+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
P04972/P11541/P16586/P22571/P23439/Q95142
(Bos taurus-Bos taurus (Bovine)) | BDBM50117715

(3-Isobutyl-1-methyl-8-[5-(4-methyl-piperazine-1-su...)Show SMILES CCCOc1ccc(cc1-c1nc2n(CC(C)C)c(=O)n(C)c(=O)c2[nH]1)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C24H34N6O5S/c1-6-13-35-19-8-7-17(36(33,34)29-11-9-27(4)10-12-29)14-18(19)21-25-20-22(26-21)30(15-16(2)3)24(32)28(5)23(20)31/h7-8,14,16H,6,9-13,15H2,1-5H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory concentration against bovine retina phosphodiesterase 6 activity |
Bioorg Med Chem Lett 12: 2587-90 (2002)
BindingDB Entry DOI: 10.7270/Q2KW5FC2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50574850
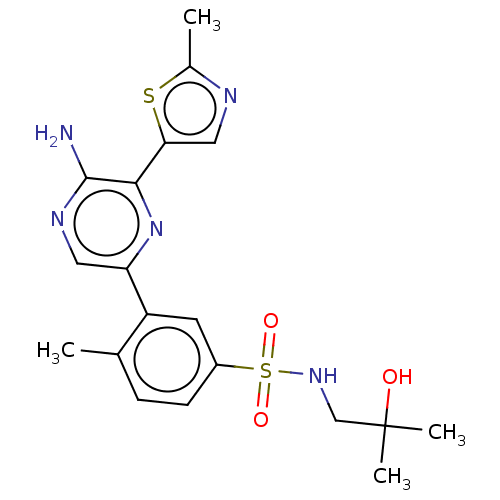
(CHEMBL4857826)Show SMILES Cc1ncc(s1)-c1nc(cnc1N)-c1cc(ccc1C)S(=O)(=O)NCC(C)(C)O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00986
BindingDB Entry DOI: 10.7270/Q27M0CRD |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50207123
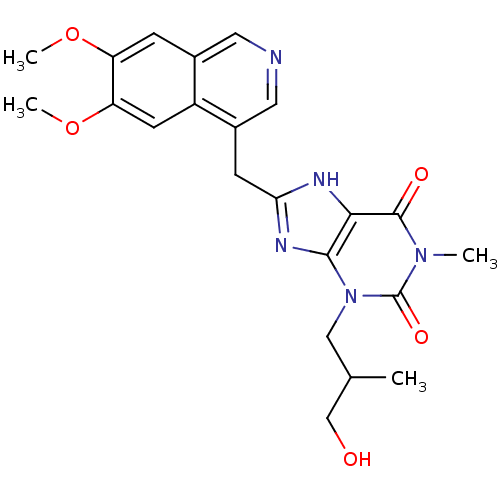
(8-((6,7-dimethoxyisoquinolin-4-yl)methyl)-3-(3-hyd...)Show SMILES COc1cc2cncc(Cc3nc4n(CC(C)CO)c(=O)n(C)c(=O)c4[nH]3)c2cc1OC Show InChI InChI=1S/C22H25N5O5/c1-12(11-28)10-27-20-19(21(29)26(2)22(27)30)24-18(25-20)6-14-9-23-8-13-5-16(31-3)17(32-4)7-15(13)14/h5,7-9,12,28H,6,10-11H2,1-4H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human platelet PDE5 by [3H]cGMP scintillation proximity assay |
Bioorg Med Chem Lett 17: 2376-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.019
BindingDB Entry DOI: 10.7270/Q2X63NSC |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285775
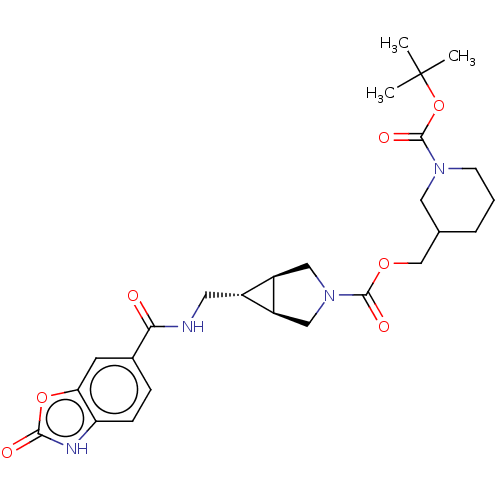
