

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor B (RAT) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50042235 (2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Endothelin A receptor | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122692 (CHEMBL282336 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122693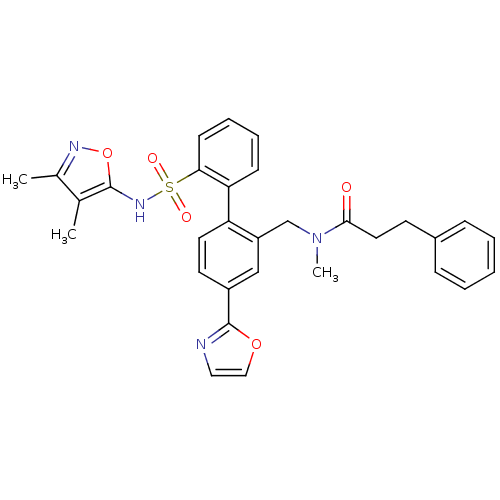 (CHEMBL29346 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122706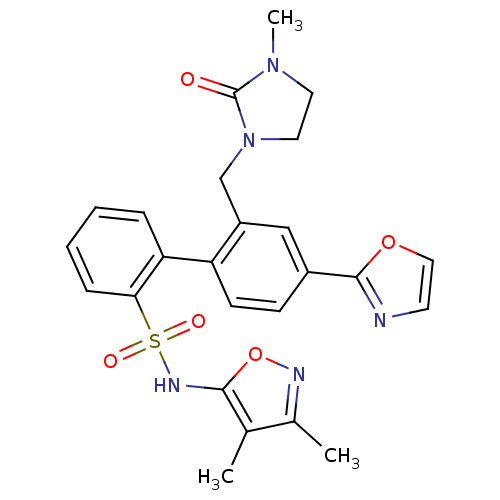 (2'-(3-Methyl-2-oxo-imidazolidin-1-ylmethyl)-4'-oxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122686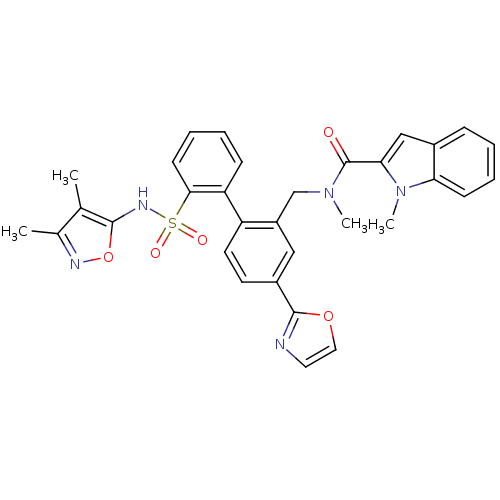 (1-Methyl-1H-indole-2-carboxylic acid [2'-(3,4-dime...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122694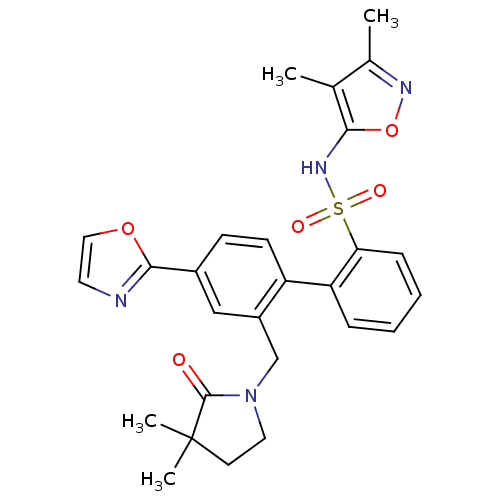 (2'-(3,3-Dimethyl-2-oxo-pyrrolidin-1-ylmethyl)-4'-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122715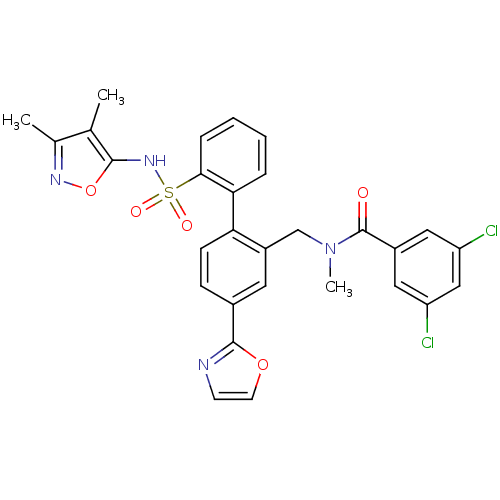 (3,5-Dichloro-N-[2'-(3,4-dimethyl-isoxazol-5-ylsulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122676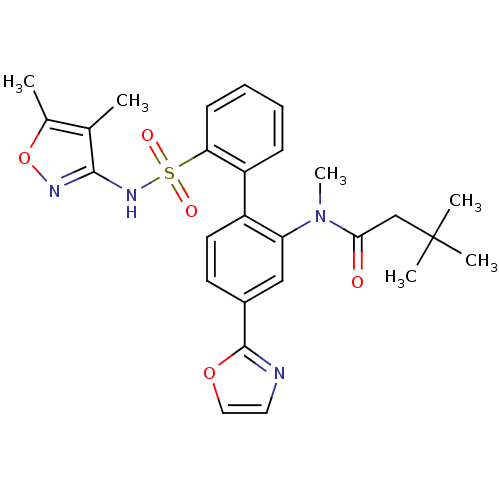 (CHEMBL274489 | N-[2'-(4,5-Dimethyl-isoxazol-3-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122712 (CHEMBL440780 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122697 (2'-[(Methyl-phenyl-amino)-methyl]-4'-oxazol-2-yl-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122707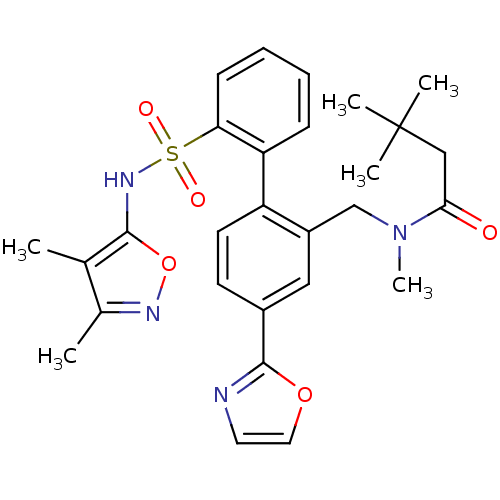 (CHEMBL281659 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122700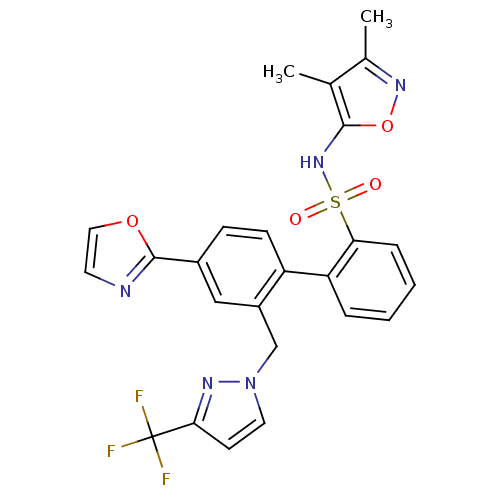 (4'-Oxazol-2-yl-2'-(3-trifluoromethyl-pyrazol-1-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122681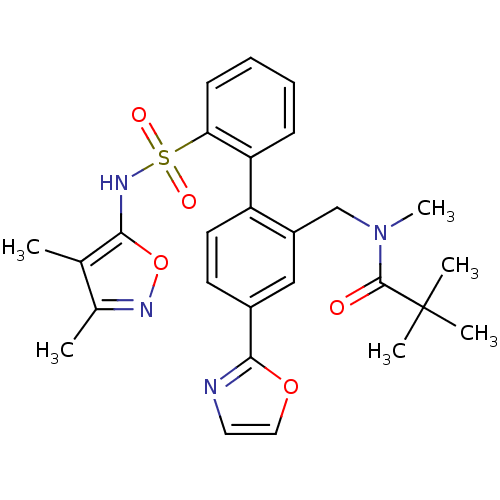 (CHEMBL27855 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50419921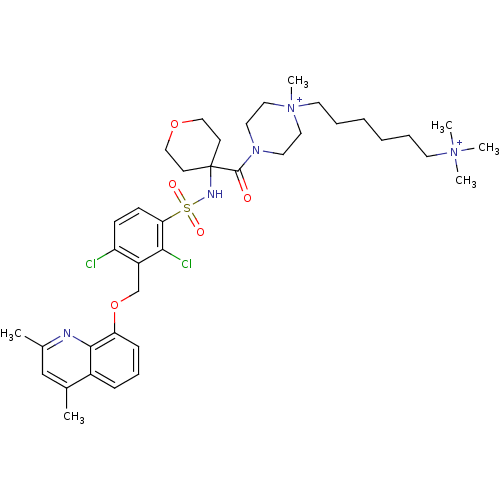 (CHEMBL1956864) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche Curated by ChEMBL | Assay Description Displacement of [3H]-Bradykinin from human bradykinin B2 receptor expressed in CHO cells membrane after 60 mins by scintillation counting | Bioorg Med Chem 20: 2091-100 (2012) Article DOI: 10.1016/j.bmc.2012.01.036 BindingDB Entry DOI: 10.7270/Q2PN96WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122698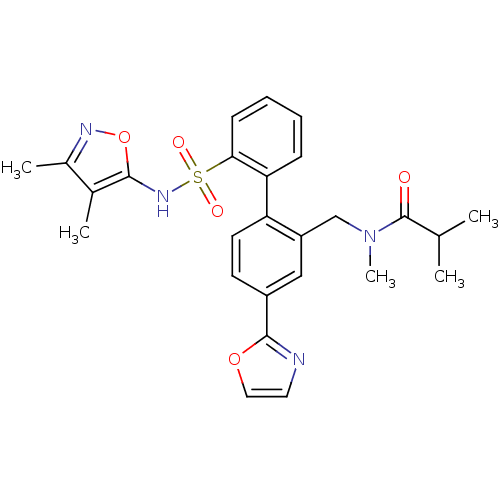 (CHEMBL28863 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122713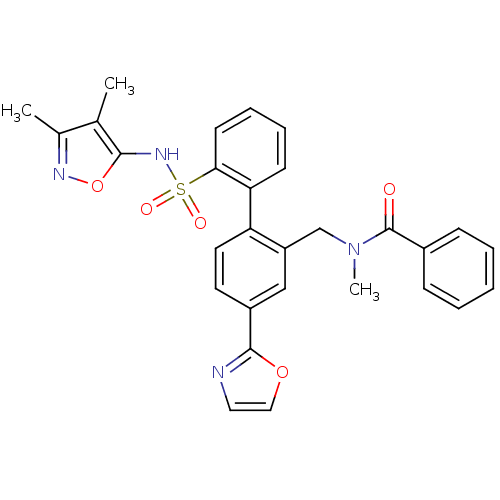 (CHEMBL282359 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122684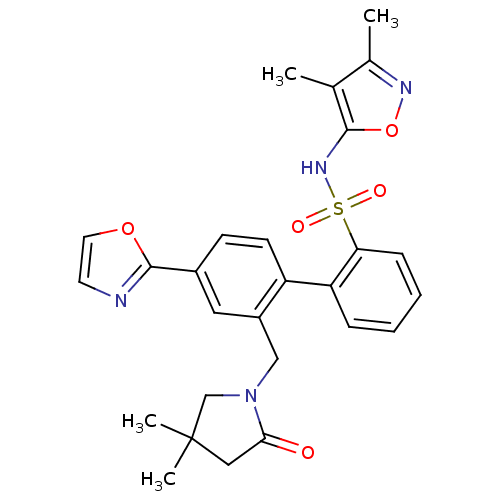 (2'-(4,4-Dimethyl-2-oxo-pyrrolidin-1-ylmethyl)-4'-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122696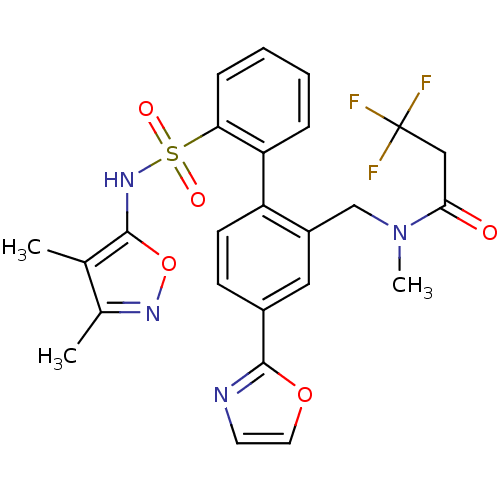 (CHEMBL281549 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122690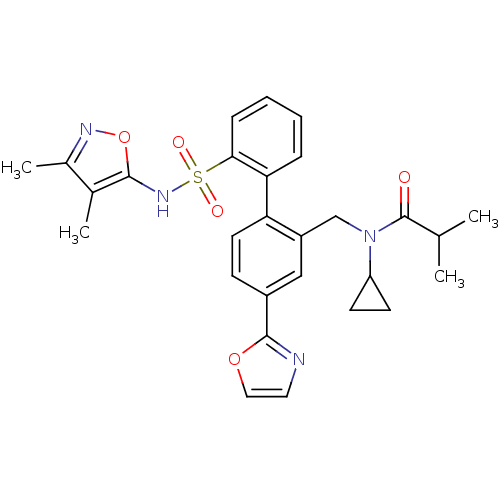 (CHEMBL28963 | N-Cyclopropyl-N-[2'-(3,4-dimethyl-is...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50419924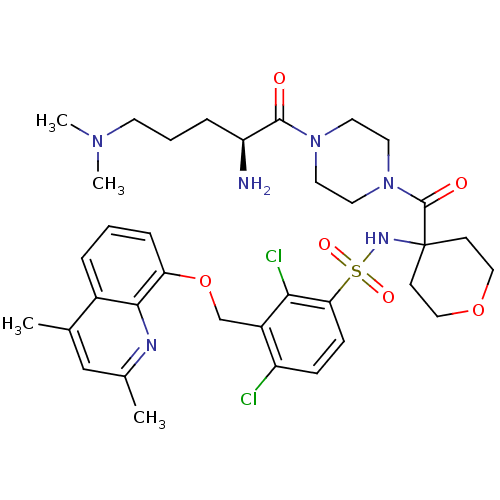 (CHEMBL1956856) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche Curated by ChEMBL | Assay Description Displacement of [3H]-Bradykinin from human bradykinin B2 receptor expressed in CHO cells membrane after 60 mins by scintillation counting | Bioorg Med Chem 20: 2091-100 (2012) Article DOI: 10.1016/j.bmc.2012.01.036 BindingDB Entry DOI: 