 Found 63 hits with Last Name = 'okuno' and Initial = 'a'
Found 63 hits with Last Name = 'okuno' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Fructose-1,6-bisphosphatase 1
(Homo sapiens (Human)) | BDBM50322036
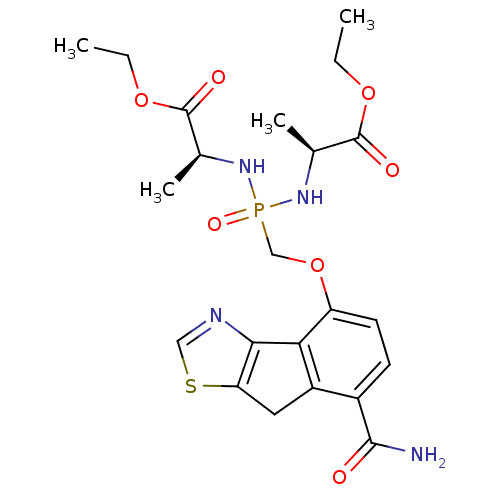
(CHEMBL1173125 | Ethyl(2S,6S)-4-{[(7-carbamoyl-8H-i...)Show SMILES CCOC(=O)[C@H](C)NP(=O)(COc1ccc(C(N)=O)c2Cc3scnc3-c12)N[C@@H](C)C(=O)OCC |r| Show InChI InChI=1S/C22H29N4O7PS/c1-5-31-21(28)12(3)25-34(30,26-13(4)22(29)32-6-2)11-33-16-8-7-14(20(23)27)15-9-17-19(18(15)16)24-10-35-17/h7-8,10,12-13H,5-6,9,11H2,1-4H3,(H2,23,27)(H2,25,26,30)/t12-,13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human FBase |
Bioorg Med Chem 18: 5346-51 (2010)
Article DOI: 10.1016/j.bmc.2010.05.041
BindingDB Entry DOI: 10.7270/Q2TB17V0 |
More data for this
Ligand-Target Pair | |
Fructose-1,6-bisphosphatase 1
(Homo sapiens (Human)) | BDBM50322037
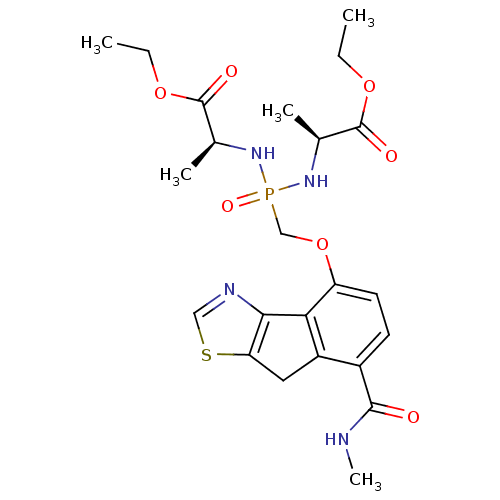
(CHEMBL1173126 | Ethyl(2S,6S)-2,6-dimethyl-4-({[7-(...)Show SMILES CCOC(=O)[C@H](C)NP(=O)(COc1ccc(C(=O)NC)c2Cc3scnc3-c12)N[C@@H](C)C(=O)OCC |r| Show InChI InChI=1S/C23H31N4O7PS/c1-6-32-22(29)13(3)26-35(31,27-14(4)23(30)33-7-2)12-34-17-9-8-15(21(28)24-5)16-10-18-20(19(16)17)25-11-36-18/h8-9,11,13-14H,6-7,10,12H2,1-5H3,(H,24,28)(H2,26,27,31)/t13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human FBase |
Bioorg Med Chem 18: 5346-51 (2010)
Article DOI: 10.1016/j.bmc.2010.05.041
BindingDB Entry DOI: 10.7270/Q2TB17V0 |
More data for this
Ligand-Target Pair | |
Fructose-1,6-bisphosphatase 1
(Homo sapiens (Human)) | BDBM50322039
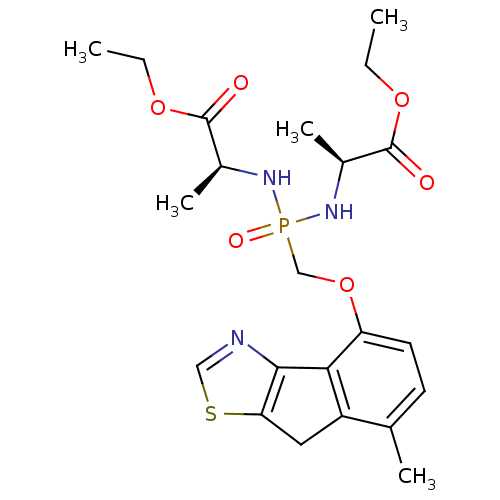
(CHEMBL1173572 | Ethyl(2S,6S)-2,6-dimethyl-4-{[(7-m...)Show SMILES CCOC(=O)[C@H](C)NP(=O)(COc1ccc(C)c2Cc3scnc3-c12)N[C@@H](C)C(=O)OCC |r| Show InChI InChI=1S/C22H30N3O6PS/c1-6-29-21(26)14(4)24-32(28,25-15(5)22(27)30-7-2)12-31-17-9-8-13(3)16-10-18-20(19(16)17)23-11-33-18/h8-9,11,14-15H,6-7,10,12H2,1-5H3,(H2,24,25,28)/t14-,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human FBase |
Bioorg Med Chem 18: 5346-51 (2010)
Article DOI: 10.1016/j.bmc.2010.05.041
BindingDB Entry DOI: 10.7270/Q2TB17V0 |
More data for this
Ligand-Target Pair | |
Fructose-1,6-bisphosphatase 1
(Homo sapiens (Human)) | BDBM50322043
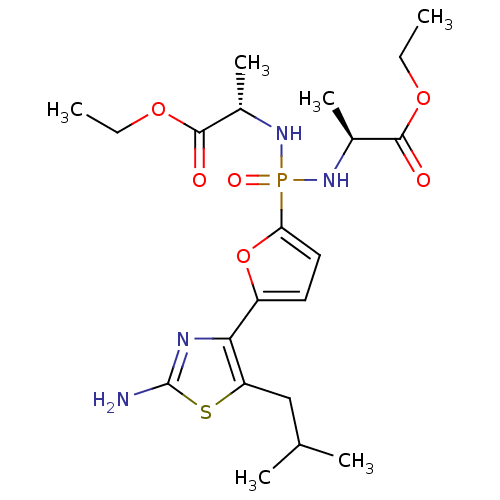
((2S,2'S)-diethyl 2,2'-((5-(2-amino-5-isobutylthiaz...)Show SMILES CCOC(=O)[C@H](C)NP(=O)(N[C@@H](C)C(=O)OCC)c1ccc(o1)-c1nc(N)sc1CC(C)C |r| Show InChI InChI=1S/C21H33N4O6PS/c1-7-29-19(26)13(5)24-32(28,25-14(6)20(27)30-8-2)17-10-9-15(31-17)18-16(11-12(3)4)33-21(22)23-18/h9-10,12-14H,7-8,11H2,1-6H3,(H2,22,23)(H2,24,25,28)/t13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human FBase |
Bioorg Med Chem 18: 5346-51 (2010)
Article DOI: 10.1016/j.bmc.2010.05.041
