

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096495 (CHEMBL85161 | N-{3-[Bis-(3-phenyl-propyl)-amino]-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana State University Curated by ChEMBL | Assay Description Tested for binding affinity against trypanothione reductase from Trypanosoma cruzi | Bioorg Med Chem Lett 11: 251-4 (2001) BindingDB Entry DOI: 10.7270/Q2FQ9VVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50103373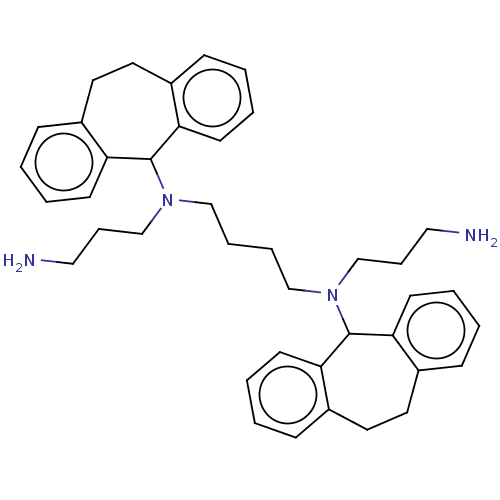 (CHEMBL3398189) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 255 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canisius College Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction of trypanothione disulfide by spectrophotometrically | Bioorg Med Chem 23: 996-1010 (2015) Article DOI: 10.1016/j.bmc.2015.01.018 BindingDB Entry DOI: 10.7270/Q27P9166 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096500 (CHEMBL86281 | N,N'-Bis-(3-phenyl-propyl)-N,N'-bis-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 614 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana State University Curated by ChEMBL | Assay Description Tested for binding affinity against trypanothione reductase from Trypanosoma cruzi | Bioorg Med Chem Lett 11: 251-4 (2001) BindingDB Entry DOI: 10.7270/Q2FQ9VVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096491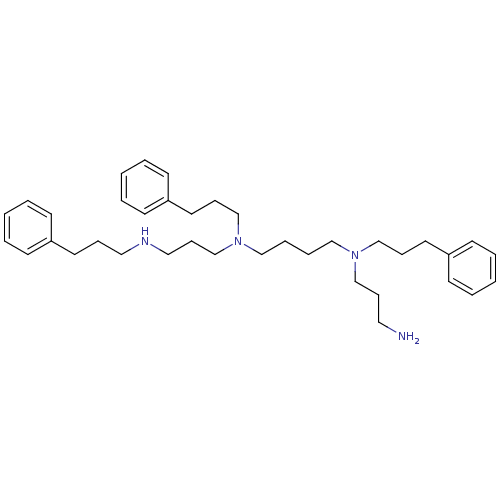 (CHEMBL85189 | N-(3-Amino-propyl)-N,N'-bis-(3-pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana State University Curated by ChEMBL | Assay Description Tested for binding affinity against trypanothione reductase from Trypanosoma cruzi | Bioorg Med Chem Lett 11: 251-4 (2001) BindingDB Entry DOI: 10.7270/Q2FQ9VVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096496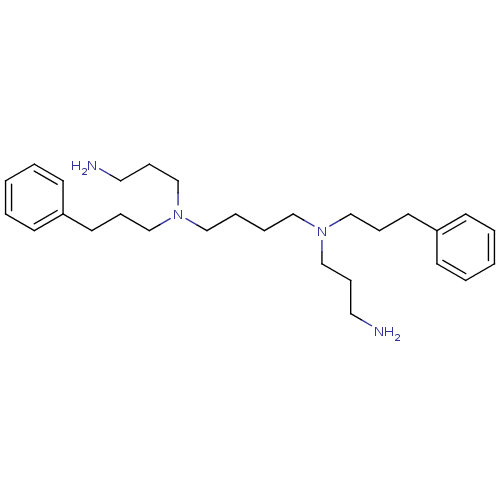 (CHEMBL62048 | N,N'-Bis-(3-amino-propyl)-N,N'-bis-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana State University Curated by ChEMBL | Assay Description Tested for binding affinity against trypanothione reductase from Trypanosoma cruzi | Bioorg Med Chem Lett 11: 251-4 (2001) BindingDB Entry DOI: 10.7270/Q2FQ9VVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096493 (CHEMBL82641 | N,N'-Bis-(3-phenyl-propyl)-N-[3-(3-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana State University Curated by ChEMBL | Assay Description Tested for binding affinity against trypanothione