

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227831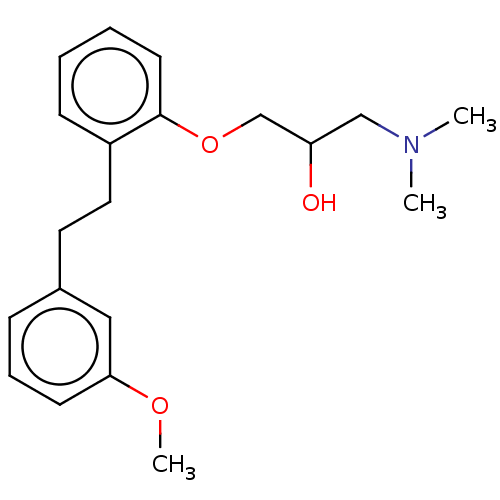 (CHEMBL52242) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227791 (CHEMBL53815) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227978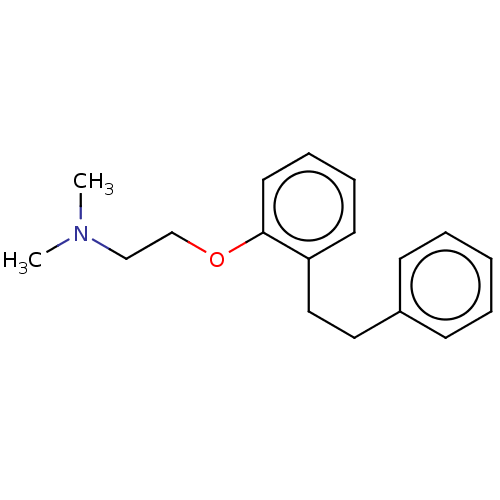 (CHEMBL53316) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50228190 (CHEMBL54263) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227787 (CHEMBL1743933) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227837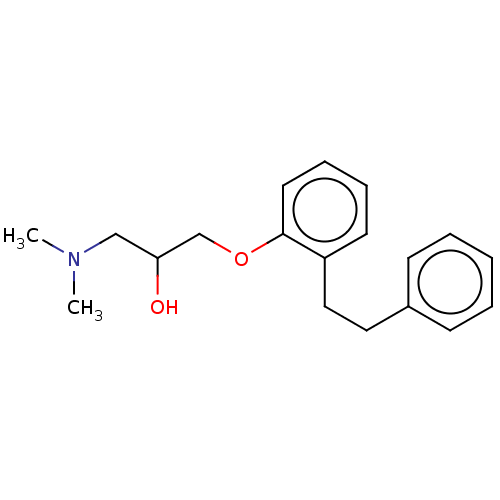 (CHEMBL52131) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50093789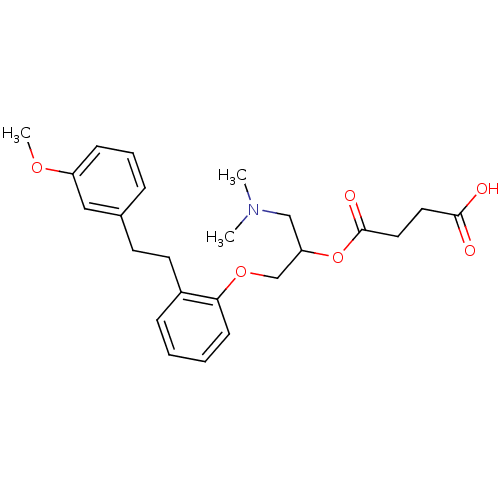 (CHEMBL541829 | Succinic acid mono-(2-dimethylamino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227788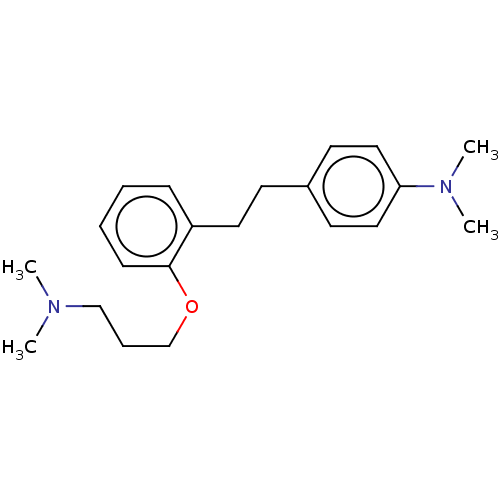 (CHEMBL1743936) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227974 (CHEMBL298554) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227976 (CHEMBL299389) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227653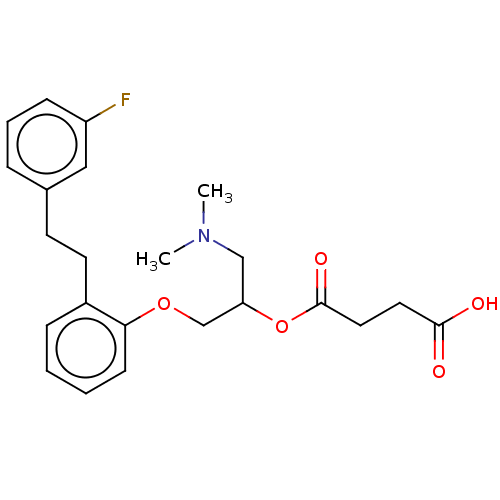 (CHEMBL299207) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227977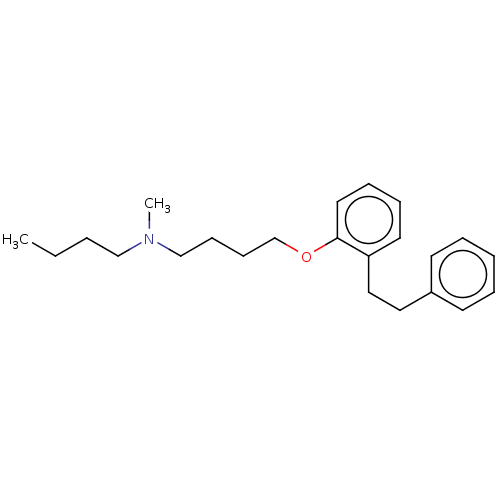 (CHEMBL300631) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227790 (CHEMBL53344) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227789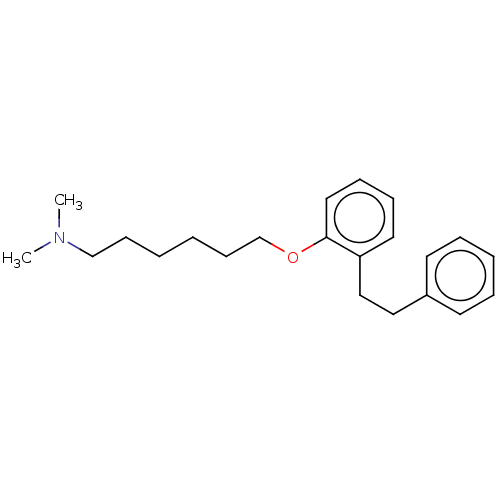 (CHEMBL412653) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50330955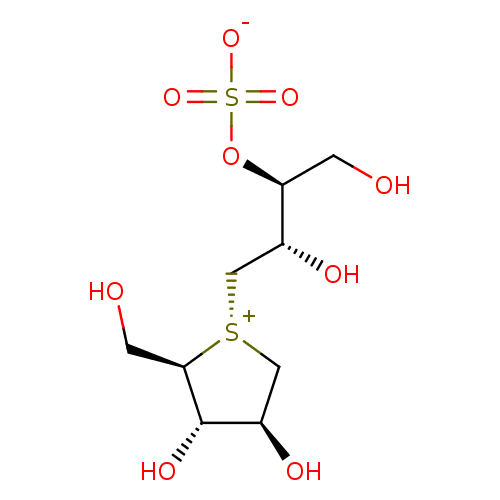 ((1S,2S)-3-[(2R,3S,4S)-3,4-dihydroxy-2-(hydroxymeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human maltase-glucoamylase using p-nitrophenyl-alpha-D-glucopyranoside as substrate incubated for 35 mins by microtiter pla... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127751 BindingDB Entry DOI: 10.7270/Q22B92NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227832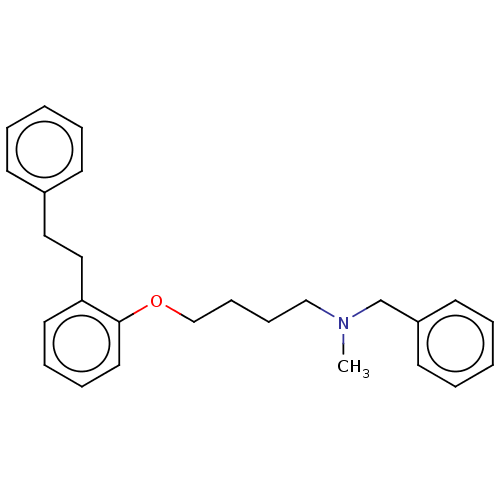 (CHEMBL54101) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 198 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50228189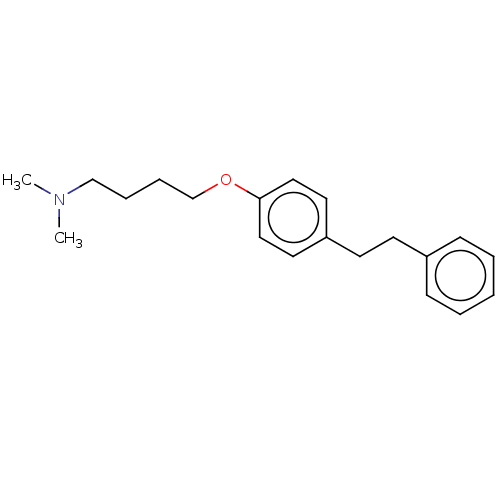 (CHEMBL52710) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 416 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50228052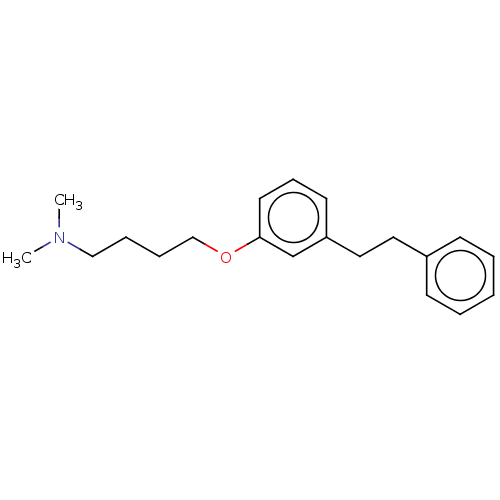 (CHEMBL52982) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 517 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Inhibition of 5-hydroxytryptamine 2 induced vasoconstriction of rat caudal artery. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50549692 (CHEMBL4749306) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human maltase-glucoamylase using p-nitrophenyl-alpha-D-glucopyranoside as substrate incubated for 35 mins by microtiter pla... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127751 BindingDB Entry DOI: 10.7270/Q22B92NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50549691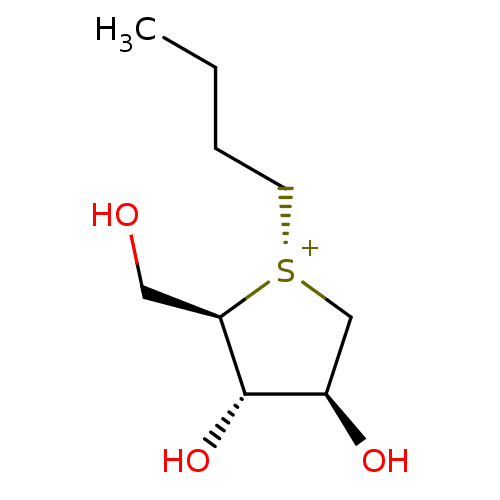 (CHEMBL4754034) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human maltase-glucoamylase using p-nitrophenyl-alpha-D-glucopyranoside as substrate incubated for 35 mins by microtiter pla... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127751 BindingDB Entry DOI: 10.7270/Q22B92NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50549693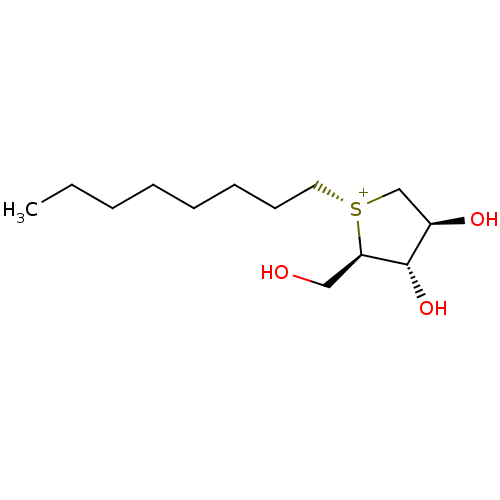 (CHEMBL4750690) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human maltase-glucoamylase using p-nitrophenyl-alpha-D-glucopyranoside as substrate incubated for 35 mins by microtiter pla... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127751 BindingDB Entry DOI: 10.7270/Q22B92NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227831 (CHEMBL52242) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Antagonistic activity against 5-hydroxytryptamine 2 receptor on rat frontal cortex membrane. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227791 (CHEMBL53815) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Antagonistic activity against 5-hydroxytryptamine 2 receptor on rat frontal cortex membrane. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50180584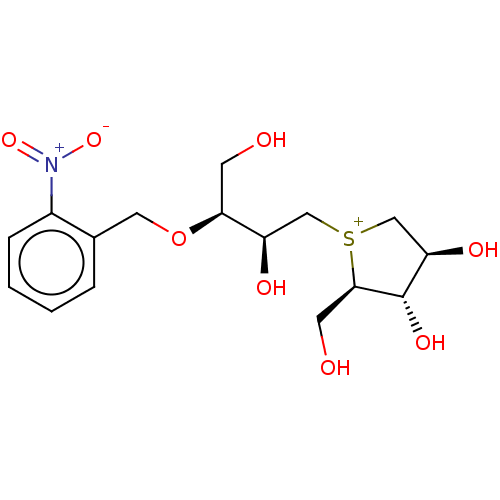 (CHEMBL3814496) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University Curated by ChEMBL | Assay Description Inhibition of rat small intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase method | Bioorg Med Chem 24: 3705-15 (2016) Article DOI: 10.1016/j.bmc.2016.06.013 BindingDB Entry DOI: 10.7270/Q241700V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50093789 (CHEMBL541829 | Succinic acid mono-(2-dimethylamino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Antagonistic activity against 5-hydroxytryptamine 2 receptor on rat frontal cortex membrane. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227788 (CHEMBL1743936) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Antagonistic activity against 5-hydroxytryptamine 2 receptor on rat frontal cortex membrane. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227837 (CHEMBL52131) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Antagonistic activity against 5-hydroxytryptamine 2 receptor on rat frontal cortex membrane. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227787 (CHEMBL1743933) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Antagonistic activity against 5-hydroxytryptamine 2 receptor on rat frontal cortex membrane. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Rattus norvegicus) | BDBM50049730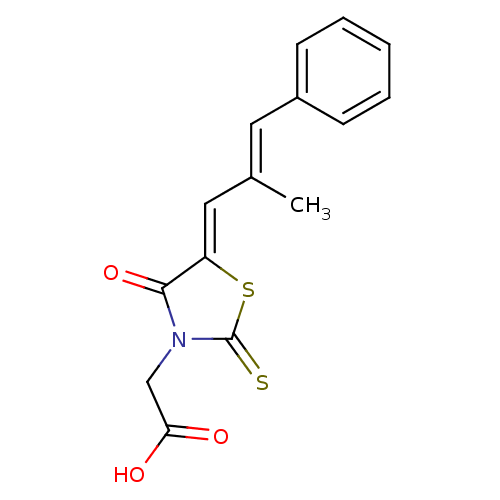 (2-(5-(2-methyl-3-phenylallylidene)-4-oxo-2-thioxot...