BindingDB Entry DOI: 10.7270/Q2TB17V0 |
More data for this
Ligand-Target Pair | |
Fructose-1,6-bisphosphatase 1
(Homo sapiens (Human)) | BDBM50322042
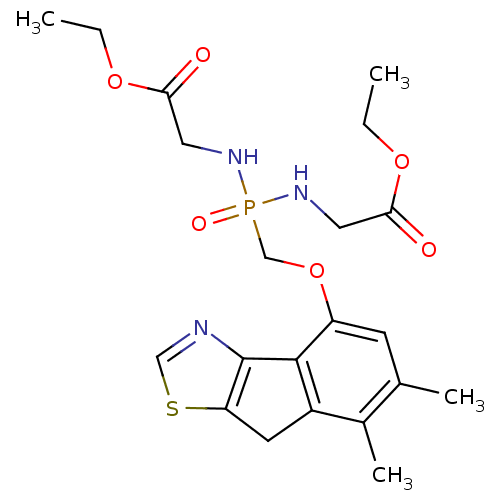
(CHEMBL1172936 | Ethyl4-{[(6,7-dimethyl-8H-indeno[1...)Show SMILES CCOC(=O)CNP(=O)(COc1cc(C)c(C)c2Cc3scnc3-c12)NCC(=O)OCC Show InChI InChI=1S/C21H28N3O6PS/c1-5-28-18(25)9-23-31(27,24-10-19(26)29-6-2)12-30-16-7-13(3)14(4)15-8-17-21(20(15)16)22-11-32-17/h7,11H,5-6,8-10,12H2,1-4H3,(H2,23,24,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human FBase |
Bioorg Med Chem 18: 5346-51 (2010)
Article DOI: 10.1016/j.bmc.2010.05.041
BindingDB Entry DOI: 10.7270/Q2TB17V0 |
More data for this
Ligand-Target Pair | |
Fructose-1,6-bisphosphatase 1
(Homo sapiens (Human)) | BDBM50317151
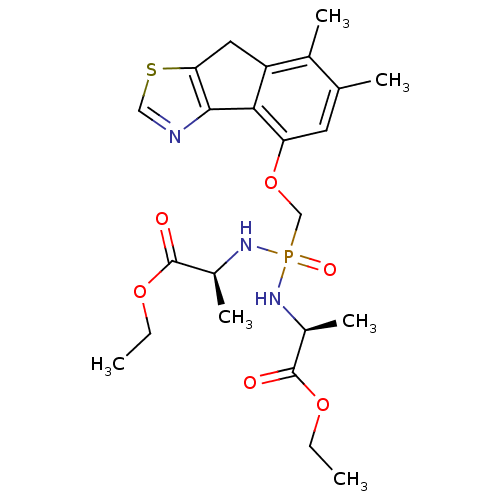
((2S,2'S)-diethyl 2,2'-(((6,7-dimethyl-8H-indeno[1,...)Show SMILES CCOC(=O)[C@H](C)NP(=O)(COc1cc(C)c(C)c2Cc3scnc3-c12)N[C@@H](C)C(=O)OCC |r| Show InChI InChI=1S/C23H32N3O6PS/c1-7-30-22(27)15(5)25-33(29,26-16(6)23(28)31-8-2)12-32-18-9-13(3)14(4)17-10-19-21(20(17)18)24-11-34-19/h9,11,15-16H,7-8,10,12H2,1-6H3,(H2,25,26,29)/t15-,16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human FBase |
Bioorg Med Chem 18: 5346-51 (2010)
Article DOI: 10.1016/j.bmc.2010.05.041
BindingDB Entry DOI: 10.7270/Q2TB17V0 |
More data for this
Ligand-Target Pair | |
Fructose-1,6-bisphosphatase 1
(Homo sapiens (Human)) | BDBM50322041
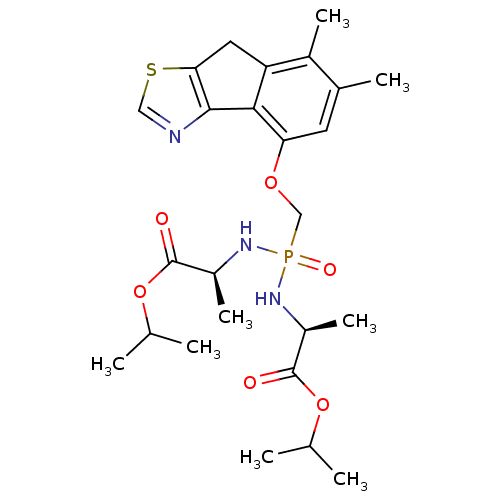
(CHEMBL1172935 | Isopropyl(2S,6S)-4-{[(6,7-dimethyl...)Show SMILES CC(C)OC(=O)[C@H](C)NP(=O)(COc1cc(C)c(C)c2Cc3scnc3-c12)N[C@@H](C)C(=O)OC(C)C |r| Show InChI InChI=1S/C25H36N3O6PS/c1-13(2)33-24(29)17(7)27-35(31,28-18(8)25(30)34-14(3)4)12-32-20-9-15(5)16(6)19-10-21-23(22(19)20)26-11-36-21/h9,11,13-14,17-18H,10,12H2,1-8H3,(H2,27,28,31)/t17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human FBase |
Bioorg Med Chem 18: 5346-51 (2010)
Article DOI: 10.1016/j.bmc.2010.05.041
BindingDB Entry DOI: 10.7270/Q2TB17V0 |
More data for this
Ligand-Target Pair | |
Fructose-1,6-bisphosphatase 1
(Homo sapiens (Human)) | BDBM50322040
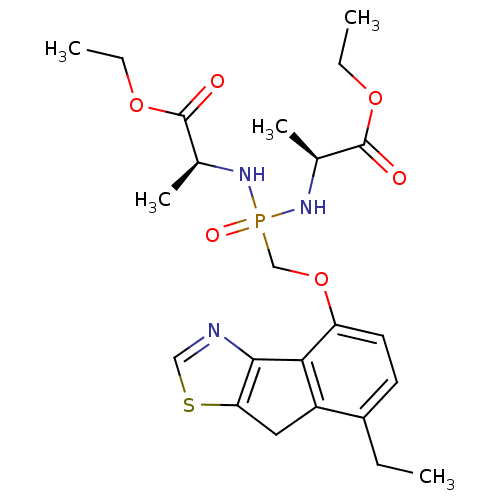
(CHEMBL1173636 | Ethyl(2S,6S)-4-{[(7-ethyl-8H-inden...)Show SMILES CCOC(=O)[C@H](C)NP(=O)(COc1ccc(CC)c2Cc3scnc3-c12)N[C@@H](C)C(=O)OCC |r| Show InChI InChI=1S/C23H32N3O6PS/c1-6-16-9-10-18(20-17(16)11-19-21(20)24-12-34-19)32-13-33(29,25-14(4)22(27)30-7-2)26-15(5)23(28)31-8-3/h9-10,12,14-15H,6-8,11,13H2,1-5H3,(H2,25,26,29)/t14-,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human FBase |
Bioorg Med Chem 18: 5346-51 (2010)
Article DOI: 10.1016/j.bmc.2010.05.041
BindingDB Entry DOI: 10.7270/Q2TB17V0 |
More data for this
Ligand-Target Pair | |
Fructose-1,6-bisphosphatase 1
(Homo sapiens (Human)) | BDBM50322038