(CHEMBL4159308)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]c(=O)oc2c1)C(=O)OCC1CCCN(C1)C(=O)OC(C)(C)C |r| Show InChI InChI=1S/C26H34N4O7/c1-26(2,3)37-25(34)29-8-4-5-15(11-29)14-35-24(33)30-12-18-17(19(18)13-30)10-27-22(31)16-6-7-20-21(9-16)36-23(32)28-20/h6-7,9,15,17-19H,4-5,8,10-14H2,1-3H3,(H,27,31)(H,28,32)/t15?,17-,18-,19+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of ATX in rat whole blood using LPA 17:0 as substrate after 1 hr by LC-MS/MS analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50574845
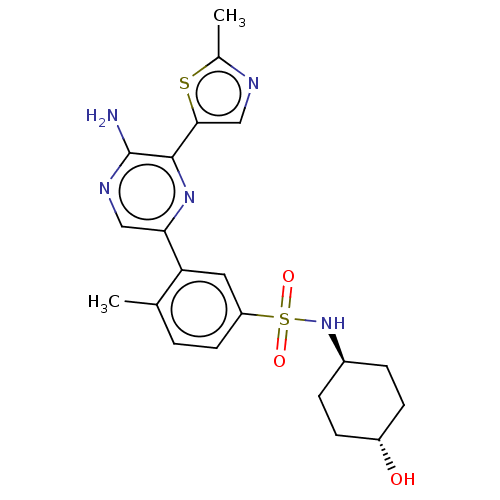
(CHEMBL4866794)Show SMILES Cc1ncc(s1)-c1nc(cnc1N)-c1cc(ccc1C)S(=O)(=O)N[C@H]1CC[C@H](O)CC1 |r,wU:27.30,wD:24.26,(31.47,-5.01,;30,-5.48,;29.52,-6.95,;27.98,-6.95,;27.51,-5.48,;28.75,-4.58,;25.73,-4.57,;24.4,-5.34,;23.07,-4.56,;23.06,-3.03,;24.39,-2.26,;25.73,-3.03,;27.06,-2.25,;21.74,-5.34,;21.74,-6.87,;20.41,-7.64,;19.07,-6.88,;19.07,-5.35,;20.4,-4.57,;20.4,-3.03,;20.41,-9.18,;19.63,-10.51,;18.87,-9.17,;21.75,-9.95,;23.08,-9.18,;24.41,-9.94,;25.74,-9.18,;25.74,-7.64,;27.07,-6.87,;24.41,-6.86,;23.08,-7.64,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kgamma in human U937 cells assessed as reduction in AKT phosphorylation preincubated for 30 mins followed by MIP1alpha stimulation f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00986
BindingDB Entry DOI: 10.7270/Q27M0CRD |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285748

(CHEMBL4173341)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]c(=O)oc2c1)C(=O)CCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C23H21Cl2N3O4/c24-14-5-12(6-15(25)8-14)1-4-21(29)28-10-17-16(18(17)11-28)9-26-22(30)13-2-3-19-20(7-13)32-23(31)27-19/h2-3,5-8,16-18H,1,4,9-11H2,(H,26,30)(H,27,31)/t16-,17-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of ATX in rat whole blood using LPA 17:0 as substrate after 1 hr by LC-MS/MS analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50207127
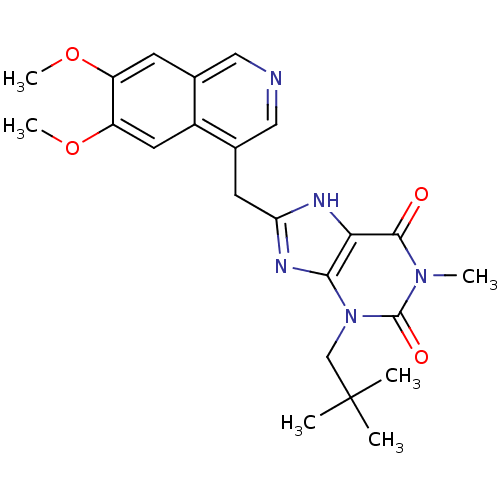
