

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153106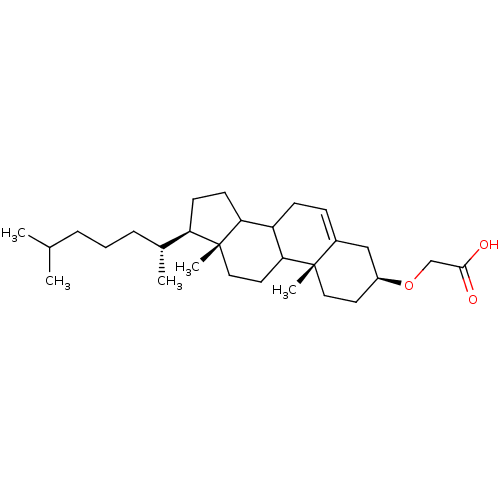 (CHEMBL189703 | [(3S,10R,13R,17R)-17-((R)-1,5-Dimet...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibition constant against DNA polymerase alpha non competitively on dNTP substrate | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50109030 (4-(3-Hydroxy-10,13-dimethyl-hexadecahydro-cyclopen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition constant of the compound was determined at a concentration of 5 microM on rat DNA polymerase beta as a function of template primer dose | Bioorg Med Chem Lett 12: 287-90 (2002) BindingDB Entry DOI: 10.7270/Q2K936TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50109030 (4-(3-Hydroxy-10,13-dimethyl-hexadecahydro-cyclopen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition constant of the compound was determined at a concentration of 10 microM on rat DNA polymerase beta as a function of nucleotide substrate c... | Bioorg Med Chem Lett 12: 287-90 (2002) BindingDB Entry DOI: 10.7270/Q2K936TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153111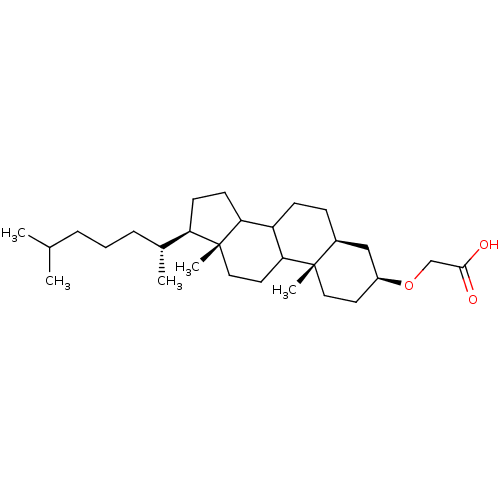 (CHEMBL189667 | [(3S,5S,10S,13R,17R)-17-((R)-1,5-Di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibition constant against DNA polymerase alpha non competitively on dNTP substrate | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153113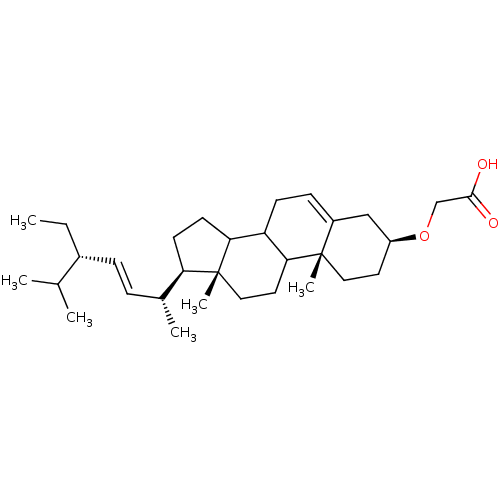 (CHEMBL364611 | [(3S,10R,13R,17R)-17-((E)-(1R,4S)-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibition constant against DNA polymerase alpha non competitively on dNTP substrate | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50109029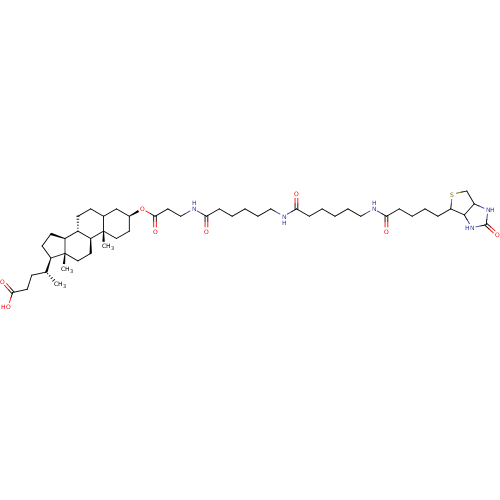 (4-{10,13-Dimethyl-3-[3-(6-{6-[5-(2-oxo-hexahydro-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition constant of the compound was determined at a concentration of 5 microM on rat DNA polymerase beta as a function of template primer dose | Bioorg Med Chem Lett 12: 287-90 (2002) BindingDB Entry DOI: 10.7270/Q2K936TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153111 (CHEMBL189667 | [(3S,5S,10S,13R,17R)-17-((R)-1,5-Di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibition constant against DNA polymerase alpha competitively on DNA template | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153106 (CHEMBL189703 | [(3S,10R,13R,17R)-17-((R)-1,5-Dimet...