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of rat lens aldose reductase using DL-glyceraldehyde as substrate after 30 mins by fluorescence microplate reader analysis | Bioorg Med Chem 20: 832-40 (2012) Article DOI: 10.1016/j.bmc.2011.11.067 BindingDB Entry DOI: 10.7270/Q20K291D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50180583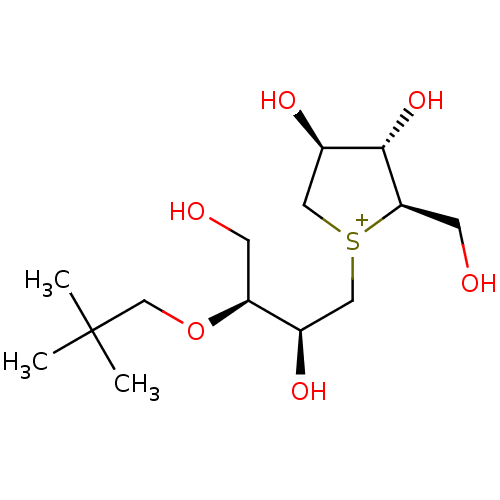 (CHEMBL3815109) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University Curated by ChEMBL | Assay Description Inhibition of rat small intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase method | Bioorg Med Chem 24: 3705-15 (2016) Article DOI: 10.1016/j.bmc.2016.06.013 BindingDB Entry DOI: 10.7270/Q241700V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50180582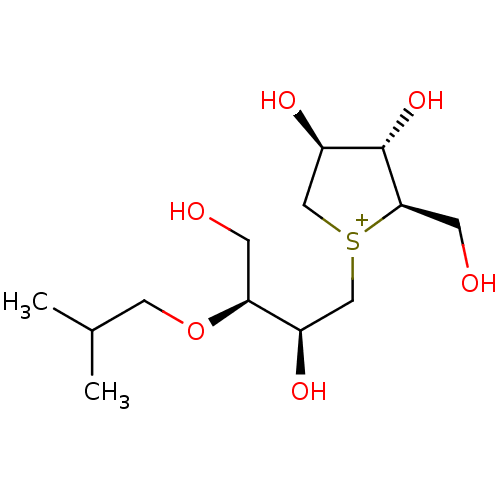 (CHEMBL3814988) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University Curated by ChEMBL | Assay Description Inhibition of rat small intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase method | Bioorg Med Chem 24: 3705-15 (2016) Article DOI: 10.1016/j.bmc.2016.06.013 BindingDB Entry DOI: 10.7270/Q241700V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227978 (CHEMBL53316) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Antagonistic activity against 5-hydroxytryptamine 2 receptor on rat frontal cortex membrane. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50228190 (CHEMBL54263) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Antagonistic activity against 5-hydroxytryptamine 2 receptor on rat frontal cortex membrane. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50180520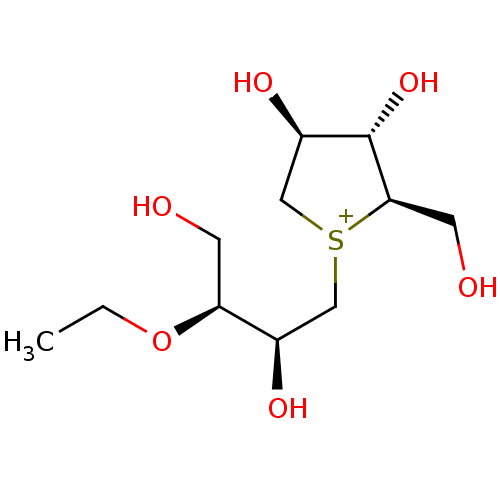 (CHEMBL3814838) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University Curated by ChEMBL | Assay Description Inhibition of rat small intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase method | Bioorg Med Chem 24: 3705-15 (2016) Article DOI: 10.1016/j.bmc.2016.06.013 BindingDB Entry DOI: 10.7270/Q241700V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50342937 ((1S,2R,3S,4S)-1-((2S,3S)-3-ethoxy-2,4-dihydroxybut...