reductase from Trypanosoma cruzi | Bioorg Med Chem Lett 11: 251-4 (2001) BindingDB Entry DOI: 10.7270/Q2FQ9VVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096496 (CHEMBL62048 | N,N'-Bis-(3-amino-propyl)-N,N'-bis-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the competitive inhibition of trypanothione reduction by recombinant Trypanothione reductase from Trypanosoma cruzi | Bioorg Med Chem Lett 5: 1957-1960 (1995) Article DOI: 10.1016/0960-894X(95)00331-M BindingDB Entry DOI: 10.7270/Q2TD9XB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096492 (CHEMBL84221 | N-{3-[Bis-(3-phenyl-propyl)-amino]-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana State University Curated by ChEMBL | Assay Description Tested for binding affinity against trypanothione reductase from Trypanosoma cruzi | Bioorg Med Chem Lett 11: 251-4 (2001) BindingDB Entry DOI: 10.7270/Q2FQ9VVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50103372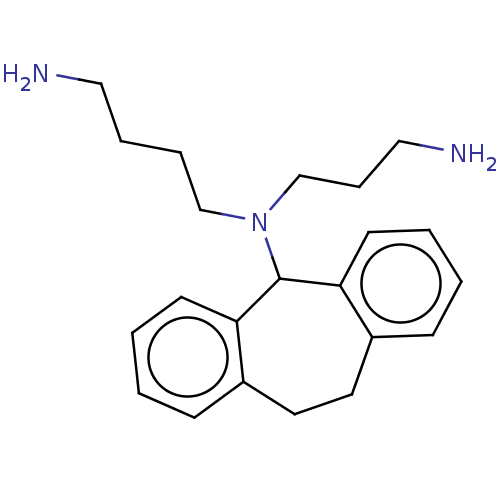 (CHEMBL378650) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canisius College Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction of trypanothione disulfide by spectrophotometrically | Bioorg Med Chem 23: 996-1010 (2015) Article DOI: 10.1016/j.bmc.2015.01.018 BindingDB Entry DOI: 10.7270/Q27P9166 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096501 (CHEMBL85198 | N*1*-(3-Phenyl-propyl)-N*1*-[3-(3-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana State University Curated by ChEMBL | Assay Description Tested for binding affinity against trypanothione reductase from Trypanosoma cruzi | Bioorg Med Chem Lett 11: 251-4 (2001) BindingDB Entry DOI: 10.7270/Q2FQ9VVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50184768 (CHEMBL61691 | N,N'-Bis-(3-amino-propyl)-N,N'-bis-n...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the competitive inhibition of trypanothione reduction by recombinant Trypanothione reductase from Trypanosoma cruzi | Bioorg Med Chem Lett 5: 1957-1960 (1995) Article DOI: 10.1016/0960-894X(95)00331-M BindingDB Entry DOI: 10.7270/Q2TD9XB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM77970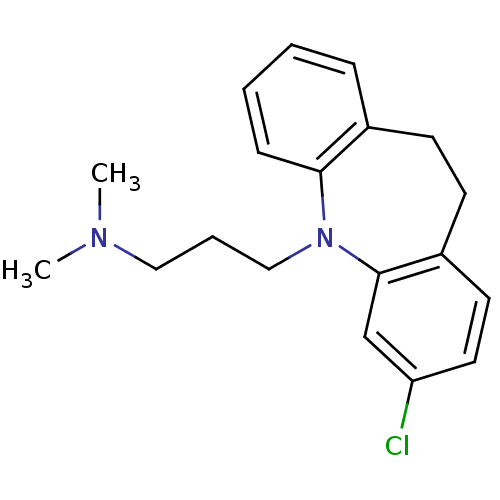 (3-(2-chloranyl-5,6-dihydrobenzo[b][1]benzazepin-11...