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibition constant against DNA polymerase alpha competitively on DNA template | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50109029 (4-{10,13-Dimethyl-3-[3-(6-{6-[5-(2-oxo-hexahydro-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibition constant of the compound was determined at a concentration of 10 microM on rat DNA polymerase beta as a function of template primer dose | Bioorg Med Chem Lett 12: 287-90 (2002) BindingDB Entry DOI: 10.7270/Q2K936TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153113 (CHEMBL364611 | [(3S,10R,13R,17R)-17-((E)-(1R,4S)-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibition constant against DNA polymerase alpha competitively on DNA template | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153113 (CHEMBL364611 | [(3S,10R,13R,17R)-17-((E)-(1R,4S)-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against human DNA polymerase alpha incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50153117 ((S)-4-((5R,10R,13R,17R)-3-Hydroxy-5,10,13-trimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against rat DNA polymerase beta incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50109030 (4-(3-Hydroxy-10,13-dimethyl-hexadecahydro-cyclopen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibitory effect of the compound on the activity of rat DNA polymerase beta was determined by inhibitory dose curves | Bioorg Med Chem Lett 12: 287-90 (2002) BindingDB Entry DOI: 10.7270/Q2K936TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153111 (CHEMBL189667 | [(3S,5S,10S,13R,17R)-17-((R)-1,5-Di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against human DNA polymerase alpha incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153106 (CHEMBL189703 | [(3S,10R,13R,17R)-17-((R)-1,5-Dimet...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against human DNA polymerase alpha incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50109031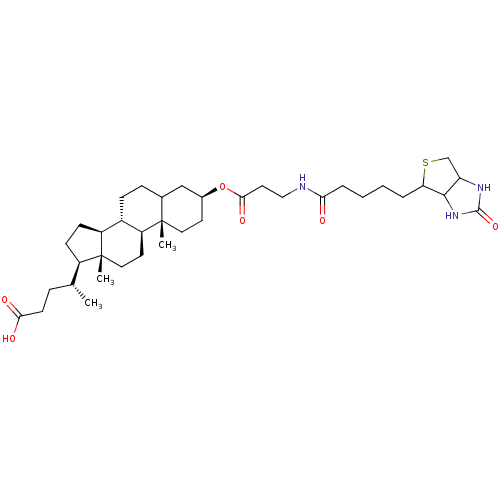 (4-(10,13-Dimethyl-3-{3-[5-(2-oxo-hexahydro-thieno[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibitory effect of the compound on the activity of rat DNA polymerase beta was determined by inhibitory dose curves | Bioorg Med Chem Lett 12: 287-90 (2002) BindingDB Entry DOI: 10.7270/Q2K936TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50109029 (4-{10,13-Dimethyl-3-[3-(6-{6-[5-(2-oxo-hexahydro-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibitory effect of the compound on the activity of mammalian DNA polymerase beta was determined by inhibitory dose curves | Bioorg Med Chem Lett 12: 287-90 (2002) BindingDB Entry DOI: 10.7270/Q2K936TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153117 ((S)-4-((5R,10R,13R,17R)-3-Hydroxy-5,10,13-trimethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against human DNA polymerase alpha incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50109032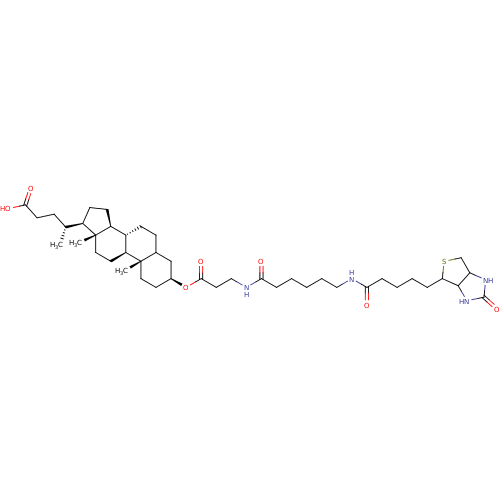 ((R)-4-[(3S,10S,13R,17R)-10,13-Dimethyl-3-(3-{6-[5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science Curated by ChEMBL | Assay Description Inhibitory effect of the compound on the activity of rat DNA polymerase beta was determined by inhibitory dose curves | Bioorg Med Chem Lett 12: 287-90 (2002) BindingDB Entry DOI: 10.7270/Q2K936TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50051836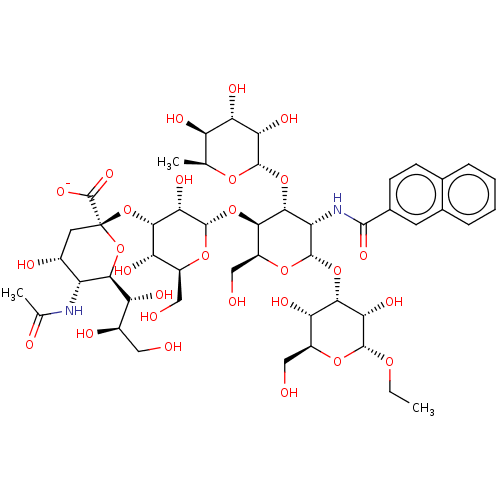 (CHEMBL2368519 | Ethyl(Sodium 5-actamido-3,5-dideox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cytel Corporation Curated by ChEMBL | Assay Description Compound was tested for the inhibition of E-selectin-mediated cellular adhesion | J Med Chem 39: 1357-60 (1996) Article DOI: 10.1021/jm9600611 BindingDB Entry DOI: 10.7270/Q22R3QQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50153113 (CHEMBL364611 | [(3S,10R,13R,17R)-17-((E)-(1R,4S)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against rat DNA polymerase beta incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50051833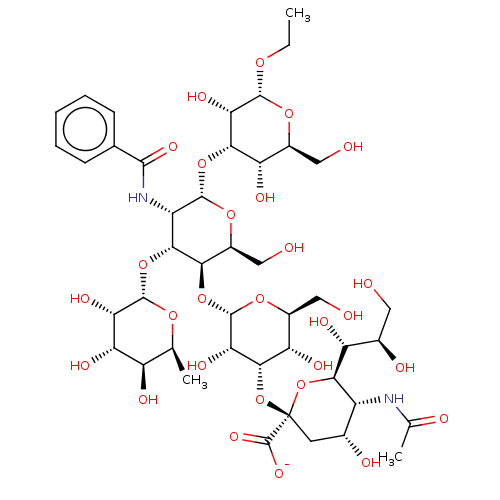 (CHEMBL2368521 | Ethyl(Sodium 5-actamido-3,5-dideox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cytel Corporation Curated by ChEMBL | Assay Description Compound was tested for the inhibition of E-selectin-mediated cellular adhesion | J Med Chem 39: 1357-60 (1996) Article DOI: 10.1021/jm9600611 BindingDB Entry DOI: 10.7270/Q22R3QQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50153106 (CHEMBL189703 | [(3S,10R,13R,17R)-17-((R)-1,5-Dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against rat DNA polymerase beta incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153115 (1-[(3S,5S,10S,13R,17R)-17-((R)-1,5-Dimethyl-hexyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against human DNA polymerase alpha incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153114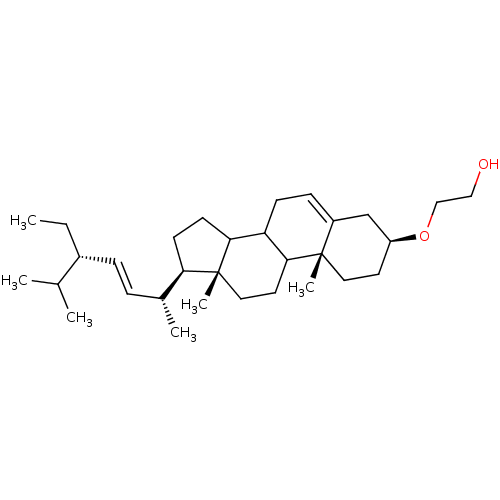 (2-[(3S,10R,13R,17R)-17-((E)-(1R,4S)-4-Ethyl-1,5-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against human DNA polymerase alpha incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153110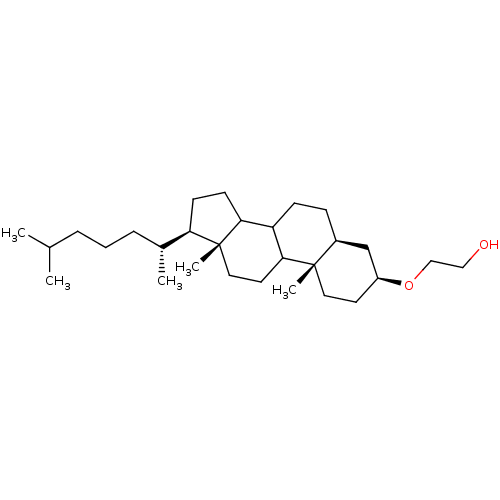 (2-[(3S,5S,10S,13R,17R)-17-((R)-1,5-Dimethyl-hexyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against human DNA polymerase alpha incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153118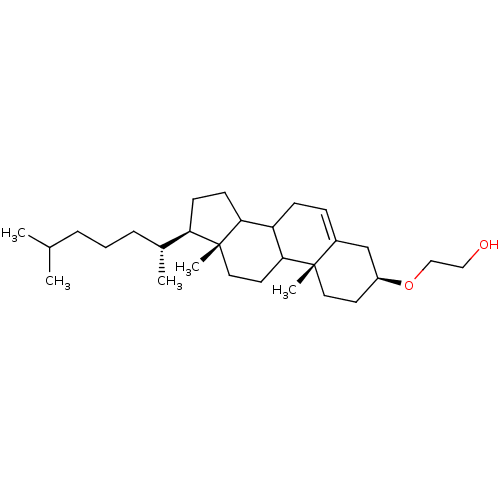 (2-[(3S,10R,13R,17R)-17-((R)-1,5-Dimethyl-hexyl)-10...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against human DNA polymerase alpha incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50153112 (1-[(3S,10R,13R,17R)-17-((R)-1,5-Dimethyl-hexyl)-10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against rat DNA polymerase beta incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50153107 ((3S,5S,10S,13R,17R)-17-((R)-1,5-Dimethyl-hexyl)-10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against rat DNA polymerase beta incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153112 (1-[(3S,10R,13R,17R)-17-((R)-1,5-Dimethyl-hexyl)-10...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against human DNA polymerase alpha incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50153118 (2-[(3S,10R,13R,17R)-17-((R)-1,5-Dimethyl-hexyl)-10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against rat DNA polymerase beta incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50153110 (2-[(3S,5S,10S,13R,17R)-17-((R)-1,5-Dimethyl-hexyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against rat DNA polymerase beta incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153109 ((3S,10R,13R,17R)-17-((E)-(1R,4S)-4-Ethyl-1,5-dimet...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against human DNA polymerase alpha incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50153111 (CHEMBL189667 | [(3S,5S,10S,13R,17R)-17-((R)-1,5-Di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against rat DNA polymerase beta incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50153108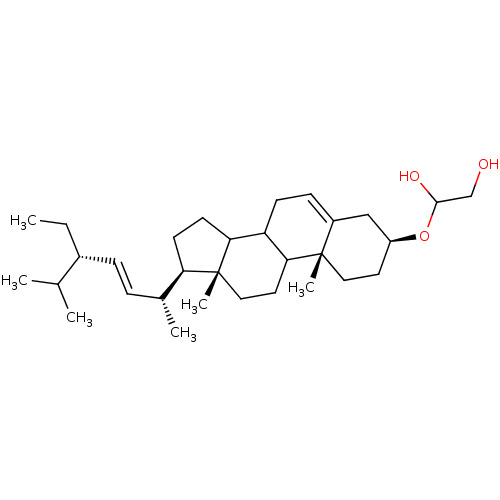 (1-[(3S,10R,13R,17R)-17-((E)-(1R,4S)-4-Ethyl-1,5-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against rat DNA polymerase beta incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153108 (1-[(3S,10R,13R,17R)-17-((E)-(1R,4S)-4-Ethyl-1,5-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against human DNA polymerase alpha incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50153109 ((3S,10R,13R,17R)-17-((E)-(1R,4S)-4-Ethyl-1,5-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against rat DNA polymerase beta incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50153115 (1-[(3S,5S,10S,13R,17R)-17-((R)-1,5-Dimethyl-hexyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against rat DNA polymerase beta incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM20192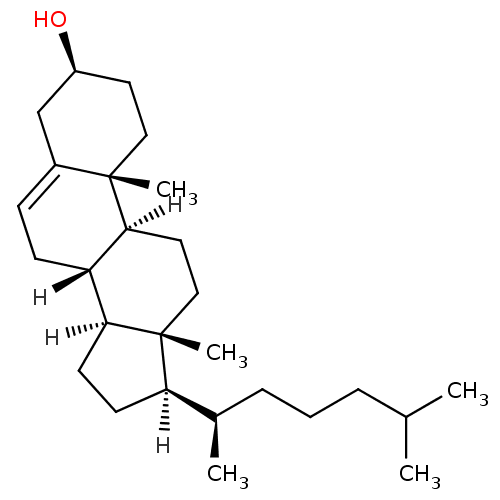 ((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against human DNA polymerase alpha incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM20192 ((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against rat DNA polymerase beta incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50153107 ((3S,5S,10S,13R,17R)-17-((R)-1,5-Dimethyl-hexyl)-10...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against human DNA polymerase alpha incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Rattus norvegicus) | BDBM50153114 (2-[(3S,10R,13R,17R)-17-((E)-(1R,4S)-4-Ethyl-1,5-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery Curated by ChEMBL | Assay Description Inhibitory concentration against rat DNA polymerase beta incubated with 0.05 units | J Med Chem 47: 4971-4 (2004) Article DOI: 10.1021/jm030553v BindingDB Entry DOI: 10.7270/Q2RN37BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50051835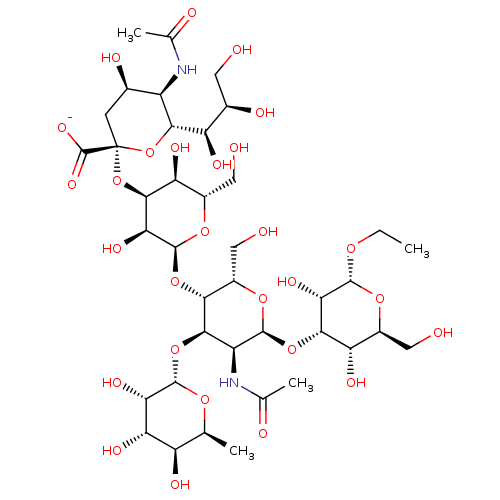 (CHEMBL2368520 | Ethyl(Sodium 5-actamido-3,5-dideox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cytel Corporation Curated by ChEMBL | Assay Description Compound was tested for the inhibition of E-selectin-mediated cellular adhesion | J Med Chem 39: 1357-60 (1996) Article DOI: 10.1021/jm9600611 BindingDB Entry DOI: 10.7270/Q22R3QQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50051834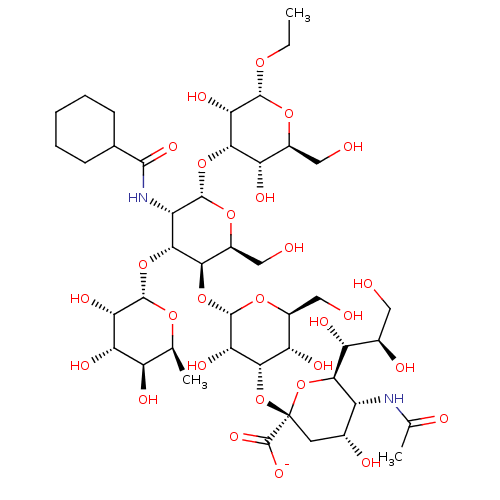 (CHEMBL2368518 | Ethyl(Sodium 5-actamido-3,5-dideox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cytel Corporation Curated by ChEMBL | Assay Description Compound was tested for the inhibition of E-selectin-mediated cellular adhesion | J Med Chem 39: 1357-60 (1996) Article DOI: 10.1021/jm9600611 BindingDB Entry DOI: 10.7270/Q22R3QQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||