10.7270/Q2PN96WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50411275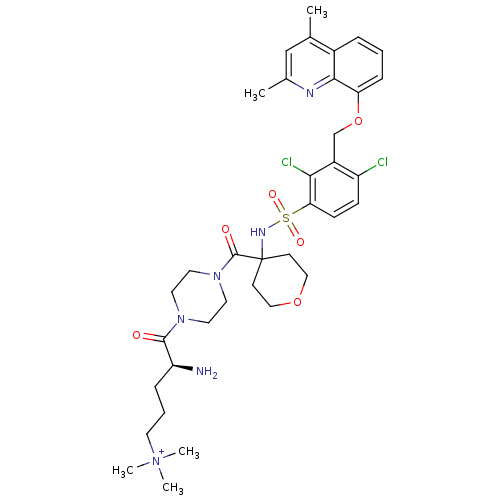 (CHEMBL218427 | MEN-16132) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche Curated by ChEMBL | Assay Description Displacement of [3H]-Bradykinin from human bradykinin B2 receptor expressed in CHO cells membrane after 60 mins by scintillation counting | Bioorg Med Chem 20: 2091-100 (2012) Article DOI: 10.1016/j.bmc.2012.01.036 BindingDB Entry DOI: 10.7270/Q2PN96WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50419907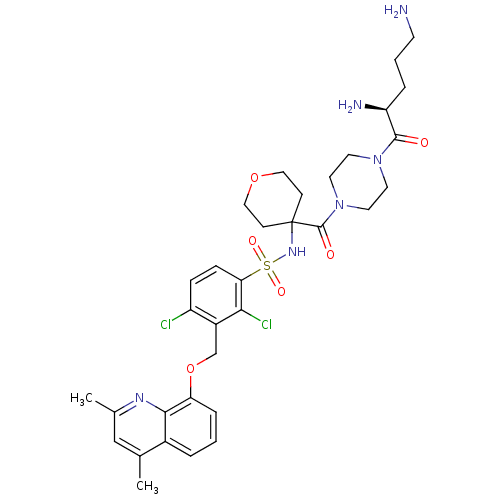 (CHEMBL1956855) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche Curated by ChEMBL | Assay Description Displacement of [3H]-Bradykinin from human bradykinin B2 receptor expressed in CHO cells membrane after 60 mins by scintillation counting | Bioorg Med Chem 20: 2091-100 (2012) Article DOI: 10.1016/j.bmc.2012.01.036 BindingDB Entry DOI: 10.7270/Q2PN96WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50419899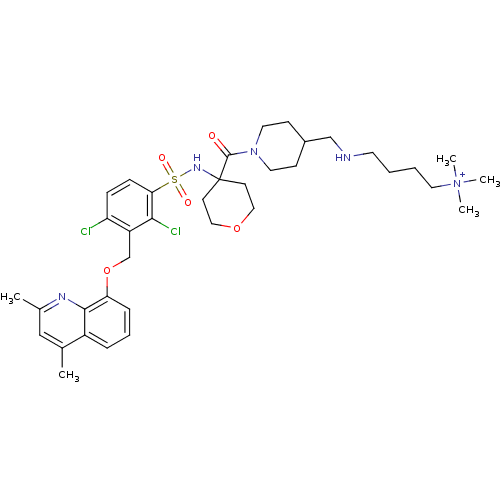 (CHEMBL1956876) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche Curated by ChEMBL | Assay Description Displacement of [3H]-Bradykinin from human bradykinin B2 receptor expressed in CHO cells membrane after 60 mins by scintillation counting | Bioorg Med Chem 20: 2091-100 (2012) Article DOI: 10.1016/j.bmc.2012.01.036 BindingDB Entry DOI: 10.7270/Q2PN96WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50415924 (CHEMBL1083850) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche Curated by ChEMBL | Assay Description Displacement of [3H]neurokinin A from human NK2 receptor | J Med Chem 53: 4148-65 (2010) Article DOI: 