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the competitive inhibition of trypanothione reduction by recombinant Trypanothione reductase from Trypanosoma cruzi | Bioorg Med Chem Lett 5: 1957-1960 (1995) Article DOI: 10.1016/0960-894X(95)00331-M BindingDB Entry DOI: 10.7270/Q2TD9XB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM77970 (3-(2-chloranyl-5,6-dihydrobenzo[b][1]benzazepin-11...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canisius College Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction of trypanothione disulfide by spectrophotometrically | Bioorg Med Chem 23: 996-1010 (2015) Article DOI: 10.1016/j.bmc.2015.01.018 BindingDB Entry DOI: 10.7270/Q27P9166 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50103371 (CHEMBL3398188) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canisius College Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction of trypanothione disulfide by spectrophotometrically | Bioorg Med Chem 23: 996-1010 (2015) Article DOI: 10.1016/j.bmc.2015.01.018 BindingDB Entry DOI: 10.7270/Q27P9166 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50285025 (CHEMBL305575 | N-Naphthalen-2-ylmethyl-N'-{3-[(nap...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the competitive inhibition of trypanothione reduction by recombinant Trypanothione reductase from Trypanosoma cruzi | Bioorg Med Chem Lett 5: 1957-1960 (1995) Article DOI: 10.1016/0960-894X(95)00331-M BindingDB Entry DOI: 10.7270/Q2TD9XB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096494 (CHEMBL310207 | N,N'-Bis-{3-[bis-(3-phenyl-propyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana State University Curated by ChEMBL | Assay Description Tested for binding affinity against trypanothione reductase from Trypanosoma cruzi | Bioorg Med Chem Lett 11: 251-4 (2001) BindingDB Entry DOI: 10.7270/Q2FQ9VVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096498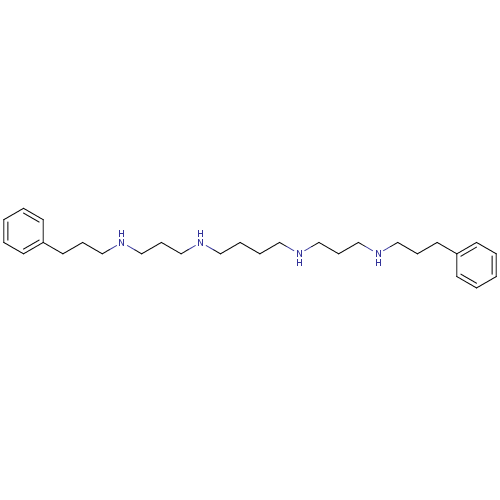 (CHEMBL86096 | N,N'-Bis-[3-(3-phenyl-propylamino)-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana State University Curated by ChEMBL | Assay Description Tested for binding affinity against trypanothione reductase from Trypanosoma cruzi | Bioorg Med Chem Lett 11: 251-4 (2001) BindingDB Entry DOI: 10.7270/Q2FQ9VVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096499 (CHEMBL85964 | N*1*-(3-Amino-propyl)-N*1*-(3-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana State University Curated by ChEMBL | Assay Description Tested for binding affinity against trypanothione reductase from