(CHEMBL1173571 | Ethyl(2S,6S)-4-[({7-[(2,2-dimethyl...)Show SMILES CCOC(=O)[C@H](C)NP(=O)(COc1ccc(C(=O)NCC(C)(C)C)c2Cc3scnc3-c12)N[C@@H](C)C(=O)OCC |r| Show InChI InChI=1S/C27H39N4O7PS/c1-8-36-25(33)16(3)30-39(35,31-17(4)26(34)37-9-2)15-38-20-11-10-18(24(32)28-13-27(5,6)7)19-12-21-23(22(19)20)29-14-40-21/h10-11,14,16-17H,8-9,12-13,15H2,1-7H3,(H,28,32)(H2,30,31,35)/t16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human FBase |
Bioorg Med Chem 18: 5346-51 (2010)
Article DOI: 10.1016/j.bmc.2010.05.041
BindingDB Entry DOI: 10.7270/Q2TB17V0 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126143

(Epacadostat | INCB-024360)Show SMILES NS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C11H13BrFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16,21H,3-4H2,(H,15,20)(H,17,18)(H2,14,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21G0QZ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126143

(Epacadostat | INCB-024360)Show SMILES NS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C11H13BrFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16,21H,3-4H2,(H,15,20)(H,17,18)(H2,14,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 incubated for 60 mins in presence of L-tryptophan by HPLC analysis |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21G0QZ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Plasminogen
(Homo sapiens (Human)) | BDBM50092333
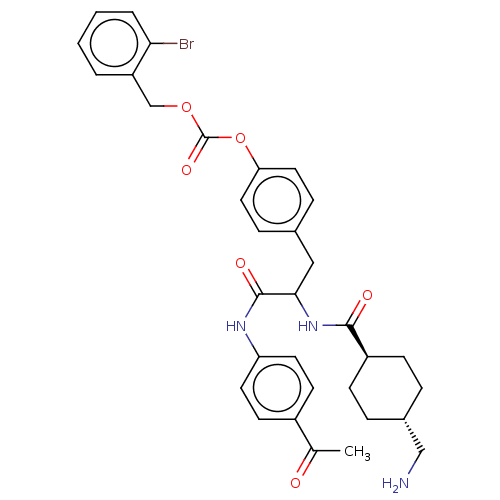
(CHEMBL3084793 | Carbonic acid 4-{2-(4-acetyl-pheny...)Show SMILES CC(=O)c1ccc(NC(=O)C(Cc2ccc(OC(=O)OCc3ccccc3Br)cc2)NC(=O)[C@H]2CC[C@H](CN)CC2)cc1 |wU:36.38,wD:33.34,(8.83,-9.49,;7.29,-9.49,;6.52,-10.82,;6.52,-8.15,;7.29,-6.82,;6.52,-5.48,;4.98,-5.48,;4.21,-4.15,;2.67,-4.15,;1.9,-5.48,;1.9,-2.82,;2.67,-1.48,;4.21,-1.48,;4.98,-2.82,;6.52,-2.82,;7.29,-1.48,;8.83,-1.48,;9.6,-2.82,;8.83,-4.15,;11.14,-2.82,;11.91,-4.15,;13.45,-4.15,;14.22,-2.82,;15.76,-2.82,;16.53,-4.15,;15.76,-5.48,;14.22,-5.48,;13.45,-6.82,;6.52,-.15,;4.98,-.15,;.36,-2.82,;-.41,-4.15,;.36,-5.48,;-1.95,-4.15,;-2.72,-2.82,;-4.26,-2.82,;-5.03,-4.15,;-6.57,-4.15,;-7.34,-2.82,;-4.26,-5.48,;-2.72,-5.48,;4.21,-6.82,;4.98,-8.15,)| Show InChI InChI=1S/C33H36BrN3O6/c1-21(38)24-12-14-27(15-13-24)36-32(40)30(37-31(39)25-10-6-23(19-35)7-11-25)18-22-8-16-28(17-9-22)43-33(41)42-20-26-4-2-3-5-29(26)34/h2-5,8-9,12-17,23,25,30H,6-7,10-11,18-20,35H2,1H3,(H,36,40)(H,37,39)/t23-,25-,30? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound was evaluated against plasmin |
Bioorg Med Chem Lett 10: 2217-21 (2001)
BindingDB Entry DOI: 10.7270/Q2125RXQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50317154
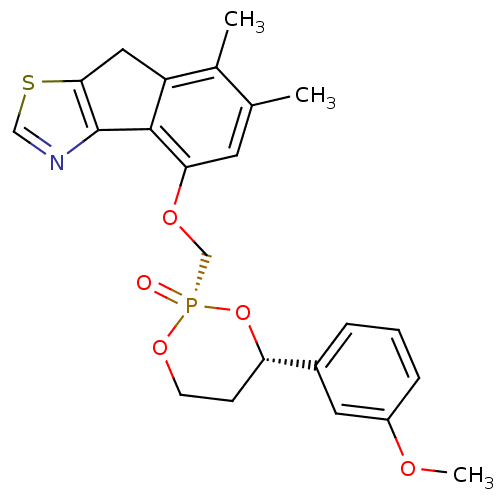
(4-[4-(3-Methoxy-phenyl)-2-oxo-2lambda*5*-[1,3,2]di...)Show SMILES COc1cccc(c1)[C@@H]1CCO[P@](=O)(COc2cc(C)c(C)c3Cc4scnc4-c23)O1 |r| Show InChI InChI=1S/C23H24NO5PS/c1-14-9-20(22-18(15(14)2)11-21-23(22)24-12-31-21)27-13-30(25)28-8-7-19(29-30)16-5-4-6-17(10-16)26-3/h4-6,9-10,12,19H,7-8,11,13H2,1-3H3/t19-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 2938-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.017
BindingDB Entry DOI: 10.7270/Q2222TXQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50317154

(4-[4-(3-Methoxy-phenyl)-2-oxo-2lambda*5*-[1,3,2]di...)Show SMILES COc1cccc(c1)[C@@H]1CCO[P@](=O)(COc2cc(C)c(C)c3Cc4scnc4-c23)O1 |r| Show InChI InChI=1S/C23H24NO5PS/c1-14-9-20(22-18(15(14)2)11-21-23(22)24-12-31-21)27-13-30(25)28-8-7-19(29-30)16-5-4-6-17(10-16)26-3/h4-6,9-10,12,19H,7-8,11,13H2,1-3H3/t19-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 2938-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.017
BindingDB Entry DOI: 10.7270/Q2222TXQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50317152
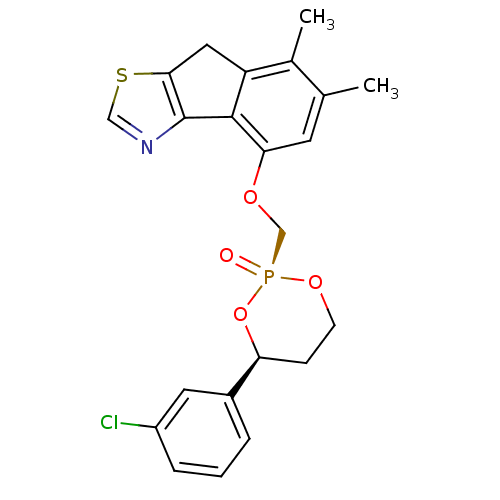
((2R,4S)-4-[4-(3-Chloro-phenyl)-2-oxo-2lambda*5*-[1...)Show SMILES Cc1cc(OC[P@@]2(=O)OCC[C@H](O2)c2cccc(Cl)c2)c-2c(Cc3scnc-23)c1C |r| Show InChI InChI=1S/C22H21ClNO4PS/c1-13-8-19(21-17(14(13)2)10-20-22(21)24-11-30-20)26-12-29(25)27-7-6-18(28-29)15-4-3-5-16(23)9-15/h3-5,8-9,11,18H,6-7,10,12H2,1-2H3/t18-,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 2938-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.017
BindingDB Entry DOI: 10.7270/Q2222TXQ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50092338