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of rat small intestinal sucrase after 30 mins by glucose-oxidase method | Bioorg Med Chem Lett 21: 3159-62 (2011) Article DOI: 10.1016/j.bmcl.2011.02.109 BindingDB Entry DOI: 10.7270/Q2DF6RJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227653 (CHEMBL299207) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Antagonistic activity against 5-hydroxytryptamine 2 receptor on rat frontal cortex membrane. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50342939 ((1S,2R,3S,4S)-1-((2S,3S)-3-(benzyloxy)-2,4-dihydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of rat small intestinal isomaltase after 30 mins by glucose-oxidase method | Bioorg Med Chem Lett 21: 3159-62 (2011) Article DOI: 10.1016/j.bmcl.2011.02.109 BindingDB Entry DOI: 10.7270/Q2DF6RJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50180584 (CHEMBL3814496) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University Curated by ChEMBL | Assay Description Inhibition of human small intestine microsomal maltase using maltose as substrate incubated for 30 mins by glucose-oxidase method | Bioorg Med Chem 24: 3705-15 (2016) Article DOI: 10.1016/j.bmc.2016.06.013 BindingDB Entry DOI: 10.7270/Q241700V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227789 (CHEMBL412653) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Antagonistic activity against 5-hydroxytryptamine 2 receptor on rat frontal cortex membrane. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227790 (CHEMBL53344) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Kasei Corporation Curated by ChEMBL | Assay Description Antagonistic activity against 5-hydroxytryptamine 2 receptor on rat frontal cortex membrane. | J Med Chem 33: 1818-23 (1990) BindingDB Entry DOI: 10.7270/Q2FB5559 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50263044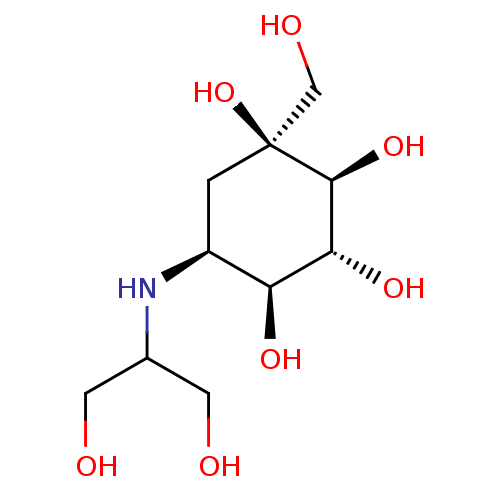 (CHEMBL476960 | Voglibose) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of rat small intestinal sucrase after 30 mins by glucose-oxidase method | Bioorg Med Chem Lett 21: 3159-62 (2011) Article DOI: 10.1016/j.bmcl.2011.02.109 BindingDB Entry DOI: 10.7270/Q2DF6RJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50263044 (CHEMBL476960 | Voglibose) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase | Bioorg Med Chem 19: 2015-22 (2011) Article DOI: 10.1016/j.bmc.2011.01.052 BindingDB Entry DOI: 10.7270/Q2J1044C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50180584 (CHEMBL3814496) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University Curated by ChEMBL | Assay Description Inhibition of rat small intestinal isomaltase using isomaltose as substrate incubated for 30 mins by glucose-oxidase method | Bioorg Med Chem 24: 3705-15 (2016) Article DOI: 10.1016/j.bmc.2016.06.013 BindingDB Entry DOI: 10.7270/Q241700V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50180580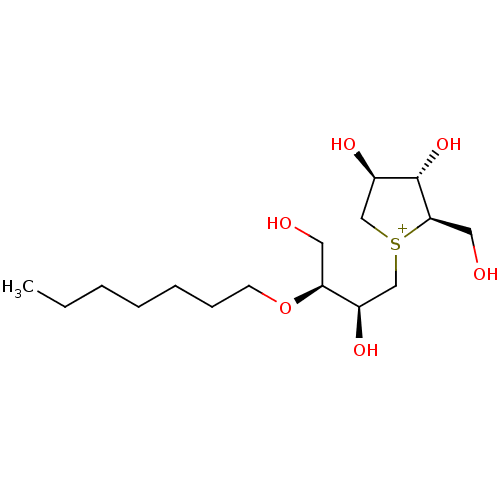 (CHEMBL3814911) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University Curated by ChEMBL | Assay Description Inhibition of rat small intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase method | Bioorg Med Chem 24: 3705-15 (2016) Article DOI: 10.1016/j.bmc.2016.06.013 BindingDB Entry DOI: 10.7270/Q241700V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50180580 (CHEMBL3814911) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University Curated by ChEMBL | Assay Description Inhibition of rat small intestinal isomaltase using isomaltose as substrate incubated for 30 mins by glucose-oxidase method | Bioorg Med Chem 24: 3705-15 (2016) Article DOI: 10.1016/j.bmc.2016.06.013 BindingDB Entry DOI: 10.7270/Q241700V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50342937 ((1S,2R,3S,4S)-1-((2S,3S)-3-ethoxy-2,4-dihydroxybut...