Trypanosoma cruzi | Bioorg Med Chem Lett 11: 251-4 (2001) BindingDB Entry DOI: 10.7270/Q2FQ9VVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50184779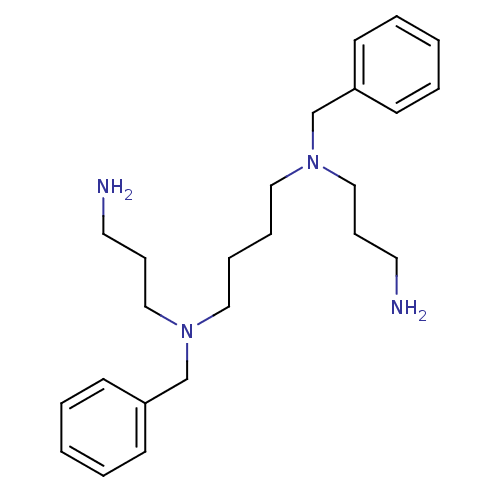 (4, 9-DB-3-4-3 | CHEMBL58084 | N,N'-Bis-(3-amino-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the competitive inhibition of trypanothione reduction by recombinant Trypanothione reductase from Trypanosoma cruzi | Bioorg Med Chem Lett 5: 1957-1960 (1995) Article DOI: 10.1016/0960-894X(95)00331-M BindingDB Entry DOI: 10.7270/Q2TD9XB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50103370 (CHEMBL3398187) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canisius College Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction of trypanothione disulfide by spectrophotometrically | Bioorg Med Chem 23: 996-1010 (2015) Article DOI: 10.1016/j.bmc.2015.01.018 BindingDB Entry DOI: 10.7270/Q27P9166 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096497 (CHEMBL85779 | N-(3-Amino-propyl)-N'-[3-(3-phenyl-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana State University Curated by ChEMBL | Assay Description Tested for binding affinity against trypanothione reductase from Trypanosoma cruzi | Bioorg Med Chem Lett 11: 251-4 (2001) BindingDB Entry DOI: 10.7270/Q2FQ9VVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50103369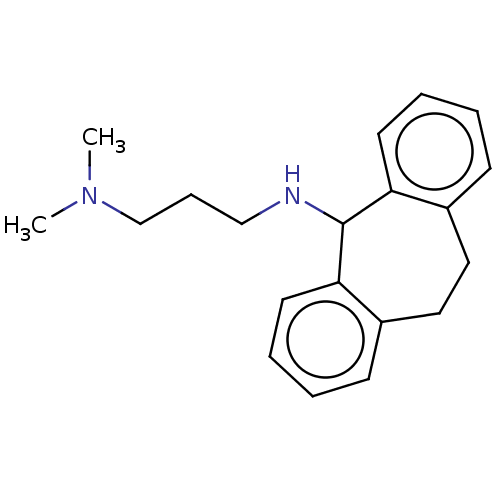 (CHEMBL3398186) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canisius College Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction of trypanothione disulfide by spectrophotometrically | Bioorg Med Chem 23: 996-1010 (2015) Article DOI: 10.1016/j.bmc.2015.01.018 BindingDB Entry DOI: 10.7270/Q27P9166 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50184783 (CHEMBL298628 | N*1*-(3-Amino-propyl)-N*1*-naphthal...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the competitive inhibition of trypanothione reduction by recombinant Trypanothione reductase from Trypanosoma cruzi | Bioorg Med Chem Lett 5: 1957-1960 (1995) Article DOI: 10.1016/0960-894X(95)00331-M BindingDB Entry DOI: 10.7270/Q2TD9XB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50103368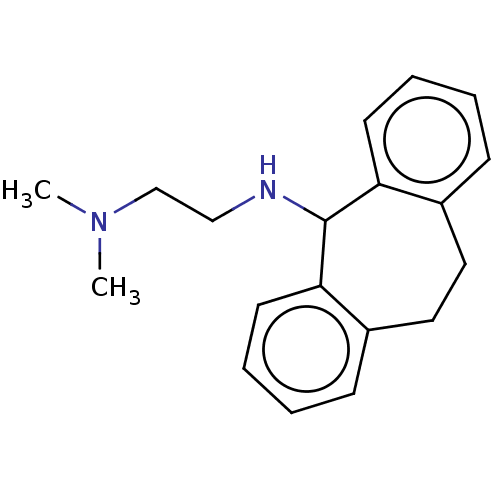 (CHEMBL332939) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canisius College Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction of trypanothione disulfide by spectrophotometrically | Bioorg Med Chem 23: 996-1010 (2015) Article DOI: 10.1016/j.bmc.2015.01.018 BindingDB Entry DOI: 10.7270/Q27P9166 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50184788 ((4-Amino-butyl)-(3-amino-propyl)-carbamic acid ben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the competitive inhibition of trypanothione reduction by recombinant Trypanothione reductase from Trypanosoma cruzi | Bioorg Med Chem Lett 5: 1957-1960 (1995) Article DOI: 10.1016/0960-894X(95)00331-M BindingDB Entry DOI: 10.7270/Q2TD9XB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50184771 (1N-[3-(4-phenylcarboxamidobutylamino)propyl]benzam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the competitive inhibition of trypanothione reduction by recombinant Trypanothione reductase from Trypanosoma cruzi | Bioorg Med Chem Lett 5: 1957-1960 (1995) Article DOI: 10.1016/0960-894X(95)00331-M BindingDB Entry DOI: 10.7270/Q2TD9XB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50436963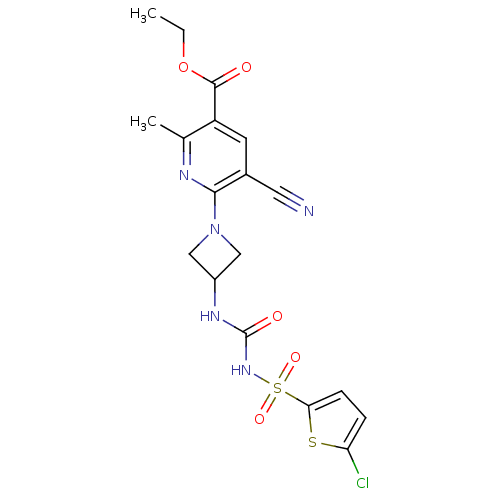 (CHEMBL2402255) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) after 1 hr by scintillation counting analysis | Eur J Med Chem 65: 360-75 (2013) Article DOI: 10.1016/j.ejmech.2013.04.007 BindingDB Entry DOI: 10.7270/Q2PK0HJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50436963 (CHEMBL2402255) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor in human washed platelets assessed as inhibition of fibrinogen-induced aggregation | Eur J Med Chem 65: 360-75 (2013) Article DOI: 10.1016/j.ejmech.2013.04.007 BindingDB Entry DOI: 10.7270/Q2PK0HJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50526962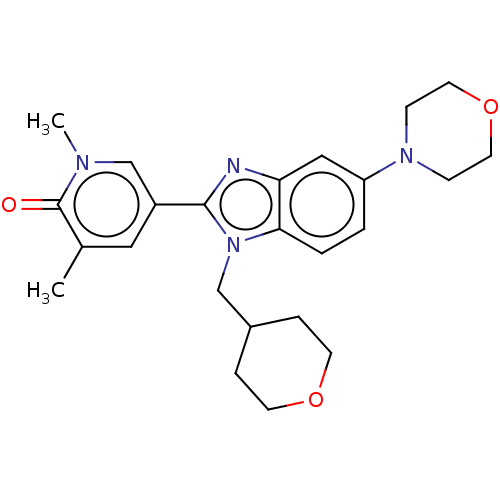 (CHEMBL4448056) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description Displacement of Alexa Fluor 647 labelled ligand from recombinant human N-terminal His6-tagged BRD4 BD1 (1 to 477 residues)/BD2 Y390A mutant incubated... | J Med Chem 63: 714-746 (2020) Article DOI: 10.1021/acs.jmedchem.9b01670 BindingDB Entry DOI: 10.7270/Q2TF01RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50526964 (CHEMBL4438171) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description Displacement of Alexa Fluor 647 labelled ligand from recombinant human N-terminal His6-tagged BRD4 BD1 (1 to 477 residues)/BD2 Y390A mutant incubated... | J Med Chem 63: 714-746 (2020) Article DOI: 10.1021/acs.jmedchem.9b01670 BindingDB