 Found 56 hits with Last Name = 'ostroski' and Initial = 'r'
Found 56 hits with Last Name = 'ostroski' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347563

(CHEMBL1801740)Show SMILES CCc1nc2c(C)cc(CC(C)C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C29H31N7/c1-5-26-31-27-18(4)15-21(14-17(2)3)30-29(27)36(26)25-13-11-20-16-19(10-12-23(20)25)22-8-6-7-9-24(22)28-32-34-35-33-28/h6-10,12,15-17,25H,5,11,13-14H2,1-4H3,(H,32,33,34,35)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human Angiotensin receptor 1 |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347563

(CHEMBL1801740)Show SMILES CCc1nc2c(C)cc(CC(C)C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C29H31N7/c1-5-26-31-27-18(4)15-21(14-17(2)3)30-29(27)36(26)25-13-11-20-16-19(10-12-23(20)25)22-8-6-7-9-24(22)28-32-34-35-33-28/h6-10,12,15-17,25H,5,11,13-14H2,1-4H3,(H,32,33,34,35)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human Angiotensin receptor 2 |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17967
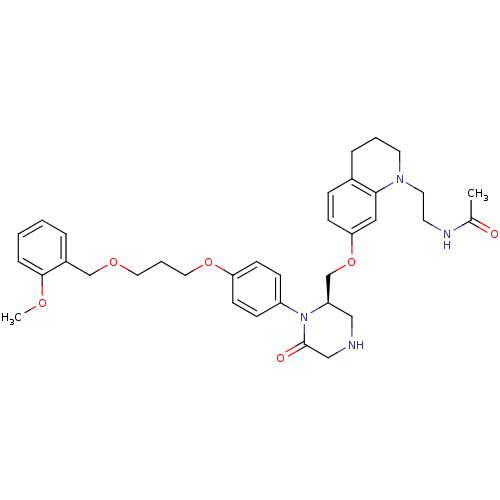
(CHEMBL411885 | Ketopiperazine-based compound, 16 |...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1[C@@H](COc2ccc3CCCN(CCNC(C)=O)c3c2)CNCC1=O |r| Show InChI InChI=1S/C35H44N4O6/c1-26(40)37-16-18-38-17-5-8-27-10-13-32(21-33(27)38)45-25-30-22-36-23-35(41)39(30)29-11-14-31(15-12-29)44-20-6-19-43-24-28-7-3-4-9-34(28)42-2/h3-4,7,9-15,21,30,36H,5-6,8,16-20,22-25H2,1-2H3,(H,37,40)/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 13: 2657-64 (2005)
Article DOI: 10.1016/j.bmc.2005.01.048
BindingDB Entry DOI: 10.7270/Q2KP80D6 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50043280
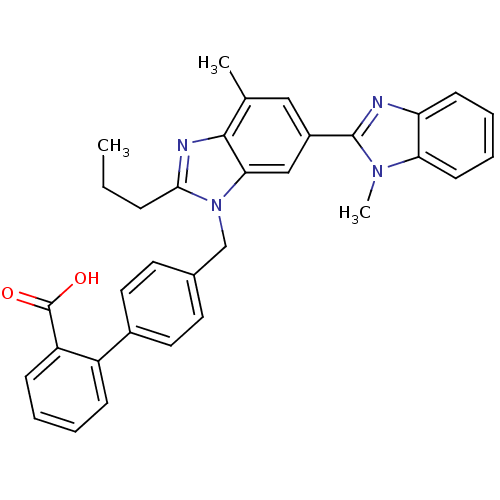