(CHEMBL3084796 | Carbonic acid 4-[2-[(4-aminomethyl...)Show SMILES CC(C)CCNC(=O)C(Cc1ccc(OC(=O)OCc2ccccc2Br)cc1)NC(=O)[C@H]1CC[C@H](CN)CC1 |wU:34.36,wD:31.32,(11.12,-6.33,;10.62,-7.79,;11.62,-8.95,;9.1,-8.08,;8.09,-6.92,;6.58,-7.21,;5.57,-6.04,;6.08,-4.59,;4.06,-6.33,;3.05,-5.17,;3.56,-3.72,;5.07,-3.42,;5.57,-1.97,;4.57,-.81,;5.07,.65,;6.58,.94,;7.59,-.22,;7.09,2.4,;8.6,2.69,;9.1,4.14,;8.09,5.31,;8.6,6.76,;10.11,7.05,;11.12,5.89,;10.62,4.43,;11.62,3.27,;3.05,-1.1,;2.55,-2.55,;3.56,-7.79,;4.57,-8.95,;6.08,-8.66,;4.06,-10.41,;2.55,-10.7,;2.05,-12.16,;3.05,-13.32,;2.55,-14.77,;1.04,-15.07,;4.57,-13.03,;5.07,-11.57,)| Show InChI InChI=1S/C30H40BrN3O5/c1-20(2)15-16-33-29(36)27(34-28(35)23-11-7-22(18-32)8-12-23)17-21-9-13-25(14-10-21)39-30(37)38-19-24-5-3-4-6-26(24)31/h3-6,9-10,13-14,20,22-23,27H,7-8,11-12,15-19,32H2,1-2H3,(H,33,36)(H,34,35)/t22-,23-,27? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound was evaluated against plasmin |
Bioorg Med Chem Lett 10: 2217-21 (2001)
BindingDB Entry DOI: 10.7270/Q2125RXQ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50092331
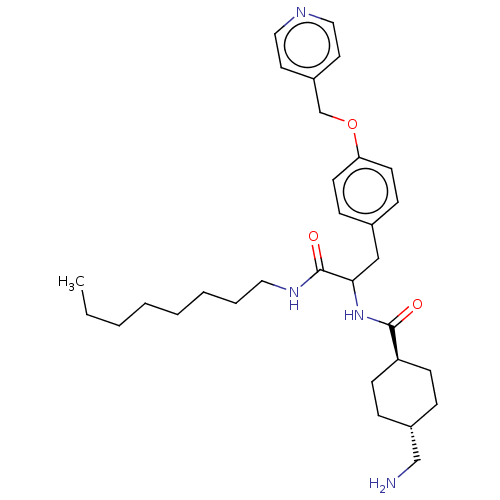
(4-Aminomethyl-cyclohexanecarboxylic acid {1-octylc...)Show SMILES CCCCCCCCNC(=O)C(Cc1ccc(OCc2ccncc2)cc1)NC(=O)[C@H]1CC[C@H](CN)CC1 |wU:33.35,wD:30.31,(22.3,-4.53,;22.3,-2.99,;20.97,-2.22,;19.64,-2.99,;18.3,-2.22,;16.97,-2.99,;15.64,-2.22,;14.3,-2.99,;12.97,-2.22,;11.63,-2.99,;11.63,-4.53,;10.3,-2.22,;10.3,-.68,;11.63,.09,;12.97,-.68,;14.3,.09,;14.3,1.63,;15.64,2.4,;16.97,1.63,;18.3,2.4,;18.3,3.94,;19.64,4.71,;20.97,3.94,;20.97,2.4,;19.64,1.63,;12.97,2.4,;11.63,1.63,;8.97,-2.99,;8.97,-4.53,;10.3,-5.3,;7.63,-5.3,;6.3,-4.53,;4.97,-5.3,;4.97,-6.84,;3.63,-7.61,;2.3,-6.84,;6.3,-7.61,;7.63,-6.84,)| Show InChI InChI=1S/C31H46N4O3/c1-2-3-4-5-6-7-18-34-31(37)29(35-30(36)27-12-8-25(22-32)9-13-27)21-24-10-14-28(15-11-24)38-23-26-16-19-33-20-17-26/h10-11,14-17,19-20,25,27,29H,2-9,12-13,18,21-23,32H2,1H3,(H,34,37)(H,35,36)/t25-,27-,29? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound was evaluated against plasmin |
Bioorg Med Chem Lett 10: 2217-21 (2001)
BindingDB Entry DOI: 10.7270/Q2125RXQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50317151

((2S,2'S)-diethyl 2,2'-(((6,7-dimethyl-8H-indeno[1,...)Show SMILES CCOC(=O)[C@H](C)NP(=O)(COc1cc(C)c(C)c2Cc3scnc3-c12)N[C@@H](C)C(=O)OCC |r| Show InChI InChI=1S/C23H32N3O6PS/c1-7-30-22(27)15(5)25-33(29,26-16(6)23(28)31-8-2)12-32-18-9-13(3)14(4)17-10-19-21(20(17)18)24-11-34-19/h9,11,15-16H,7-8,10,12H2,1-6H3,(H2,25,26,29)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 2938-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.017
BindingDB Entry DOI: 10.7270/Q2222TXQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50317153
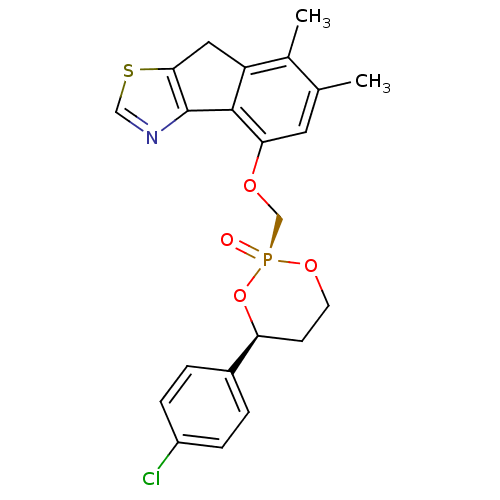
(4-[4-(4-Chloro-phenyl)-2-oxo-2lambda*5*-[1,3,2]dio...)Show SMILES Cc1cc(OC[P@@]2(=O)OCC[C@H](O2)c2ccc(Cl)cc2)c-2c(Cc3scnc-23)c1C |r| Show InChI InChI=1S/C22H21ClNO4PS/c1-13-9-19(21-17(14(13)2)10-20-22(21)24-11-30-20)26-12-29(25)27-8-7-18(28-29)15-3-5-16(23)6-4-15/h3-6,9,11,18H,7-8,10,12H2,1-2H3/t18-,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 2938-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.017
BindingDB Entry DOI: 10.7270/Q2222TXQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50317153

(4-[4-(4-Chloro-phenyl)-2-oxo-2lambda*5*-[1,3,2]dio...)Show SMILES Cc1cc(OC[P@@]2(=O)OCC[C@H](O2)c2ccc(Cl)cc2)c-2c(Cc3scnc-23)c1C |r| Show InChI InChI=1S/C22H21ClNO4PS/c1-13-9-19(21-17(14(13)2)10-20-22(21)24-11-30-20)26-12-29(25)27-8-7-18(28-29)15-3-5-16(23)6-4-15/h3-6,9,11,18H,7-8,10,12H2,1-2H3/t18-,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 2938-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.017
BindingDB Entry DOI: 10.7270/Q2222TXQ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50092335
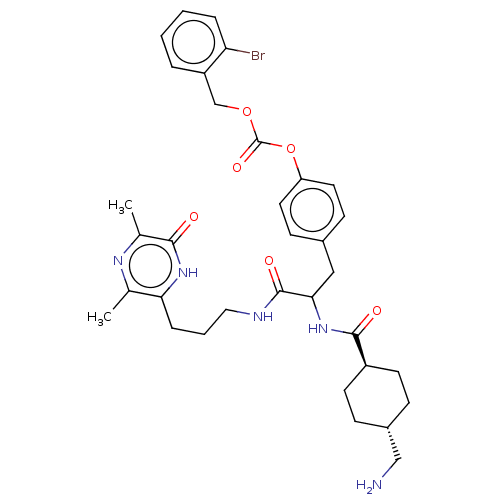
(CHEMBL3084794 | Carbonic acid 4-{2-[(4-aminomethyl...)Show SMILES Cc1nc(C)c(CCCNC(=O)C(Cc2ccc(OC(=O)OCc3ccccc3Br)cc2)NC(=O)[C@H]2CC[C@H](CN)CC2)nc1O |wU:35.36,wD:38.40,(3.86,-9.45,;3.86,-7.91,;5.19,-7.14,;5.19,-5.6,;6.53,-4.83,;3.86,-4.83,;3.86,-3.29,;2.53,-2.52,;1.19,-3.29,;-.14,-2.52,;-1.47,-3.29,;-1.47,-4.83,;-2.81,-2.52,;-2.81,-.98,;-1.47,-.21,;-.14,-.98,;1.19,-.21,;1.19,1.33,;2.53,2.1,;3.86,1.33,;3.86,-.21,;5.19,2.1,;6.53,1.33,;7.86,2.1,;7.86,3.64,;9.2,4.41,;10.53,3.64,;10.53,2.1,;9.2,1.33,;9.2,-.21,;-.14,2.1,;-1.47,1.33,;-4.14,-3.29,;-4.14,-4.83,;-2.81,-5.6,;-5.48,-5.6,;-5.48,-7.14,;-6.81,-7.91,;-8.14,-7.14,;-9.48,-7.91,;-10.81,-7.14,;-8.14,-5.6,;-6.81,-4.83,;2.53,-5.6,;2.53,-7.14,;1.19,-7.91,)| Show InChI InChI=1S/C34H42BrN5O6/c1-21-29(39-31(41)22(2)38-21)8-5-17-37-33(43)30(40-32(42)25-13-9-24(19-36)10-14-25)18-23-11-15-27(16-12-23)46-34(44)45-20-26-6-3-4-7-28(26)35/h3-4,6-7,11-12,15-16,24-25,30H,5,8-10,13-14,17-20,36H2,1-2H3,(H,37,43)(H,39,41)(H,40,42)/t24-,25-,30? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound was evaluated against plasmin; not detectable |
Bioorg Med Chem Lett 10: 2217-21 (2001)
BindingDB Entry DOI: 10.7270/Q2125RXQ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50092332