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of rat small intestinal isomaltase after 30 mins by glucose-oxidase method | Bioorg Med Chem Lett 21: 3159-62 (2011) Article DOI: 10.1016/j.bmcl.2011.02.109 BindingDB Entry DOI: 10.7270/Q2DF6RJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50180520 (CHEMBL3814838) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University Curated by ChEMBL | Assay Description Inhibition of rat small intestinal isomaltase using isomaltose as substrate incubated for 30 mins by glucose-oxidase method | Bioorg Med Chem 24: 3705-15 (2016) Article DOI: 10.1016/j.bmc.2016.06.013 BindingDB Entry DOI: 10.7270/Q241700V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50180583 (CHEMBL3815109) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University Curated by ChEMBL | Assay Description Inhibition of rat small intestinal isomaltase using isomaltose as substrate incubated for 30 mins by glucose-oxidase method | Bioorg Med Chem 24: 3705-15 (2016) Article DOI: 10.1016/j.bmc.2016.06.013 BindingDB Entry DOI: 10.7270/Q241700V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50327501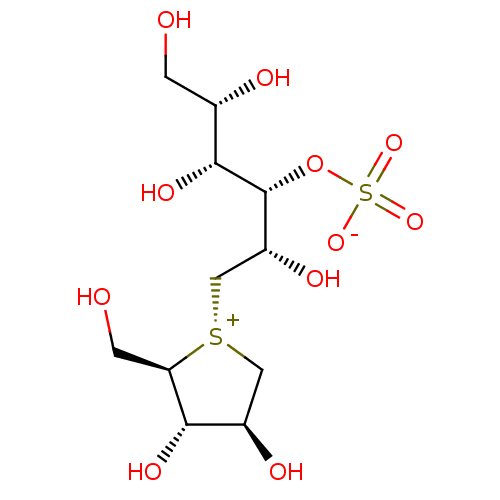 (CHEMBL1258528 | ponkoranol) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase | Bioorg Med Chem 19: 2015-22 (2011) Article DOI: 10.1016/j.bmc.2011.01.052 BindingDB Entry DOI: 10.7270/Q2J1044C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50180586 (CHEMBL1182462) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University Curated by ChEMBL | Assay Description Inhibition of rat small intestinal isomaltase using isomaltose as substrate incubated for 30 mins by glucose-oxidase method | Bioorg Med Chem 24: 3705-15 (2016) Article DOI: 10.1016/j.bmc.2016.06.013 BindingDB Entry DOI: 10.7270/Q241700V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 235 total ) | Next | Last >> |