Entry DOI: 10.7270/Q2TF01RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50436962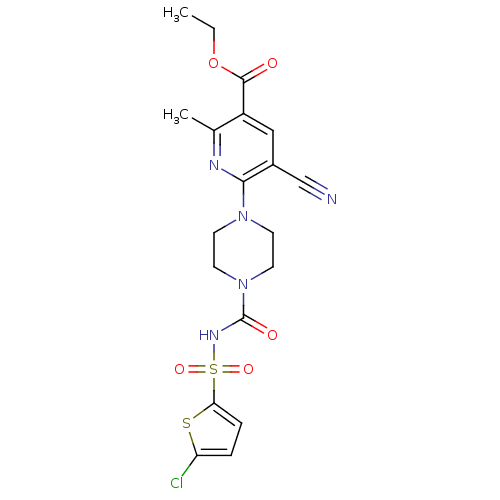 (CHEMBL2402264) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor in human washed platelets assessed as inhibition of fibrinogen-induced aggregation | Eur J Med Chem 65: 360-75 (2013) Article DOI: 10.1016/j.ejmech.2013.04.007 BindingDB Entry DOI: 10.7270/Q2PK0HJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50436961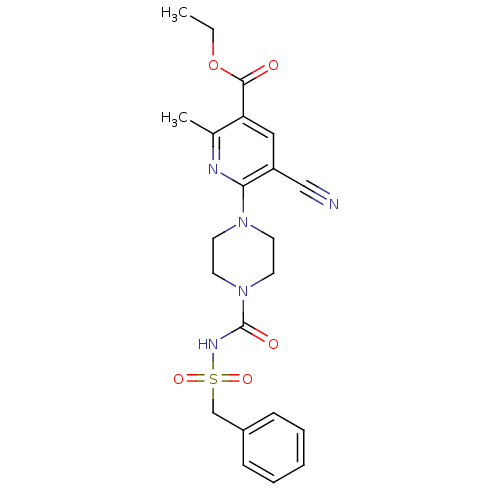 (CHEMBL2402266) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor in human washed platelets assessed as inhibition of fibrinogen-induced aggregation | Eur J Med Chem 65: 360-75 (2013) Article DOI: 10.1016/j.ejmech.2013.04.007 BindingDB Entry DOI: 10.7270/Q2PK0HJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50436963 (CHEMBL2402255) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of GTPgammaS binding after 1 hr by scintillation counting analysis | Eur J Med Chem 65: 360-75 (2013) Article DOI: 10.1016/j.ejmech.2013.04.007 BindingDB Entry DOI: 10.7270/Q2PK0HJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50436960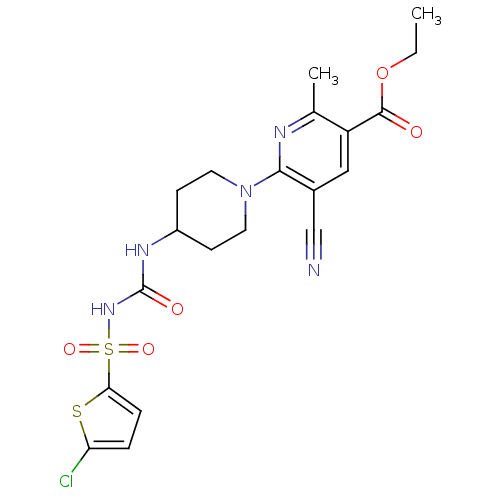 (CHEMBL2402260) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor in human washed platelets assessed as inhibition of fibrinogen-induced aggregation | Eur J Med Chem 65: 360-75 (2013) Article DOI: 10.1016/j.ejmech.2013.04.007 BindingDB Entry DOI: 10.7270/Q2PK0HJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50436960 (CHEMBL2402260) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) after 1 hr by scintillation counting analysis | Eur J Med Chem 65: 360-75 (2013) Article DOI: 10.1016/j.ejmech.2013.04.007 BindingDB Entry DOI: 10.7270/Q2PK0HJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50436952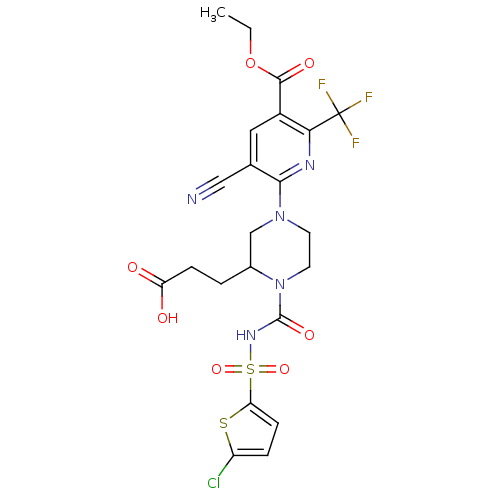 (CHEMBL2402142) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor in human washed platelets assessed as inhibition of fibrinogen-induced aggregation | Eur J Med Chem 65: 360-75 (2013) Article DOI: 10.1016/j.ejmech.2013.04.007 BindingDB Entry DOI: 10.7270/Q2PK0HJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50436958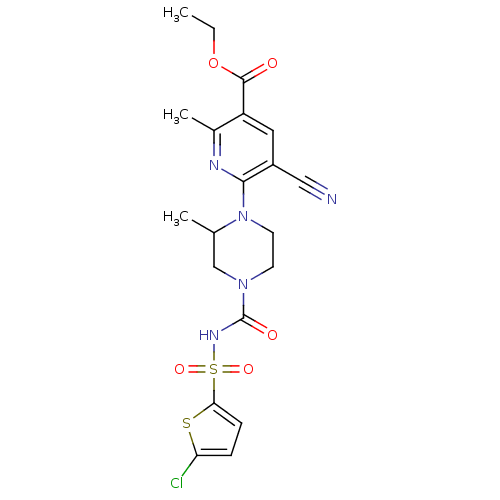 (CHEMBL2402144) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor in human washed platelets assessed as inhibition of fibrinogen-induced aggregation | Eur J Med Chem 65: 360-75 (2013) Article