(4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)-c1nc2ccccc2n1C Show InChI InChI=1S/C33H30N4O2/c1-4-9-30-35-31-21(2)18-24(32-34-27-12-7-8-13-28(27)36(32)3)19-29(31)37(30)20-22-14-16-23(17-15-22)25-10-5-6-11-26(25)33(38)39/h5-8,10-19H,4,9,20H2,1-3H3,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17965
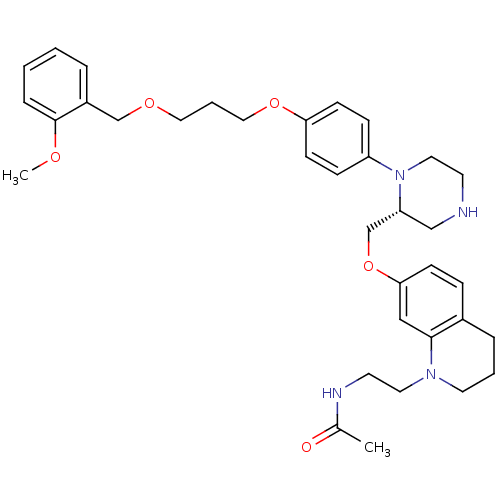
(N-[2-(7-{[(2R)-1-(4-{3-[(2-methoxyphenyl)methoxy]p...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1CCNC[C@@H]1COc1ccc2CCCN(CCNC(C)=O)c2c1 |r| Show InChI InChI=1S/C35H46N4O5/c1-27(40)37-17-19-38-18-5-8-28-10-13-33(23-34(28)38)44-26-31-24-36-16-20-39(31)30-11-14-32(15-12-30)43-22-6-21-42-25-29-7-3-4-9-35(29)41-2/h3-4,7,9-15,23,31,36H,5-6,8,16-22,24-26H2,1-2H3,(H,37,40)/t31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 13: 2657-64 (2005)
Article DOI: 10.1016/j.bmc.2005.01.048
BindingDB Entry DOI: 10.7270/Q2KP80D6 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347563

(CHEMBL1801740)Show SMILES CCc1nc2c(C)cc(CC(C)C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C29H31N7/c1-5-26-31-27-18(4)15-21(14-17(2)3)30-29(27)36(26)25-13-11-20-16-19(10-12-23(20)25)22-8-6-7-9-24(22)28-32-34-35-33-28/h6-10,12,15-17,25H,5,11,13-14H2,1-4H3,(H,32,33,34,35)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50009718
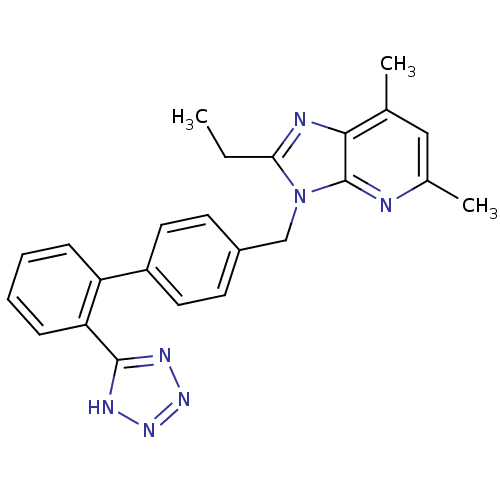
(2-Ethyl-5,7-dimethyl-3-[2'-(1H-tetrazol-5-yl)-biph...)Show SMILES CCc1nc2c(C)cc(C)nc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H23N7/c1-4-21-26-22-15(2)13-16(3)25-24(22)31(21)14-17-9-11-18(12-10-17)19-7-5-6-8-20(19)23-27-29-30-28-23/h5-13H,4,14H2,1-3H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347564
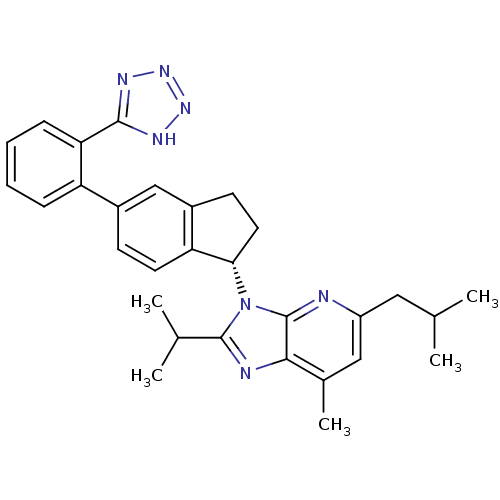
(CHEMBL1801741)Show SMILES CC(C)Cc1cc(C)c2nc(C(C)C)n([C@H]3CCc4cc(ccc34)-c3ccccc3-c3nnn[nH]3)c2n1 |r| Show InChI InChI=1S/C30H33N7/c1-17(2)14-22-15-19(5)27-30(31-22)37(29(32-27)18(3)4)26-13-11-21-16-20(10-12-24(21)26)23-8-6-7-9-25(23)28-33-35-36-34-28/h6-10,12,15-18,26H,11,13-14H2,1-5H3,(H,33,34,35,36)/t26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347565