(CHEMBL3084795 | Carbonic acid 4-{2-[(4-aminomethyl...)Show SMILES CCCCCCCCNC(=O)C(Cc1ccc(OC(=O)OCc2ccccc2Br)cc1)NC(=O)[C@H]1CC[C@H](CN)CC1 |wU:37.39,wD:34.35,(15.04,-7.34,;15.04,-5.8,;13.71,-5.03,;12.37,-5.8,;11.04,-5.03,;9.71,-5.8,;8.37,-5.03,;7.04,-5.8,;5.71,-5.03,;4.37,-5.8,;4.37,-7.34,;3.04,-5.03,;3.04,-3.49,;4.37,-2.72,;5.71,-3.49,;7.04,-2.72,;7.04,-1.18,;8.37,-.41,;9.71,-1.18,;9.71,-2.72,;11.04,-.41,;12.37,-1.18,;13.71,-.41,;13.71,1.13,;15.04,1.9,;16.37,1.13,;16.37,-.41,;15.04,-1.18,;15.04,-2.72,;5.71,-.41,;4.37,-1.18,;1.7,-5.8,;1.7,-7.34,;3.04,-8.11,;.37,-8.11,;-.96,-7.34,;-2.3,-8.11,;-2.3,-9.65,;-3.63,-10.42,;-4.96,-9.65,;-.96,-10.42,;.37,-9.65,)| Show InChI InChI=1S/C33H46BrN3O5/c1-2-3-4-5-6-9-20-36-32(39)30(37-31(38)26-16-12-25(22-35)13-17-26)21-24-14-18-28(19-15-24)42-33(40)41-23-27-10-7-8-11-29(27)34/h7-8,10-11,14-15,18-19,25-26,30H,2-6,9,12-13,16-17,20-23,35H2,1H3,(H,36,39)(H,37,38)/t25-,26-,30? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound was evaluated against plasmin |
Bioorg Med Chem Lett 10: 2217-21 (2001)
BindingDB Entry DOI: 10.7270/Q2125RXQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Rattus norvegicus) | BDBM50016703
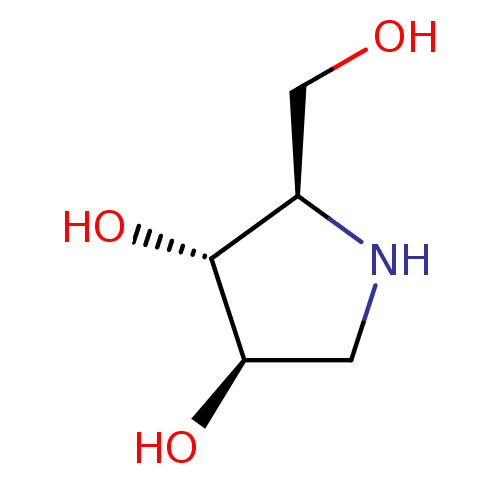
(2-Hydroxymethyl-pyrrolidine-3,4-diol | BDBM5003148...)Show InChI InChI=1S/C5H11NO3/c7-2-3-5(9)4(8)1-6-3/h3-9H,1-2H2/t3-,4-,5?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of glycogen phosphorylase in rat hepatocytes assessed as reduction in basal glycogenolysis |
Drug Metab Dispos 41: 878-87 (2013)
Article DOI: 10.1124/dmd.112.050591
BindingDB Entry DOI: 10.7270/Q2TB18M8 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Rattus norvegicus) | BDBM50016703

(2-Hydroxymethyl-pyrrolidine-3,4-diol | BDBM5003148...)Show InChI InChI=1S/C5H11NO3/c7-2-3-5(9)4(8)1-6-3/h3-9H,1-2H2/t3-,4-,5?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of glycogen phosphorylase in rat hepatocytes assessed as reduction in glucagom-induced glycogenolysis |
Drug Metab Dispos 41: 878-87 (2013)
Article DOI: 10.1124/dmd.112.050591
BindingDB Entry DOI: 10.7270/Q2TB18M8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50317157
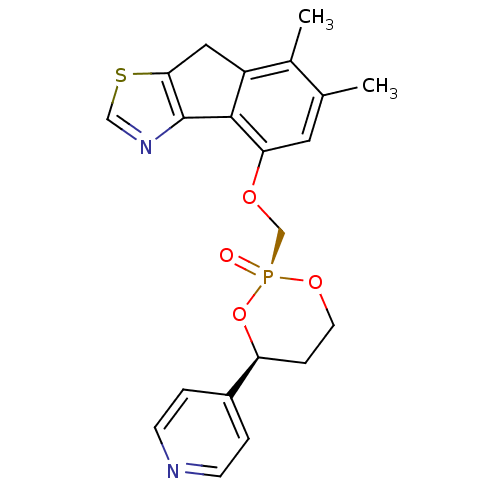
(6,7-Dimethyl-4-(2-oxo-4-pyridin-4-yl-2lambda*5*-[1...)Show SMILES Cc1cc(OC[P@@]2(=O)OCC[C@H](O2)c2ccncc2)c-2c(Cc3scnc-23)c1C |r| Show InChI InChI=1S/C21H21N2O4PS/c1-13-9-18(20-16(14(13)2)10-19-21(20)23-11-29-19)25-12-28(24)26-8-5-17(27-28)15-3-6-22-7-4-15/h3-4,6-7,9,11,17H,5,8,10,12H2,1-2H3/t17-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 2938-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.017
BindingDB Entry DOI: 10.7270/Q2222TXQ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50092334
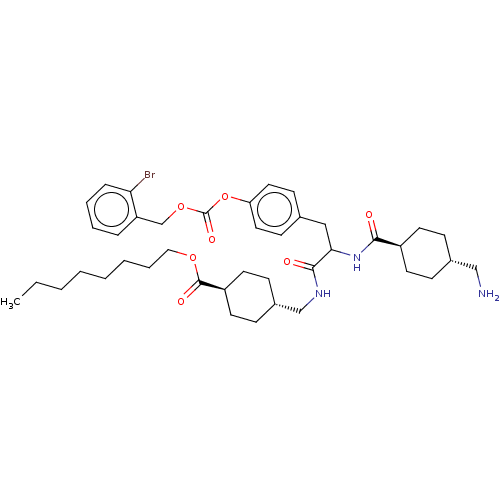
(4-({2-[(4-Aminomethyl-cyclohexanecarbonyl)-amino]-...)Show SMILES CCCCCCCCOC(=O)[C@H]1CC[C@H](CNC(=O)C(Cc2ccc(OC(=O)OCc3ccccc3Br)cc2)NC(=O)[C@H]2CC[C@H](CN)CC2)CC1 |wU:42.43,14.14,wD:11.10,45.47,(22.77,-3.21,;21.44,-2.44,;20.1,-3.21,;18.77,-2.44,;17.44,-3.21,;16.1,-2.44,;14.77,-3.21,;13.44,-2.44,;12.1,-3.21,;10.77,-2.44,;10.77,-.9,;9.44,-3.21,;8.1,-2.44,;6.77,-3.21,;6.77,-4.75,;5.43,-5.52,;4.1,-4.75,;2.77,-5.52,;2.77,-7.06,;1.43,-4.75,;1.43,-3.21,;.1,-2.44,;.1,-.9,;-1.23,-.13,;-2.57,-.9,;-3.9,-.13,;-3.9,1.41,;-2.57,2.18,;-5.24,2.18,;-5.24,3.72,;-6.57,4.49,;-7.9,3.72,;-9.24,4.49,;-9.24,6.03,;-7.9,6.8,;-6.57,6.03,;-5.24,6.8,;-2.57,-2.44,;-1.23,-3.21,;.1,-5.52,;.1,-7.06,;1.43,-7.83,;-1.23,-7.83,;-1.23,-9.37,;-2.57,-10.14,;-3.9,-9.37,;-5.24,-10.14,;-6.57,-9.37,;-3.9,-7.83,;-2.57,-7.06,;8.1,-5.52,;9.44,-4.75,)| Show InChI InChI=1S/C41H58BrN3O7/c1-2-3-4-5-6-9-24-50-40(48)33-20-14-31(15-21-33)27-44-39(47)37(45-38(46)32-18-12-30(26-43)13-19-32)25-29-16-22-35(23-17-29)52-41(49)51-28-34-10-7-8-11-36(34)42/h7-8,10-11,16-17,22-23,30-33,37H,2-6,9,12-15,18-21,24-28,43H2,1H3,(H,44,47)(H,45,46)/t30-,31-,32-,33-,37? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound was evaluated against plasmin |
Bioorg Med Chem Lett 10: 2217-21 (2001)
BindingDB Entry DOI: 10.7270/Q2125RXQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50317152