DOI: 10.1016/j.ejmech.2013.04.007 BindingDB Entry DOI: 10.7270/Q2PK0HJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50526959 (CHEMBL4522991) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description Displacement of Alexa Fluor 647 labelled ligand from recombinant human N-terminal His6-tagged BRD4 BD1 (1 to 477 residues)/BD2 Y390A mutant incubated... | J Med Chem 63: 714-746 (2020) Article DOI: 10.1021/acs.jmedchem.9b01670 BindingDB Entry DOI: 10.7270/Q2TF01RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50526959 (CHEMBL4522991) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description Displacement of Alexa Fluor 647 labelled ligand from recombinant human N-terminal His6-tagged BRD4 BD1 (1 to 477 residues)/BD2 Y390A mutant incubated... | J Med Chem 63: 714-746 (2020) Article DOI: 10.1021/acs.jmedchem.9b01670 BindingDB Entry DOI: 10.7270/Q2TF01RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50436961 (CHEMBL2402266) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) after 1 hr by scintillation counting analysis | Eur J Med Chem 65: 360-75 (2013) Article DOI: 10.1016/j.ejmech.2013.04.007 BindingDB Entry DOI: 10.7270/Q2PK0HJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50436962 (CHEMBL2402264) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of GTPgammaS binding after 1 hr by scintillation counting analysis | Eur J Med Chem 65: 360-75 (2013) Article DOI: 10.1016/j.ejmech.2013.04.007 BindingDB Entry DOI: 10.7270/Q2PK0HJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50436958 (CHEMBL2402144) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of GTPgammaS binding after 1 hr by scintillation counting analysis | Eur J Med Chem 65: 360-75 (2013) Article DOI: 10.1016/j.ejmech.2013.04.007 BindingDB Entry DOI: 10.7270/Q2PK0HJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50436957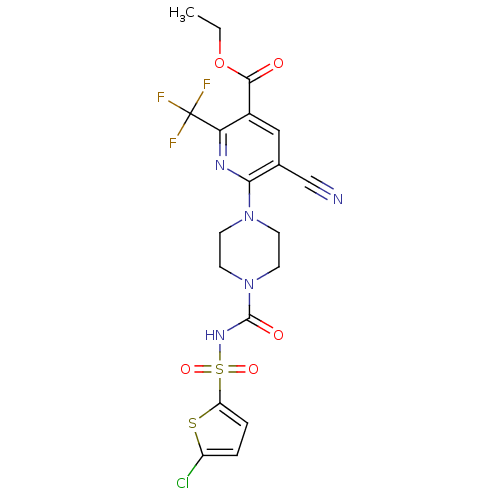 (CHEMBL2402244) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor in human washed platelets assessed as inhibition of fibrinogen-induced aggregation | Eur J Med Chem 65: 360-75 (2013) Article DOI: 10.1016/j.ejmech.2013.04.007 BindingDB Entry DOI: 10.7270/Q2PK0HJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50436957 (CHEMBL2402244) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of GTPgammaS binding after 1 hr by scintillation counting analysis | Eur J Med Chem 65: 360-75 (2013) Article