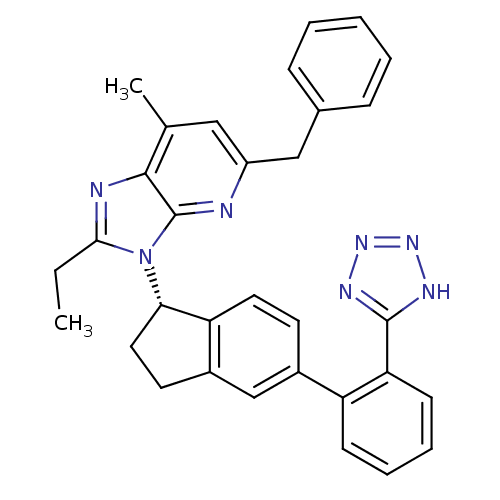
(CHEMBL1801743)Show SMILES CCc1nc2c(C)cc(Cc3ccccc3)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C32H29N7/c1-3-29-34-30-20(2)17-24(18-21-9-5-4-6-10-21)33-32(30)39(29)28-16-14-23-19-22(13-15-26(23)28)25-11-7-8-12-27(25)31-35-37-38-36-31/h4-13,15,17,19,28H,3,14,16,18H2,1-2H3,(H,35,36,37,38)/t28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347566
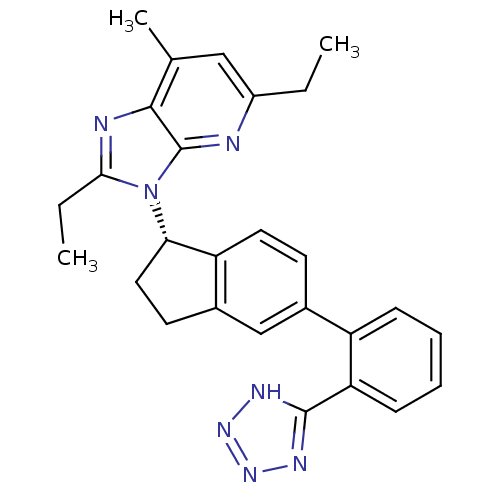
(CHEMBL1801738)Show SMILES CCc1nc2c(C)cc(CC)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C27H27N7/c1-4-19-14-16(3)25-27(28-19)34(24(5-2)29-25)23-13-11-18-15-17(10-12-21(18)23)20-8-6-7-9-22(20)26-30-32-33-31-26/h6-10,12,14-15,23H,4-5,11,13H2,1-3H3,(H,30,31,32,33)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347567

(CHEMBL1801712)Show SMILES CCc1nc2c(C)cc(C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C26H25N7/c1-4-23-28-24-15(2)13-16(3)27-26(24)33(23)22-12-10-18-14-17(9-11-20(18)22)19-7-5-6-8-21(19)25-29-31-32-30-25/h5-9,11,13-14,22H,4,10,12H2,1-3H3,(H,29,30,31,32)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347568
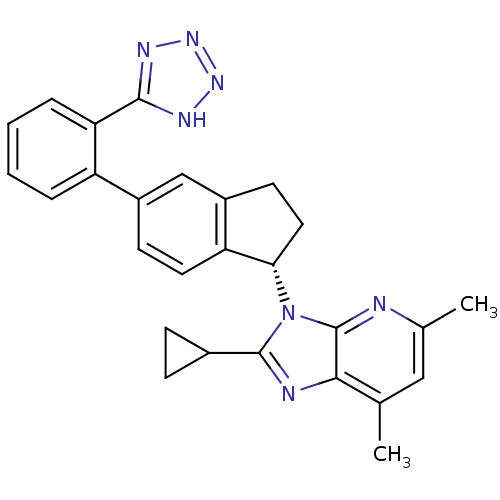
(CHEMBL1801735)Show SMILES Cc1cc(C)c2nc(C3CC3)n([C@H]3CCc4cc(ccc34)-c3ccccc3-c3nnn[nH]3)c2n1 |r| Show InChI InChI=1S/C27H25N7/c1-15-13-16(2)28-27-24(15)29-26(17-7-8-17)34(27)23-12-10-19-14-18(9-11-21(19)23)20-5-3-4-6-22(20)25-30-32-33-31-25/h3-6,9,11,13-14,17,23H,7-8,10,12H2,1-2H3,(H,30,31,32,33)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347569

(CHEMBL1801734)Show SMILES CC(C)c1nc2c(C)cc(C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C27H27N7/c1-15(2)26-29-24-16(3)13-17(4)28-27(24)34(26)23-12-10-19-14-18(9-11-21(19)23)20-7-5-6-8-22(20)25-30-32-33-31-25/h5-9,11,13-15,23H,10,12H2,1-4H3,(H,30,31,32,33)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347570