((2R,4S)-4-[4-(3-Chloro-phenyl)-2-oxo-2lambda*5*-[1...)Show SMILES Cc1cc(OC[P@@]2(=O)OCC[C@H](O2)c2cccc(Cl)c2)c-2c(Cc3scnc-23)c1C |r| Show InChI InChI=1S/C22H21ClNO4PS/c1-13-8-19(21-17(14(13)2)10-20-22(21)24-11-30-20)26-12-29(25)27-7-6-18(28-29)15-4-3-5-16(23)9-15/h3-5,8-9,11,18H,6-7,10,12H2,1-2H3/t18-,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 2938-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.017
BindingDB Entry DOI: 10.7270/Q2222TXQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50317156
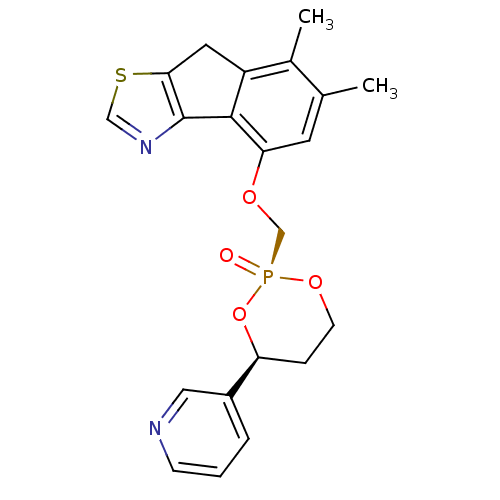
(6,7-Dimethyl-4-(2-oxo-4-pyridin-3-yl-2lambda*5*-[1...)Show SMILES Cc1cc(OC[P@@]2(=O)OCC[C@H](O2)c2cccnc2)c-2c(Cc3scnc-23)c1C |r| Show InChI InChI=1S/C21H21N2O4PS/c1-13-8-18(20-16(14(13)2)9-19-21(20)23-11-29-19)25-12-28(24)26-7-5-17(27-28)15-4-3-6-22-10-15/h3-4,6,8,10-11,17H,5,7,9,12H2,1-2H3/t17-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 2938-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.017
BindingDB Entry DOI: 10.7270/Q2222TXQ |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50030791
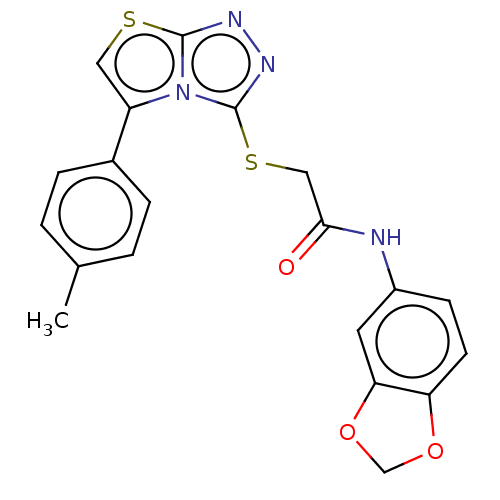
(CHEMBL3342402)Show SMILES Cc1ccc(cc1)-c1csc2nnc(SCC(=O)Nc3ccc4OCOc4c3)n12 Show InChI InChI=1S/C20H16N4O3S2/c1-12-2-4-13(5-3-12)15-9-28-19-22-23-20(24(15)19)29-10-18(25)21-14-6-7-16-17(8-14)27-11-26-16/h2-9H,10-11H2,1H3,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21G0QZ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50030791

(CHEMBL3342402)Show SMILES Cc1ccc(cc1)-c1csc2nnc(SCC(=O)Nc3ccc4OCOc4c3)n12 Show InChI InChI=1S/C20H16N4O3S2/c1-12-2-4-13(5-3-12)15-9-28-19-22-23-20(24(15)19)29-10-18(25)21-14-6-7-16-17(8-14)27-11-26-16/h2-9H,10-11H2,1H3,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 incubated for 60 mins in presence of L-tryptophan by HPLC analysis |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21G0QZ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50317159

(6,7-Dimethyl-4-[4-(6-methyl-pyridin-3-yl)-2-oxo-2l...)Show SMILES Cc1ccc(cn1)[C@@H]1CCO[P@](=O)(COc2cc(C)c(C)c3Cc4scnc4-c23)O1 |r| Show InChI InChI=1S/C22H23N2O4PS/c1-13-8-19(21-17(15(13)3)9-20-22(21)24-11-30-20)26-12-29(25)27-7-6-18(28-29)16-5-4-14(2)23-10-16/h4-5,8,10-11,18H,6-7,9,12H2,1-3H3/t18-,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 2938-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.017
BindingDB Entry DOI: 10.7270/Q2222TXQ |
More data for this
Ligand-Target Pair | |
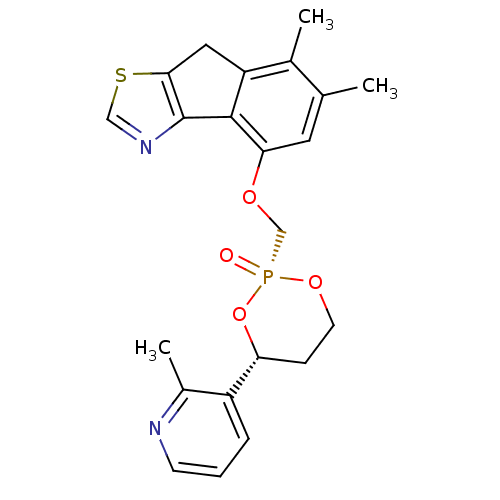
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50317158
(6,7-Dimethyl-4-[4-(2-methyl-pyridin-3-yl)-2-oxo-2l...)Show SMILES Cc1cc(OC[P@]2(=O)OCC[C@@H](O2)c2cccnc2C)c-2c(Cc3scnc-23)c1C |r| Show InChI InChI=1S/C22H23N2O4PS/c1-13-9-19(21-17(14(13)2)10-20-22(21)24-11-30-20)26-12-29(25)27-8-6-18(28-29)16-5-4-7-23-15(16)3/h4-5,7,9,11,18H,6,8,10,12H2,1-3H3/t18-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 2938-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.017
BindingDB Entry DOI: 10.7270/Q2222TXQ |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50558530