(8-((6,7-dimethoxyisoquinolin-4-yl)methyl)-1-methyl...)Show SMILES COc1cc2cncc(Cc3nc4n(CC(C)(C)C)c(=O)n(C)c(=O)c4[nH]3)c2cc1OC Show InChI InChI=1S/C23H27N5O4/c1-23(2,3)12-28-20-19(21(29)27(4)22(28)30)25-18(26-20)8-14-11-24-10-13-7-16(31-5)17(32-6)9-15(13)14/h7,9-11H,8,12H2,1-6H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human platelet PDE5 by [3H]cGMP scintillation proximity assay |
Bioorg Med Chem Lett 17: 2376-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.019
BindingDB Entry DOI: 10.7270/Q2X63NSC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50574848
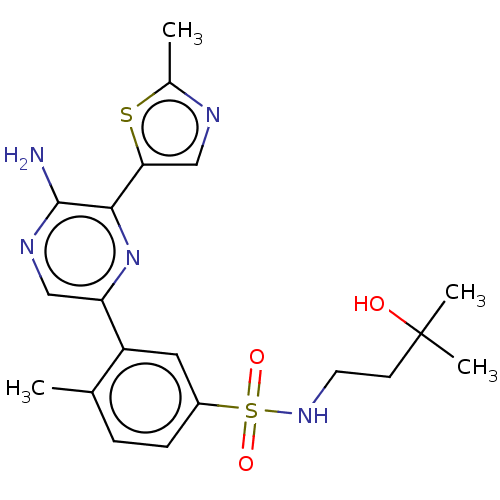
(CHEMBL4850655)Show SMILES Cc1ncc(s1)-c1nc(cnc1N)-c1cc(ccc1C)S(=O)(=O)NCCC(C)(C)O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00986
BindingDB Entry DOI: 10.7270/Q27M0CRD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50574856

(CHEMBL4864109)Show SMILES Cc1nn(C)nc1-c1nc(cnc1N)-c1cc(ccc1C)S(=O)(=O)NCC(C)(C)O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kgamma in human U937 cells assessed as reduction in AKT phosphorylation preincubated for 30 mins followed by MIP1alpha stimulation f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00986
BindingDB Entry DOI: 10.7270/Q27M0CRD |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50207132
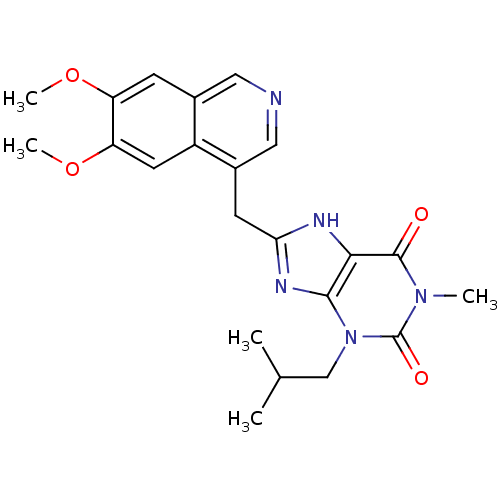
(8-((6,7-dimethoxyisoquinolin-4-yl)methyl)-3-isobut...)Show SMILES COc1cc2cncc(Cc3nc4n(CC(C)C)c(=O)n(C)c(=O)c4[nH]3)c2cc1OC Show InChI InChI=1S/C22H25N5O4/c1-12(2)11-27-20-19(21(28)26(3)22(27)29)24-18(25-20)7-14-10-23-9-13-6-16(30-4)17(31-5)8-15(13)14/h6,8-10,12H,7,11H2,1-5H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human platelet PDE5 by [3H]cGMP scintillation proximity assay |
Bioorg Med Chem Lett 17: 2376-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.019
BindingDB Entry DOI: 10.7270/Q2X63NSC |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50117707
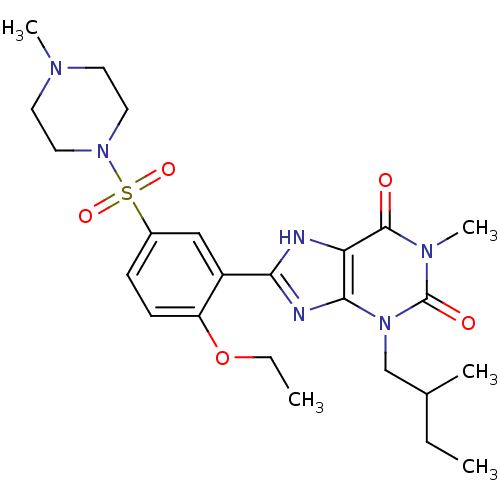