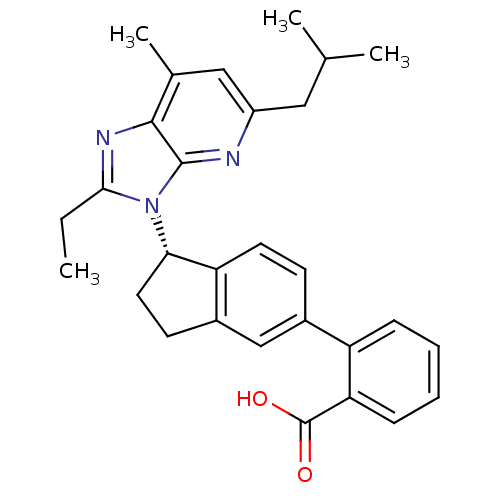
(CHEMBL1801744)Show SMILES CCc1nc2c(C)cc(CC(C)C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1C(O)=O |r| Show InChI InChI=1S/C29H31N3O2/c1-5-26-31-27-18(4)15-21(14-17(2)3)30-28(27)32(26)25-13-11-20-16-19(10-12-23(20)25)22-8-6-7-9-24(22)29(33)34/h6-10,12,15-17,25H,5,11,13-14H2,1-4H3,(H,33,34)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347571
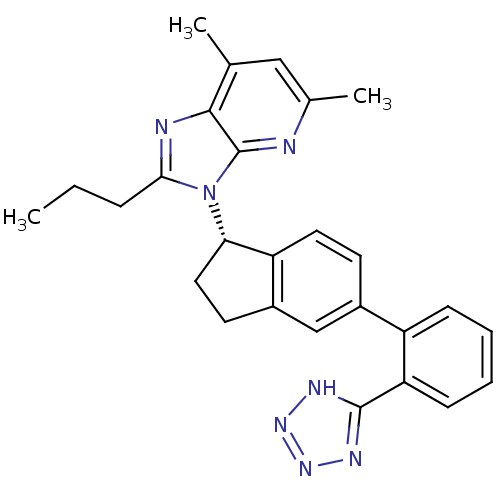
(CHEMBL1801714)Show SMILES CCCc1nc2c(C)cc(C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C27H27N7/c1-4-7-24-29-25-16(2)14-17(3)28-27(25)34(24)23-13-11-19-15-18(10-12-21(19)23)20-8-5-6-9-22(20)26-30-32-33-31-26/h5-6,8-10,12,14-15,23H,4,7,11,13H2,1-3H3,(H,30,31,32,33)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347572

(CHEMBL1801737)Show SMILES CCc1nc2c(C)c(CC)c(C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C28H29N7/c1-5-20-16(3)26-28(29-17(20)4)35(25(6-2)30-26)24-14-12-19-15-18(11-13-22(19)24)21-9-7-8-10-23(21)27-31-33-34-32-27/h7-11,13,15,24H,5-6,12,14H2,1-4H3,(H,31,32,33,34)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347573

(CHEMBL1801739)Show SMILES CCc1cc(C)c2nc(C(C)C)n([C@H]3CCc4cc(ccc34)-c3ccccc3-c3nnn[nH]3)c2n1 |r| Show InChI InChI=1S/C28H29N7/c1-5-20-14-17(4)25-28(29-20)35(27(30-25)16(2)3)24-13-11-19-15-18(10-12-22(19)24)21-8-6-7-9-23(21)26-31-33-34-32-26/h6-10,12,14-16,24H,5,11,13H2,1-4H3,(H,31,32,33,34)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17966
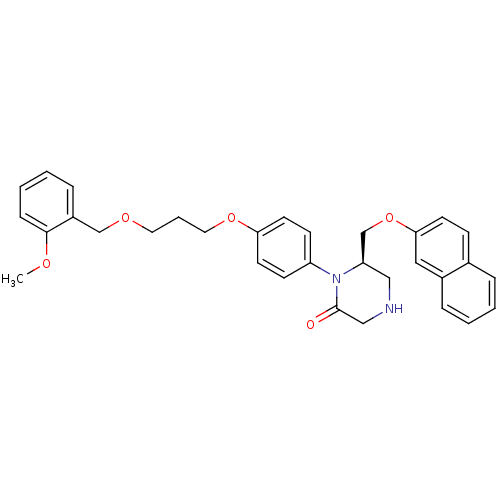
((6R)-1-(4-{3-[(2-methoxyphenyl)methoxy]propoxy}phe...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1[C@@H](COc2ccc3ccccc3c2)CNCC1=O |r| Show InChI InChI=1S/C32H34N2O5/c1-36-31-10-5-4-9-26(31)22-37-17-6-18-38-29-15-12-27(13-16-29)34-28(20-33-21-32(34)35)23-39-30-14-11-24-7-2-3-8-25(24)19-30/h2-5,7-16,19,28,33H,6,17-18,20-23H2,1H3/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 13: 2657-64 (2005)