10.1021/jm100176s BindingDB Entry DOI: 10.7270/Q2MP54JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50419920 (CHEMBL1956863) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche Curated by ChEMBL | Assay Description Displacement of [3H]-Bradykinin from human bradykinin B2 receptor expressed in CHO cells membrane after 60 mins by scintillation counting | Bioorg Med Chem 20: 2091-100 (2012) Article DOI: 10.1016/j.bmc.2012.01.036 BindingDB Entry DOI: 10.7270/Q2PN96WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50419918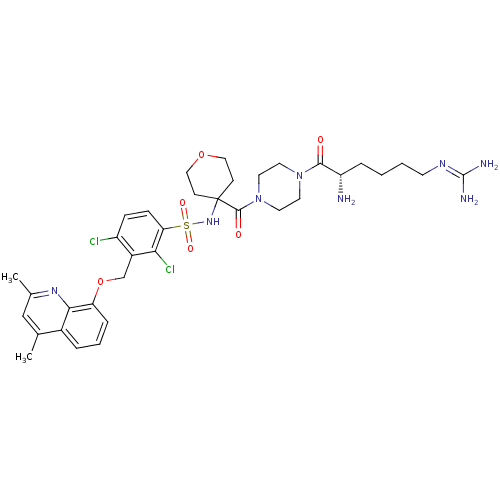 (CHEMBL1956719) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche Curated by ChEMBL | Assay Description Displacement of [3H]-Bradykinin from human bradykinin B2 receptor expressed in CHO cells membrane after 60 mins by scintillation counting | Bioorg Med Chem 20: 2091-100 (2012) Article DOI: 10.1016/j.bmc.2012.01.036 BindingDB Entry DOI: 10.7270/Q2PN96WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50419922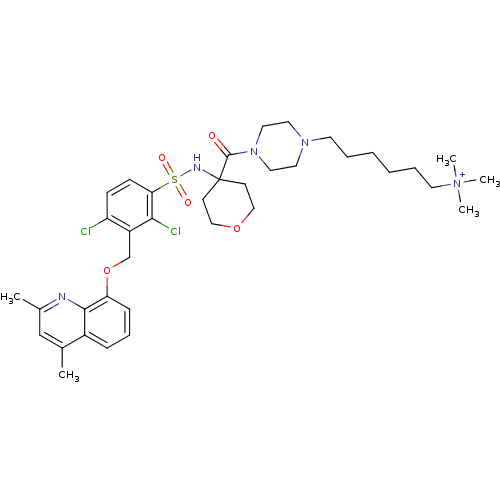 (CHEMBL1956870) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche Curated by ChEMBL | Assay Description Displacement of [3H]-Bradykinin from human bradykinin B2 receptor expressed in CHO cells membrane after 60 mins by scintillation counting | Bioorg Med Chem 20: 2091-100 (2012) Article DOI: 10.1016/j.bmc.2012.01.036 BindingDB Entry DOI: 10.7270/Q2PN96WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50419909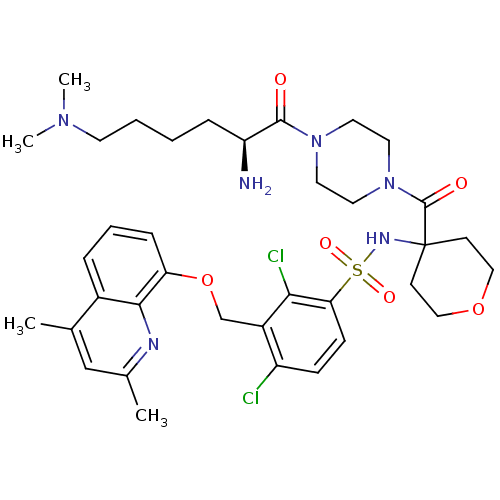 (CHEMBL1956858) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche Curated by ChEMBL | Assay Description Displacement of [3H]-Bradykinin