(8-[2-Ethoxy-5-(4-methyl-piperazine-1-sulfonyl)-phe...)Show SMILES CCOc1ccc(cc1-c1nc2n(CC(C)CC)c(=O)n(C)c(=O)c2[nH]1)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C24H34N6O5S/c1-6-16(3)15-30-22-20(23(31)28(5)24(30)32)25-21(26-22)18-14-17(8-9-19(18)35-7-2)36(33,34)29-12-10-27(4)11-13-29/h8-9,14,16H,6-7,10-13,15H2,1-5H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against human platelet Phosphodiesterase 5 (PDE5) |
Bioorg Med Chem Lett 12: 2587-90 (2002)
BindingDB Entry DOI: 10.7270/Q2KW5FC2 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285778
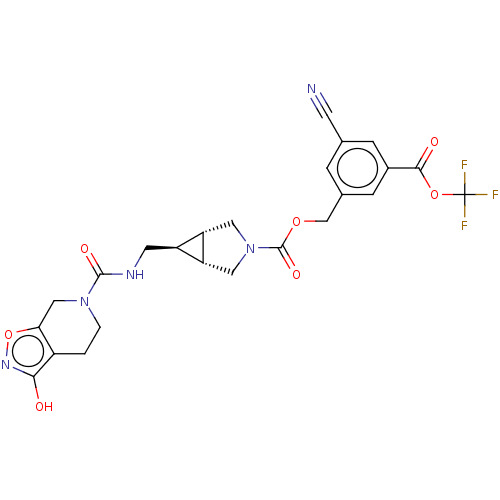
(CHEMBL4164935)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)N1CCc2c(O)noc2C1)C(=O)OCc1cc(cc(c1)C(=O)OC(F)(F)F)C#N |r| Show InChI InChI=1S/C24H22F3N5O7/c25-24(26,27)38-21(34)14-4-12(6-28)3-13(5-14)11-37-23(36)32-8-17-16(18(17)9-32)7-29-22(35)31-2-1-15-19(10-31)39-30-20(15)33/h3-5,16-18H,1-2,7-11H2,(H,29,35)(H,30,33)/t16-,17-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50117715

(3-Isobutyl-1-methyl-8-[5-(4-methyl-piperazine-1-su...)Show SMILES CCCOc1ccc(cc1-c1nc2n(CC(C)C)c(=O)n(C)c(=O)c2[nH]1)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C24H34N6O5S/c1-6-13-35-19-8-7-17(36(33,34)29-11-9-27(4)10-12-29)14-18(19)21-25-20-22(26-21)30(15-16(2)3)24(32)28(5)23(20)31/h7-8,14,16H,6,9-13,15H2,1-5H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against human platelet Phosphodiesterase 5 (PDE5) |
Bioorg Med Chem Lett 12: 2587-90 (2002)
BindingDB Entry DOI: 10.7270/Q2KW5FC2 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50117715

(3-Isobutyl-1-methyl-8-[5-(4-methyl-piperazine-1-su...)Show SMILES CCCOc1ccc(cc1-c1nc2n(CC(C)C)c(=O)n(C)c(=O)c2[nH]1)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C24H34N6O5S/c1-6-13-35-19-8-7-17(36(33,34)29-11-9-27(4)10-12-29)14-18(19)21-25-20-22(26-21)30(15-16(2)3)24(32)28(5)23(20)31/h7-8,14,16H,6,9-13,15H2,1-5H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against human platelet Phosphodiesterase 5 (PDE5) |