DOI: 10.1016/j.ejmech.2013.04.007 BindingDB Entry DOI: 10.7270/Q2PK0HJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50526961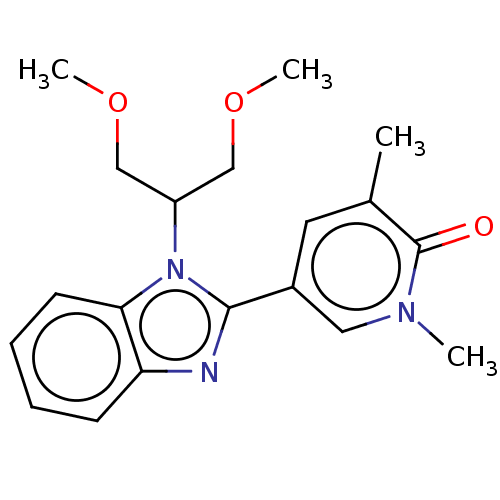 (CHEMBL4576013) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description Displacement of Alexa Fluor 647 labelled ligand from recombinant human N-terminal His6-tagged BRD4 BD1 (1 to 477 residues)/BD2 Y390A mutant incubated... | J Med Chem 63: 714-746 (2020) Article DOI: 10.1021/acs.jmedchem.9b01670 BindingDB Entry DOI: 10.7270/Q2TF01RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50436962 (CHEMBL2402264) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) after 1 hr by scintillation counting analysis | Eur J Med Chem 65: 360-75 (2013) Article DOI: 10.1016/j.ejmech.2013.04.007 BindingDB Entry DOI: 10.7270/Q2PK0HJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50526950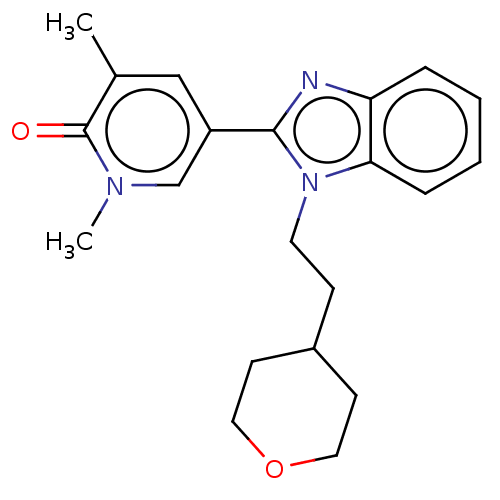 (CHEMBL4547614) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description Displacement of Alexa Fluor 647 labelled ligand from recombinant human N-terminal His6-tagged BRD4 BD1 (1 to 477 residues)/BD2 Y390A mutant incubated... | J Med Chem 63: 714-746 (2020) Article DOI: 10.1021/acs.jmedchem.9b01670 BindingDB Entry DOI: 10.7270/Q2TF01RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50526967 (CHEMBL4579229) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description Displacement of Alexa Fluor 647 labelled ligand from recombinant human N-terminal His6-tagged BRD4 BD1 (1 to 477 residues)/BD2 Y390A mutant incubated... | J Med Chem 63: 714-746 (2020) Article DOI: 10.1021/acs.jmedchem.9b01670 BindingDB Entry DOI: 10.7270/Q2TF01RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50436961 (CHEMBL2402266) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of GTPgammaS binding after 1 hr by scintillation counting analysis | Eur J Med Chem 65: 360-75 (2013) Article DOI: 10.1016/j.ejmech.2013.04.007 BindingDB Entry DOI: 10.7270/Q2PK0HJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50436960 (CHEMBL2402260) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor (unknown origin) assessed as inhibition of GTPgammaS binding after 1 hr by scintillation counting analysis | Eur J Med Chem 65: 360-75 (2013) Article DOI: 10.1016/j.ejmech.2013.04.007 BindingDB Entry DOI: 10.7270/Q2PK0HJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 236 total ) | Next | Last >> |