from human bradykinin B2 receptor expressed in CHO cells membrane after 60 mins by scintillation counting | Bioorg Med Chem 20: 2091-100 (2012) Article DOI: 10.1016/j.bmc.2012.01.036 BindingDB Entry DOI: 10.7270/Q2PN96WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004193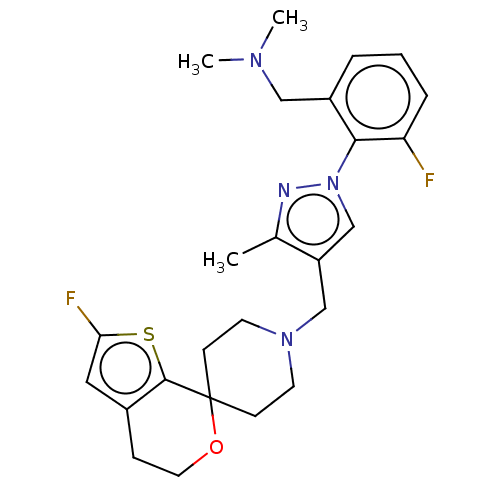 (CHEMBL3236476) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0648 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50122703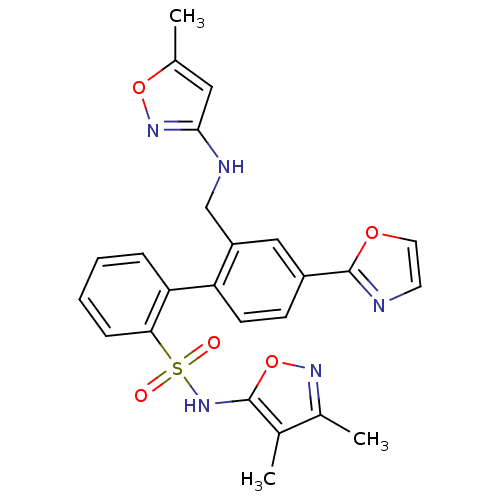 (2'-[(5-Methyl-isoxazol-3-ylamino)-methyl]-4'-oxazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human endothelin A receptor expressed in CHO cells | J Med Chem 46: 125-37 (2002) Article DOI: 10.1021/jm020289q BindingDB Entry DOI: 10.7270/Q2348M3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50167011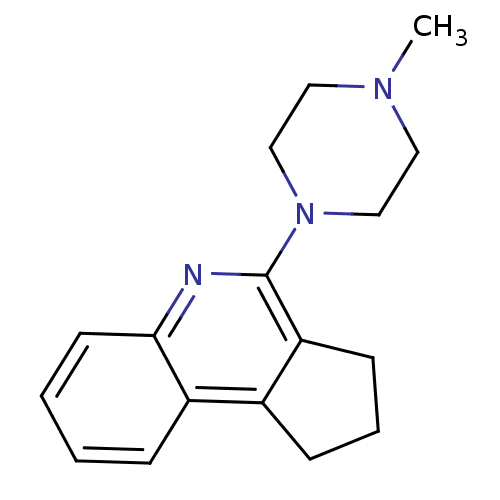 (4-(4-Methyl-piperazin-1-yl)-2,3-dihydro-1H-cyclope...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Rat 5-hydroxytryptamine 3 receptor (5-HT3) antagonist | J Med Chem 41: 2029-39 (1998) Article DOI: 10.1021/jm970745o BindingDB Entry DOI: 10.7270/Q2CJ8H7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004180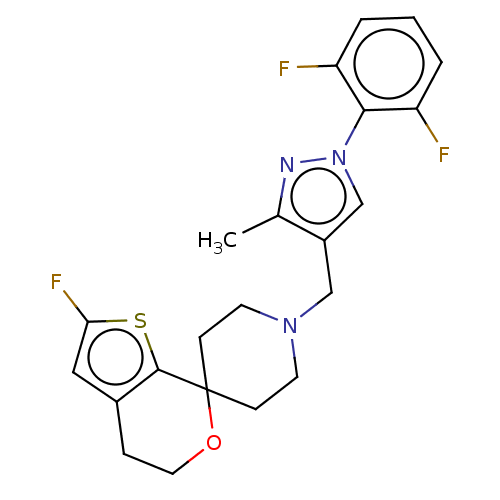 (CHEMBL3236474) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0711 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50415954 (CHEMBL1083858) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche Curated by ChEMBL | Assay Description Displacement of [3H]neurokinin A from human NK2 receptor | J Med Chem 53: 4148-65 (2010) Article DOI: 10.1021/jm100176s BindingDB Entry DOI: 