Article DOI: 10.1016/j.bmc.2005.01.048
BindingDB Entry DOI: 10.7270/Q2KP80D6 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17951

((3S,4R)-4-(4-{3-[(2-methoxyphenyl)methoxy]propoxy}...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)[C@H]1CCNC[C@H]1NCc1ccc2ccccc2c1 |r| Show InChI InChI=1S/C33H38N2O3/c1-36-33-10-5-4-9-29(33)24-37-19-6-20-38-30-15-13-27(14-16-30)31-17-18-34-23-32(31)35-22-25-11-12-26-7-2-3-8-28(26)21-25/h2-5,7-16,21,31-32,34-35H,6,17-20,22-24H2,1H3/t31-,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 13: 59-68 (2005)
Article DOI: 10.1016/j.bmc.2004.09.056
BindingDB Entry DOI: 10.7270/Q2QC01SN |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17952
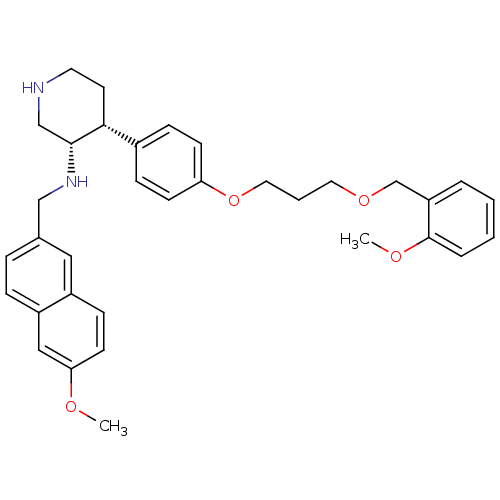
((3S,4R)-N-[(6-methoxynaphthalen-2-yl)methyl]-4-(4-...)Show SMILES COc1ccc2cc(CN[C@@H]3CNCC[C@@H]3c3ccc(OCCCOCc4ccccc4OC)cc3)ccc2c1 |r| Show InChI InChI=1S/C34H40N2O4/c1-37-31-15-12-27-20-25(8-9-28(27)21-31)22-36-33-23-35-17-16-32(33)26-10-13-30(14-11-26)40-19-5-18-39-24-29-6-3-4-7-34(29)38-2/h3-4,6-15,20-21,32-33,35-36H,5,16-19,22-24H2,1-2H3/t32-,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 13: 59-68 (2005)
Article DOI: 10.1016/j.bmc.2004.09.056
BindingDB Entry DOI: 10.7270/Q2QC01SN |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50282484
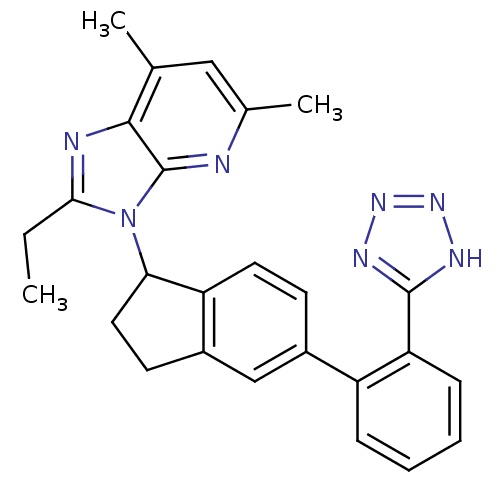
(2-Ethyl-5,7-dimethyl-3-{5-[2-(1H-tetrazol-5-yl)-ph...)Show SMILES CCc1nc2c(C)cc(C)nc2n1C1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C26H25N7/c1-4-23-28-24-15(2)13-16(3)27-26(24)33(23)22-12-10-18-14-17(9-11-20(18)22)19-7-5-6-8-21(19)25-29-31-32-30-25/h5-9,11,13-14,22H,4,10,12H2,1-3H3,(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17953