Bioorg Med Chem Lett 12: 2587-90 (2002)
BindingDB Entry DOI: 10.7270/Q2KW5FC2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50395821
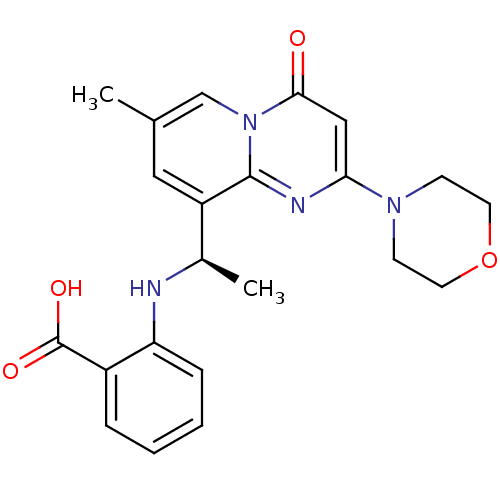
(CHEMBL2165191)Show SMILES C[C@@H](Nc1ccccc1C(O)=O)c1cc(C)cn2c1nc(cc2=O)N1CCOCC1 |r| Show InChI InChI=1S/C22H24N4O4/c1-14-11-17(15(2)23-18-6-4-3-5-16(18)22(28)29)21-24-19(12-20(27)26(21)13-14)25-7-9-30-10-8-25/h3-6,11-13,15,23H,7-10H2,1-2H3,(H,28,29)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kbeta assessed as reduction in PIP3 formation by AlphaScreen assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00986
BindingDB Entry DOI: 10.7270/Q27M0CRD |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50117714

(CHEMBL90164 | N-(2-Dimethylamino-ethyl)-N-ethyl-3-...)Show SMILES CCCOc1ccc(cc1-c1nc2n(CC(C)C)c(=O)n(C)c(=O)c2[nH]1)S(=O)(=O)N(CC)CCN(C)C Show InChI InChI=1S/C25H38N6O5S/c1-8-14-36-20-11-10-18(37(34,35)30(9-2)13-12-28(5)6)15-19(20)22-26-21-23(27-22)31(16-17(3)4)25(33)29(7)24(21)32/h10-11,15,17H,8-9,12-14,16H2,1-7H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against human platelet Phosphodiesterase 5 (PDE5) |
Bioorg Med Chem Lett 12: 2587-90 (2002)
BindingDB Entry DOI: 10.7270/Q2KW5FC2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM295061
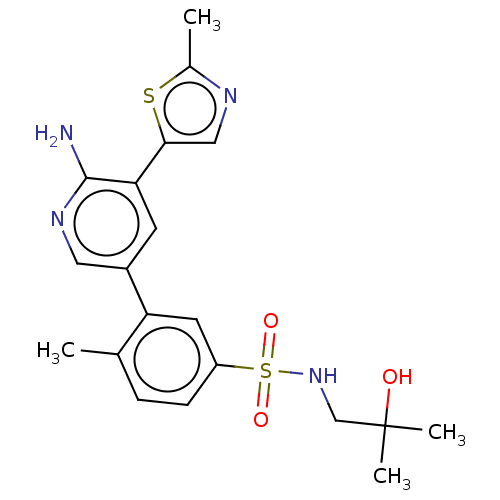
(3-[6-Amino-5-(2-methyl-thiazol-5-yl)-pyridin-3-yl]...)Show SMILES Cc1ncc(s1)-c1cc(cnc1N)-c1cc(ccc1C)S(=O)(=O)NCC(C)(C)O Show InChI InChI=1S/C20H24N4O3S2/c1-12-5-6-15(29(26,27)24-11-20(3,4)25)8-16(12)14-7-17(19(21)23-9-14)18-10-22-13(2)28-18/h5-10,24-25H,11H2,1-4H3,(H2,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00986
BindingDB Entry DOI: 10.7270/Q27M0CRD |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50117729