10.7270/Q2MP54JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50415966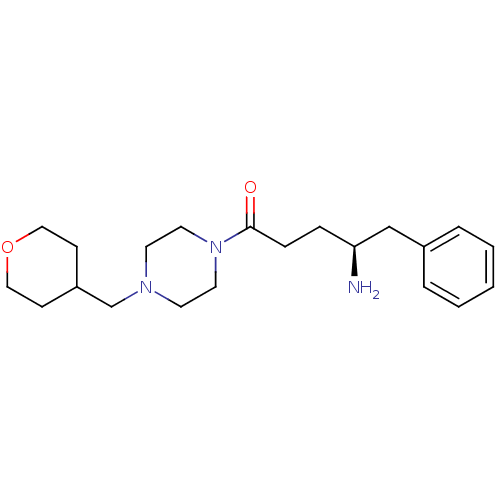 (CHEMBL1083974) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche Curated by ChEMBL | Assay Description Displacement of [3H]neurokinin A from human NK2 receptor | J Med Chem 53: 4148-65 (2010) Article DOI: 10.1021/jm100176s BindingDB Entry DOI: 10.7270/Q2MP54JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50419901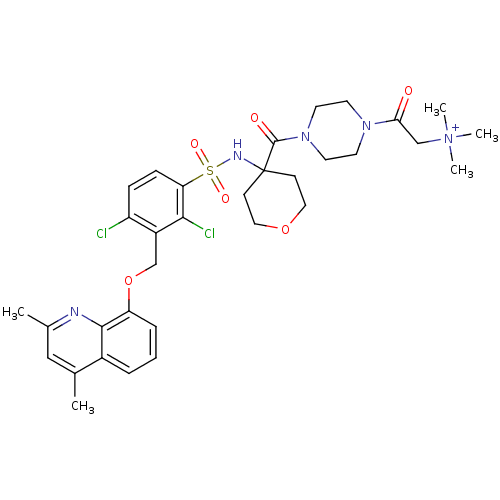 (CHEMBL1956720) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche Curated by ChEMBL | Assay Description Displacement of [3H]-Bradykinin from human bradykinin B2 receptor expressed in CHO cells membrane after 60 mins by scintillation counting | Bioorg Med Chem 20: 2091-100 (2012) Article DOI: 10.1016/j.bmc.2012.01.036 BindingDB Entry DOI: 10.7270/Q2PN96WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50419902 (CHEMBL1956722) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche Curated by ChEMBL | Assay Description Displacement of [3H]-Bradykinin from human bradykinin B2 receptor expressed in CHO cells membrane after 60 mins by scintillation counting | Bioorg Med Chem 20: 2091-100 (2012) Article DOI: 10.1016/j.bmc.2012.01.036 BindingDB Entry DOI: 10.7270/Q2PN96WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50419906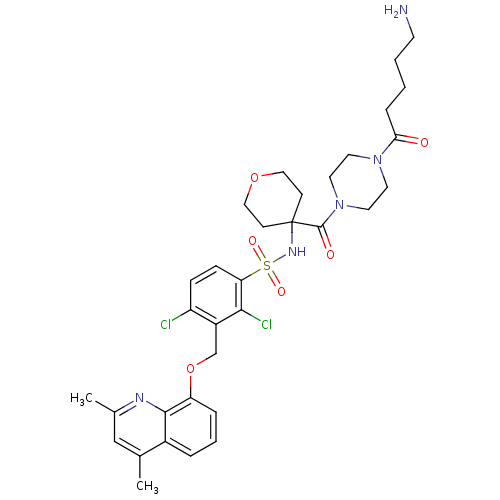 (CHEMBL1956854) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche Curated by ChEMBL | Assay Description Displacement of [3H]-Bradykinin from human bradykinin B2 receptor expressed in CHO cells membrane after 60 mins by scintillation counting | Bioorg Med Chem 20: 2091-100 (2012) Article DOI: 10.1016/j.bmc.2012.01.036 BindingDB