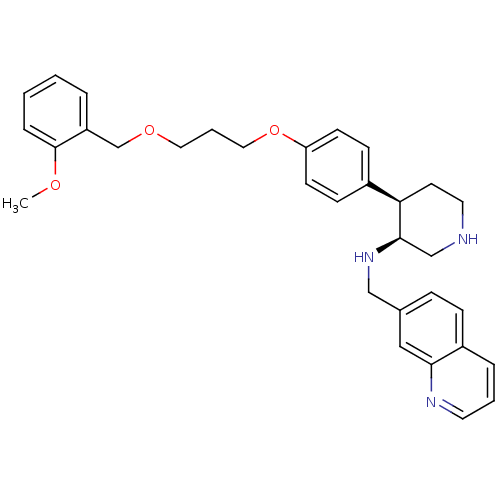
((3S,4R)-4-(4-{3-[(2-methoxyphenyl)methoxy]propoxy}...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)[C@H]1CCNC[C@H]1NCc1ccc2cccnc2c1 |r| Show InChI InChI=1S/C32H37N3O3/c1-36-32-8-3-2-6-27(32)23-37-18-5-19-38-28-13-11-25(12-14-28)29-15-17-33-22-31(29)35-21-24-9-10-26-7-4-16-34-30(26)20-24/h2-4,6-14,16,20,29,31,33,35H,5,15,17-19,21-23H2,1H3/t29-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 175 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 13: 59-68 (2005)
Article DOI: 10.1016/j.bmc.2004.09.056
BindingDB Entry DOI: 10.7270/Q2QC01SN |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17961

((2R)-1-(4-{3-[(2-methoxyphenyl)methoxy]propoxy}phe...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1CCNC[C@@H]1COc1ccc2ccccc2c1 |r| Show InChI InChI=1S/C32H36N2O4/c1-35-32-10-5-4-9-27(32)23-36-19-6-20-37-30-15-12-28(13-16-30)34-18-17-33-22-29(34)24-38-31-14-11-25-7-2-3-8-26(25)21-31/h2-5,7-16,21,29,33H,6,17-20,22-24H2,1H3/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 13: 2657-64 (2005)
Article DOI: 10.1016/j.bmc.2005.01.048
BindingDB Entry DOI: 10.7270/Q2KP80D6 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347575
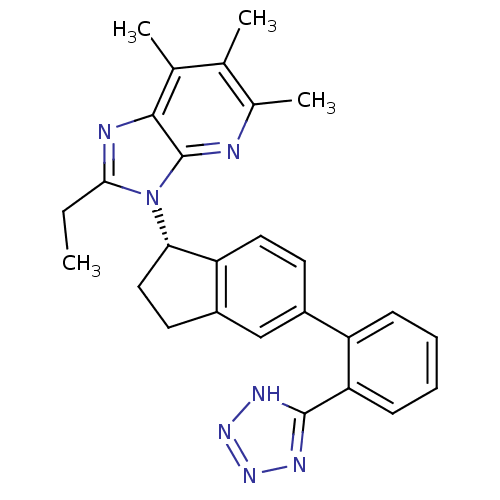
(CHEMBL1801736)Show SMILES CCc1nc2c(C)c(C)c(C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C27H27N7/c1-5-24-29-25-16(3)15(2)17(4)28-27(25)34(24)23-13-11-19-14-18(10-12-21(19)23)20-8-6-7-9-22(20)26-30-32-33-31-26/h6-10,12,14,23H,5,11,13H2,1-4H3,(H,30,31,32,33)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 202 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50282478
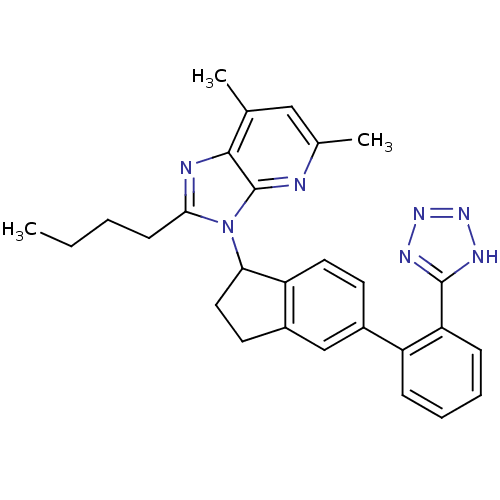