(3-Isobutyl-8-{5-[4-(2-methoxy-ethyl)-piperazine-1-...)Show SMILES CCCOc1ccc(cc1-c1nc2n(CC(C)C)c(=O)n(C)c(=O)c2[nH]1)S(=O)(=O)N1CCN(CCOC)CC1 Show InChI InChI=1S/C26H38N6O6S/c1-6-14-38-21-8-7-19(39(35,36)31-11-9-30(10-12-31)13-15-37-5)16-20(21)23-27-22-24(28-23)32(17-18(2)3)26(34)29(4)25(22)33/h7-8,16,18H,6,9-15,17H2,1-5H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory activity against human platelet Phosphodiesterase 5 (PDE5) |
Bioorg Med Chem Lett 12: 2587-90 (2002)
BindingDB Entry DOI: 10.7270/Q2KW5FC2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50574847
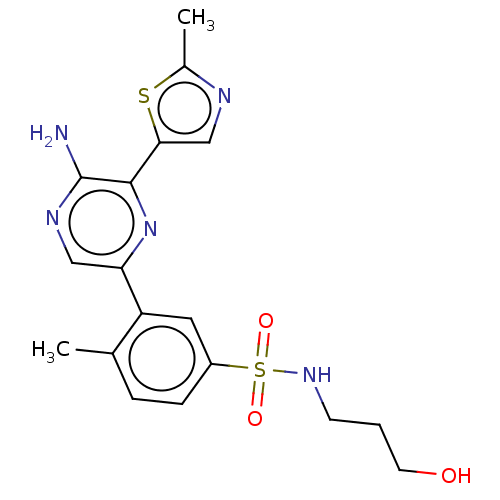
(CHEMBL4855904)Show SMILES Cc1ncc(s1)-c1nc(cnc1N)-c1cc(ccc1C)S(=O)(=O)NCCCO | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00986
BindingDB Entry DOI: 10.7270/Q27M0CRD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50574845

(CHEMBL4866794)Show SMILES Cc1ncc(s1)-c1nc(cnc1N)-c1cc(ccc1C)S(=O)(=O)N[C@H]1CC[C@H](O)CC1 |r,wU:27.30,wD:24.26,(31.47,-5.01,;30,-5.48,;29.52,-6.95,;27.98,-6.95,;27.51,-5.48,;28.75,-4.58,;25.73,-4.57,;24.4,-5.34,;23.07,-4.56,;23.06,-3.03,;24.39,-2.26,;25.73,-3.03,;27.06,-2.25,;21.74,-5.34,;21.74,-6.87,;20.41,-7.64,;19.07,-6.88,;19.07,-5.35,;20.4,-4.57,;20.4,-3.03,;20.41,-9.18,;19.63,-10.51,;18.87,-9.17,;21.75,-9.95,;23.08,-9.18,;24.41,-9.94,;25.74,-9.18,;25.74,-7.64,;27.07,-6.87,;24.41,-6.86,;23.08,-7.64,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00986
BindingDB Entry DOI: 10.7270/Q27M0CRD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50574849
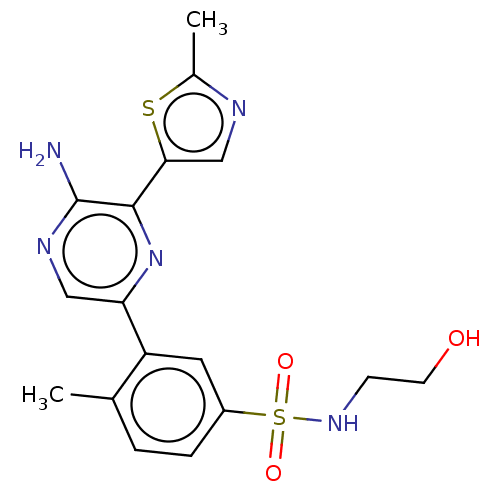
(CHEMBL4863328)Show SMILES Cc1ncc(s1)-c1nc(cnc1N)-c1cc(ccc1C)S(=O)(=O)NCCO | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00986
BindingDB Entry DOI: 10.7270/Q27M0CRD |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285742

(CHEMBL4163050)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]c(=O)oc2c1)C(=O)NCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C22H20Cl2N4O4/c23-13-3-11(4-14(24)6-13)7-26-21(30)28-9-16-15(17(16)10-28)8-25-20(29)12-1-2-18-19(5-12)32-22(31)27-18/h1-6,15-17H,7-10H2,(H,25,29)(H,26,30)(H,27,31)/t15-,16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