(CHEMBL4749159) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 incubated for 60 mins in presence of L-tryptophan by HPLC analysis |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21G0QZ0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50317155

(6,7-Dimethyl-4-(2-oxo-4-pyridin-2-yl-2lambda*5*-[1...)Show SMILES Cc1cc(OC[P@@]2(=O)OCC[C@H](O2)c2ccccn2)c-2c(Cc3scnc-23)c1C |r| Show InChI InChI=1S/C21H21N2O4PS/c1-13-9-18(20-15(14(13)2)10-19-21(20)23-11-29-19)25-12-28(24)26-8-6-17(27-28)16-5-3-4-7-22-16/h3-5,7,9,11,17H,6,8,10,12H2,1-2H3/t17-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 2938-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.017
BindingDB Entry DOI: 10.7270/Q2222TXQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50317160
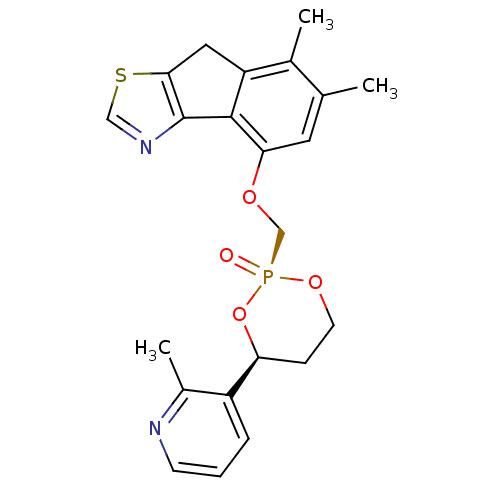
((2R,4S)-6,7-Dimethyl-4-[4-(2-methyl-pyridin-3-yl)-...)Show SMILES Cc1cc(OC[P@@]2(=O)OCC[C@H](O2)c2cccnc2C)c-2c(Cc3scnc-23)c1C |r| Show InChI InChI=1S/C22H23N2O4PS/c1-13-9-19(21-17(14(13)2)10-20-22(21)24-11-30-20)26-12-29(25)27-8-6-18(28-29)16-5-4-7-23-15(16)3/h4-5,7,9,11,18H,6,8,10,12H2,1-3H3/t18-,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 2938-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.017
BindingDB Entry DOI: 10.7270/Q2222TXQ |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50558522
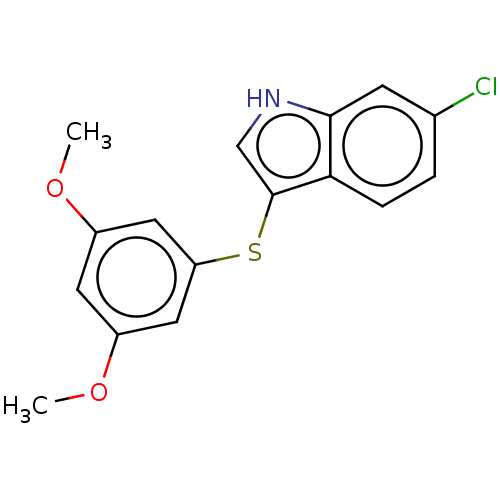
(CHEMBL4790386) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 incubated for 60 mins in presence of L-tryptophan by HPLC analysis |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21G0QZ0 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50558539

(CHEMBL4744327) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 incubated for 60 mins in presence of L-tryptophan by HPLC analysis |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21G0QZ0 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50558546
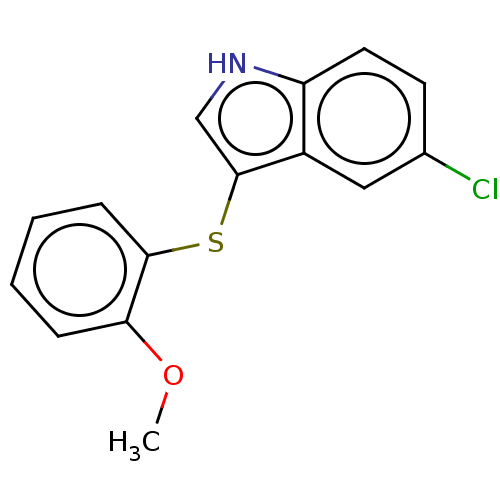
(CHEMBL374404) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 incubated for 60 mins in presence of L-tryptophan by HPLC analysis |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21G0QZ0 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50558528

(CHEMBL229510) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 incubated for 60 mins in presence of L-tryptophan by HPLC analysis |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21G0QZ0 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50558540
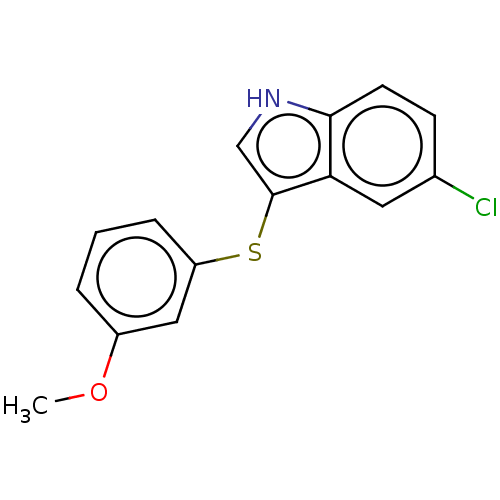
(CHEMBL4782372) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 incubated for 60 mins in presence of L-tryptophan by HPLC analysis |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21G0QZ0 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50558538
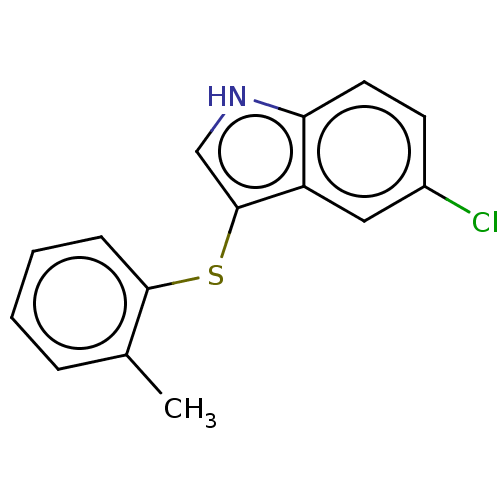
(CHEMBL4800450) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 incubated for 60 mins in presence of L-tryptophan by HPLC analysis |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21G0QZ0 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50558534