(2-Butyl-5,7-dimethyl-3-{5-[2-(1H-tetrazol-5-yl)-ph...)Show SMILES CCCCc1nc2c(C)cc(C)nc2n1C1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C28H29N7/c1-4-5-10-25-30-26-17(2)15-18(3)29-28(26)35(25)24-14-12-20-16-19(11-13-22(20)24)21-8-6-7-9-23(21)27-31-33-34-32-27/h6-9,11,13,15-16,24H,4-5,10,12,14H2,1-3H3,(H,31,32,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17954
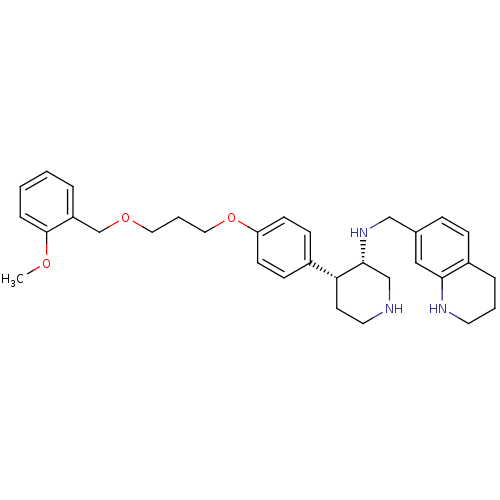
((3S,4R)-4-(4-{3-[(2-methoxyphenyl)methoxy]propoxy}...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)[C@H]1CCNC[C@H]1NCc1ccc2CCCNc2c1 |r| Show InChI InChI=1S/C32H41N3O3/c1-36-32-8-3-2-6-27(32)23-37-18-5-19-38-28-13-11-25(12-14-28)29-15-17-33-22-31(29)35-21-24-9-10-26-7-4-16-34-30(26)20-24/h2-3,6,8-14,20,29,31,33-35H,4-5,7,15-19,21-23H2,1H3/t29-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 255 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 13: 59-68 (2005)
Article DOI: 10.1016/j.bmc.2004.09.056
BindingDB Entry DOI: 10.7270/Q2QC01SN |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17955
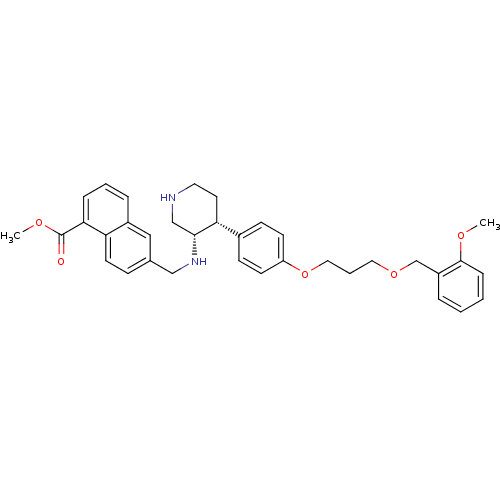
(amino-aryl-piperidine-based compound, 20 | methyl ...)Show SMILES COC(=O)c1cccc2cc(CN[C@@H]3CNCC[C@@H]3c3ccc(OCCCOCc4ccccc4OC)cc3)ccc12 |r| Show InChI InChI=1S/C35H40N2O5/c1-39-34-10-4-3-7-28(34)24-41-19-6-20-42-29-14-12-26(13-15-29)31-17-18-36-23-33(31)37-22-25-11-16-30-27(21-25)8-5-9-32(30)35(38)40-2/h3-5,7-16,21,31,33,36-37H,6,17-20,22-24H2,1-2H3/t31-,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 282 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 13: 59-68 (2005)
Article DOI: 10.1016/j.bmc.2004.09.056
BindingDB Entry DOI: 10.7270/Q2QC01SN |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17956
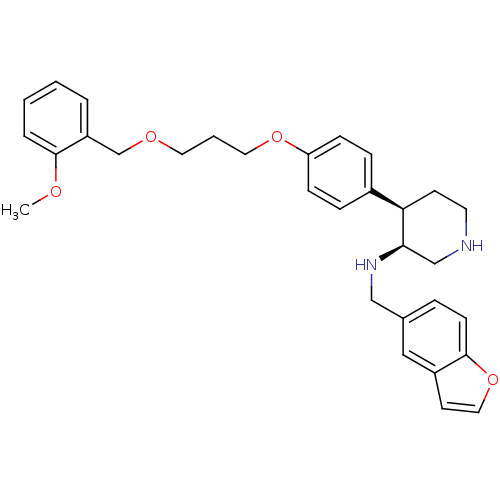