Entry DOI: 10.7270/Q2PN96WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50419913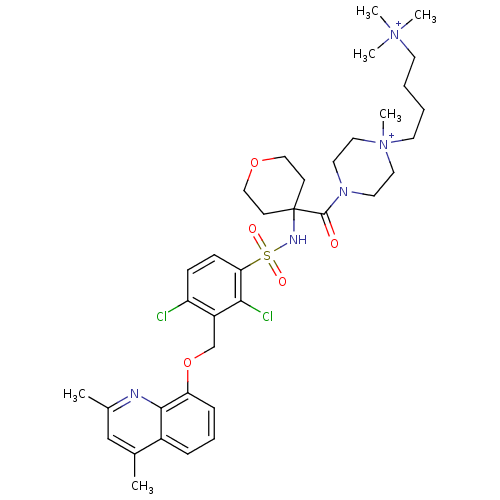 (CHEMBL1956862) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche Curated by ChEMBL | Assay Description Displacement of [3H]-Bradykinin from human bradykinin B2 receptor expressed in CHO cells membrane after 60 mins by scintillation counting | Bioorg Med Chem 20: 2091-100 (2012) Article DOI: 10.1016/j.bmc.2012.01.036 BindingDB Entry DOI: 10.7270/Q2PN96WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004195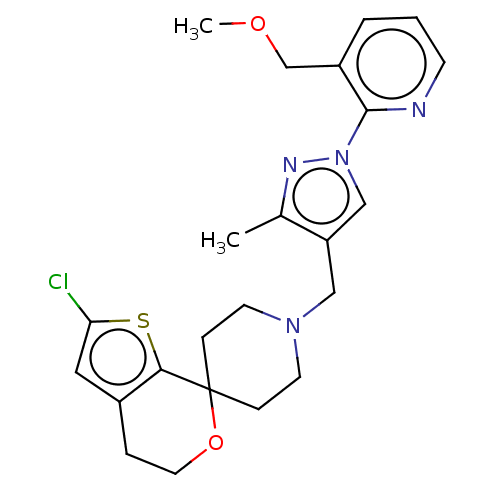 (CHEMBL3236478) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0908 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004175 (CHEMBL3236486) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0916 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004196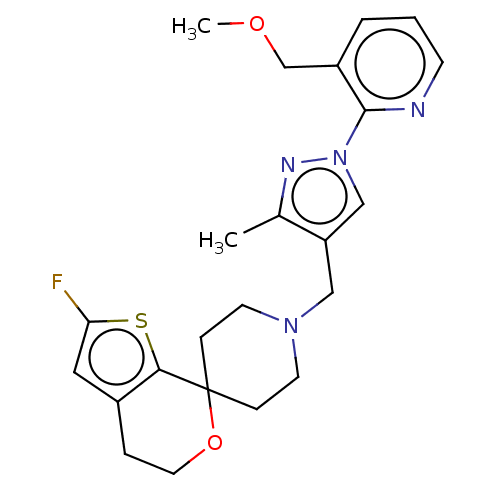 (CHEMBL3236479) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0939 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004181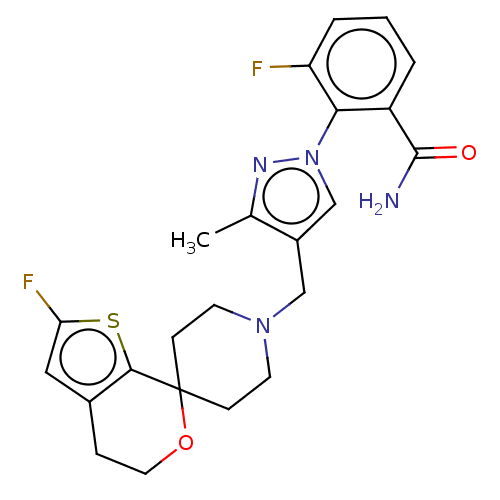 (CHEMBL3236475) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0954 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004174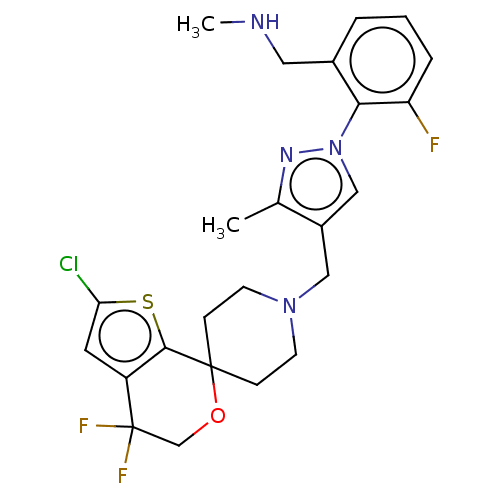 (CHEMBL3236485) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50063263 (6-(4-Methyl-piperazin-1-yl)-7,8,9,10-tetrahydro-ph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0977 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Rat 5-hydroxytryptamine 3 receptor (5-HT3) antagonist | J Med Chem 41: 2029-39 (1998) Article DOI: 10.1021/jm970745o BindingDB Entry DOI: 10.7270/Q2CJ8H7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50163035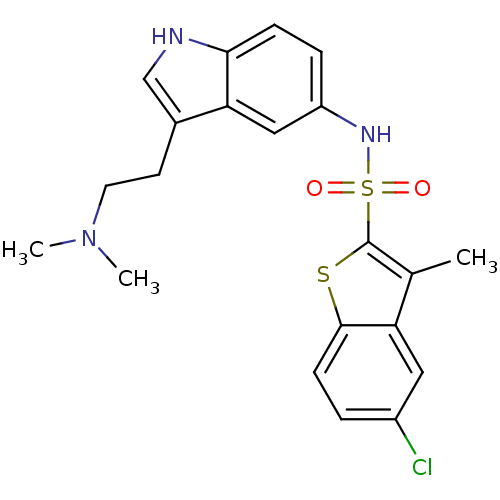 (5-Chloro-3-methyl-benzo[b]thiophene-2-sulfonic aci...