(CHEMBL4759143) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 incubated for 60 mins in presence of L-tryptophan by HPLC analysis |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21G0QZ0 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50558526
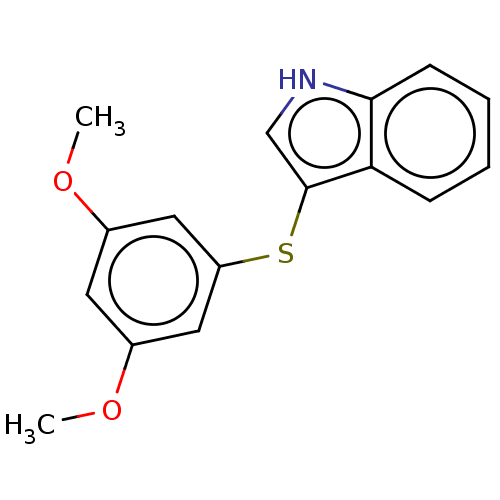
(CHEMBL4760664) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 incubated for 60 mins in presence of L-tryptophan by HPLC analysis |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21G0QZ0 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50451117
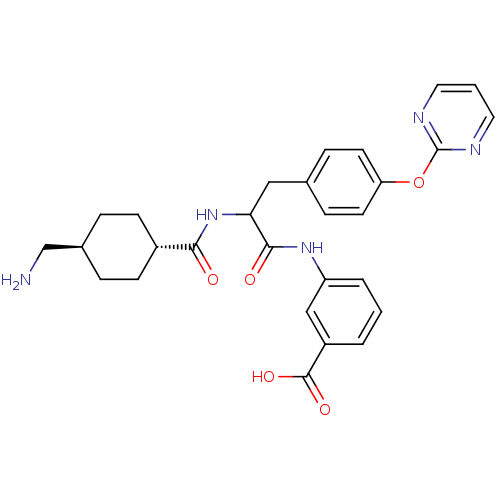
(CHEMBL3084798)Show SMILES NC[C@H]1CC[C@@H](CC1)C(=O)NC(Cc1ccc(Oc2ncccn2)cc1)C(=O)Nc1cccc(c1)C(O)=O |wU:2.1,wD:5.8,(-5.57,-3.35,;-4.8,-4.68,;-3.26,-4.68,;-2.49,-3.35,;-.95,-3.35,;-.18,-4.68,;-.95,-6.02,;-2.49,-6.02,;1.36,-4.68,;2.13,-6.02,;2.13,-3.35,;3.67,-3.35,;4.44,-2.02,;5.98,-2.02,;6.75,-3.35,;8.29,-3.35,;9.06,-2.02,;10.6,-2.02,;11.37,-.68,;12.91,-.68,;13.68,.65,;12.91,1.99,;11.37,1.99,;10.6,.65,;8.29,-.68,;6.75,-.68,;4.44,-4.68,;3.67,-6.02,;5.98,-4.68,;6.75,-6.02,;8.29,-6.02,;9.06,-7.35,;8.29,-8.68,;6.75,-8.68,;5.98,-7.35,;5.98,-10.02,;4.44,-10.02,;6.75,-11.35,)| Show InChI InChI=1S/C28H31N5O5/c29-17-19-5-9-20(10-6-19)25(34)33-24(26(35)32-22-4-1-3-21(16-22)27(36)37)15-18-7-11-23(12-8-18)38-28-30-13-2-14-31-28/h1-4,7-8,11-14,16,19-20,24H,5-6,9-10,15,17,29H2,(H,32,35)(H,33,34)(H,36,37)/t19-,20-,24? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound was evaluated against plasmin |
Bioorg Med Chem Lett 10: 2217-21 (2001)
BindingDB Entry DOI: 10.7270/Q2125RXQ |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50558535
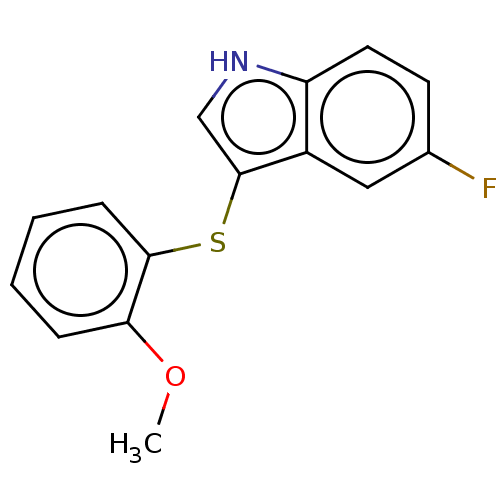
(CHEMBL4757189) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 incubated for 60 mins in presence of L-tryptophan by HPLC analysis |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21G0QZ0 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50558527
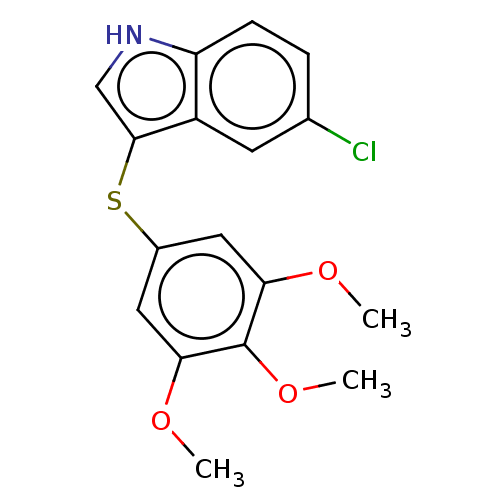
(CHEMBL389506) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 incubated for 60 mins in presence of L-tryptophan by HPLC analysis |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21G0QZ0 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50558533
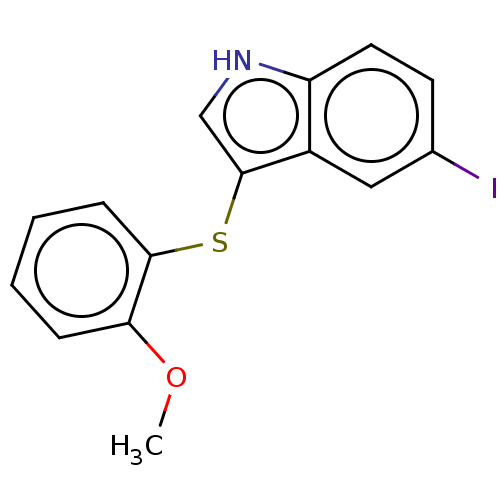
(CHEMBL4791547) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 incubated for 60 mins in presence of L-tryptophan by HPLC analysis |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21G0QZ0 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50558529
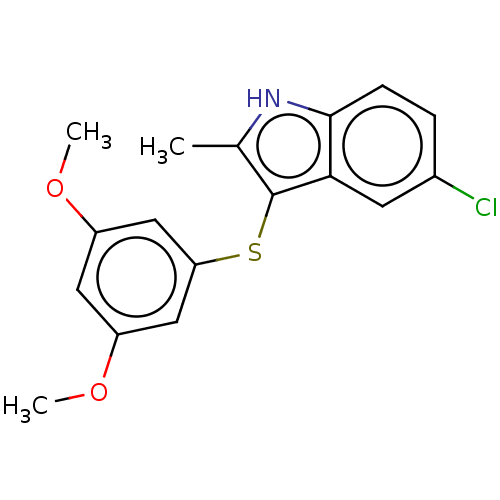
(CHEMBL4754880) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 incubated for 60 mins in presence of L-tryptophan by HPLC analysis |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21G0QZ0 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50558531

(CHEMBL4747897) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 incubated for 60 mins in presence of L-tryptophan by HPLC analysis |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21G0QZ0 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50558525
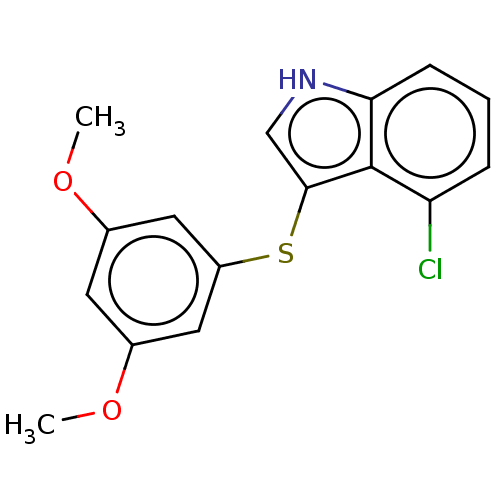
(CHEMBL4776062) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 incubated for 60 mins in presence of L-tryptophan by HPLC analysis |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21G0QZ0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data