P04972/P11541/P16586/P22571/P23439/Q95142
(Bos taurus-Bos taurus (Bovine)) | BDBM50117719

(4-[3-(3-Isobutyl-1-methyl-2,6-dioxo-2,3,6,7-tetrah...)Show SMILES CCCOc1ccc(cc1-c1nc2n(CC(C)C)c(=O)n(C)c(=O)c2[nH]1)S(=O)(=O)N1CCN(CC1)C(=O)N(C)C Show InChI InChI=1S/C26H37N7O6S/c1-7-14-39-20-9-8-18(40(37,38)32-12-10-31(11-13-32)25(35)29(4)5)15-19(20)22-27-21-23(28-22)33(16-17(2)3)26(36)30(6)24(21)34/h8-9,15,17H,7,10-14,16H2,1-6H3,(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory concentration against bovine retina phosphodiesterase 6 activity |
Bioorg Med Chem Lett 12: 2587-90 (2002)
BindingDB Entry DOI: 10.7270/Q2KW5FC2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50574854
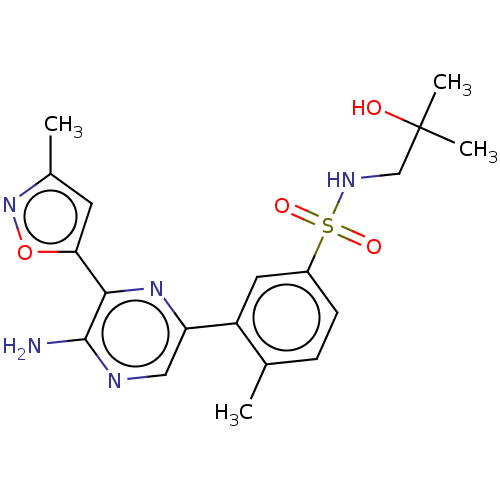
(CHEMBL4849042)Show SMILES Cc1cc(on1)-c1nc(cnc1N)-c1cc(ccc1C)S(=O)(=O)NCC(C)(C)O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00986
BindingDB Entry DOI: 10.7270/Q27M0CRD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50574860

(CHEMBL4859017)Show SMILES Cc1ccc(cc1-c1cnc(N)c(n1)C(=O)C1CC1)S(=O)(=O)N[C@H]1CC[C@H](O)CC1 |r,wU:23.25,wD:26.29,(8.47,-3.48,;7.14,-4.25,;5.8,-3.49,;4.47,-4.26,;4.48,-5.79,;5.81,-6.57,;7.15,-5.79,;8.48,-6.56,;8.48,-8.1,;9.81,-8.87,;11.14,-8.09,;12.48,-8.86,;11.14,-6.54,;9.8,-5.78,;12.46,-5.77,;12.46,-4.23,;13.8,-6.53,;14.57,-7.86,;15.34,-6.52,;3.14,-6.56,;1.6,-6.56,;2.38,-5.23,;3.15,-8.11,;4.49,-8.87,;5.82,-8.1,;7.15,-8.87,;7.15,-10.41,;8.48,-11.19,;5.82,-11.18,;4.49,-10.41,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kgamma in human U937 cells assessed as reduction in AKT phosphorylation preincubated for 30 mins followed by MIP1alpha stimulation f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00986
BindingDB Entry DOI: 10.7270/Q27M0CRD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data