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorios Dr. Esteve S.A. Curated by ChEMBL | Assay Description Inhibition of [3H]-LSD binding to human 5-hydroxytryptamine 6 receptor expressed in HEK293 cells | J Med Chem 48: 1781-95 (2005) Article DOI: 10.1021/jm049615n BindingDB Entry DOI: 10.7270/Q2ZP45N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50063264 (6-Piperazin-1-yl-7,8,9,10-tetrahydro-phenanthridin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Rat 5-hydroxytryptamine 3 receptor (5-HT3) antagonist | J Med Chem 41: 2029-39 (1998) Article DOI: 10.1021/jm970745o BindingDB Entry DOI: 10.7270/Q2CJ8H7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50415928 (CHEMBL1083879) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche Curated by ChEMBL | Assay Description Displacement of [3H]neurokinin A from human NK2 receptor | J Med Chem 53: 4148-65 (2010) Article DOI: 10.1021/jm100176s BindingDB Entry DOI: 10.7270/Q2MP54JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50419912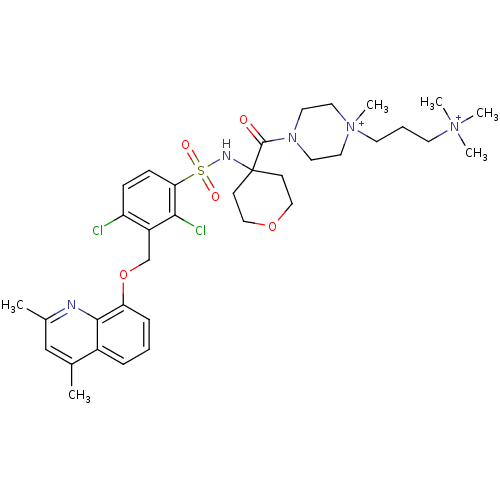 (CHEMBL1956861) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche Curated by ChEMBL | Assay Description Displacement of [3H]-Bradykinin from human bradykinin B2 receptor expressed in CHO cells membrane after 60 mins by scintillation counting | Bioorg Med Chem 20: 2091-100 (2012) Article DOI: 10.1016/j.bmc.2012.01.036 BindingDB Entry DOI: 10.7270/Q2PN96WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 10524 total ) | Next | Last >> |