((3S,4R)-N-(1-benzofuran-5-ylmethyl)-4-(4-{3-[(2-me...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)[C@H]1CCNC[C@H]1NCc1ccc2occc2c1 |r| Show InChI InChI=1S/C31H36N2O4/c1-34-30-6-3-2-5-26(30)22-35-16-4-17-36-27-10-8-24(9-11-27)28-13-15-32-21-29(28)33-20-23-7-12-31-25(19-23)14-18-37-31/h2-3,5-12,14,18-19,28-29,32-33H,4,13,15-17,20-22H2,1H3/t28-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 393 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 13: 59-68 (2005)
Article DOI: 10.1016/j.bmc.2004.09.056
BindingDB Entry DOI: 10.7270/Q2QC01SN |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17964
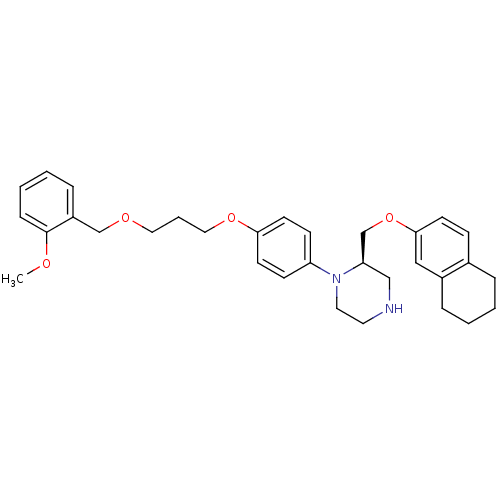
((2R)-1-(4-{3-[(2-methoxyphenyl)methoxy]propoxy}phe...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1CCNC[C@@H]1COc1ccc2CCCCc2c1 |r| Show InChI InChI=1S/C32H40N2O4/c1-35-32-10-5-4-9-27(32)23-36-19-6-20-37-30-15-12-28(13-16-30)34-18-17-33-22-29(34)24-38-31-14-11-25-7-2-3-8-26(25)21-31/h4-5,9-16,21,29,33H,2-3,6-8,17-20,22-24H2,1H3/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 13: 2657-64 (2005)
Article DOI: 10.1016/j.bmc.2005.01.048
BindingDB Entry DOI: 10.7270/Q2KP80D6 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347576
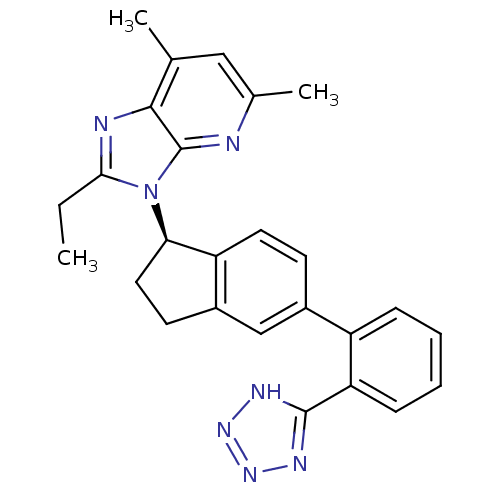
(CHEMBL1801713)Show SMILES CCc1nc2c(C)cc(C)nc2n1[C@@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C26H25N7/c1-4-23-28-24-15(2)13-16(3)27-26(24)33(23)22-12-10-18-14-17(9-11-20(18)22)19-7-5-6-8-21(19)25-29-31-32-30-25/h5-9,11,13-14,22H,4,10,12H2,1-3H3,(H,29,30,31,32)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 661 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347574

(CHEMBL1801742)Show SMILES CCc1nc2c(CC(C)C)cc(C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C29H31N7/c1-5-26-31-27-21(14-17(2)3)15-18(4)30-29(27)36(26)25-13-11-20-16-19(10-12-23(20)25)22-8-6-7-9-24(22)28-32-34-35-33-28/h6-10,12,15-17,25H,5,11,13-14H2,1-4H3,(H,32,33,34,35)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 748 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17963
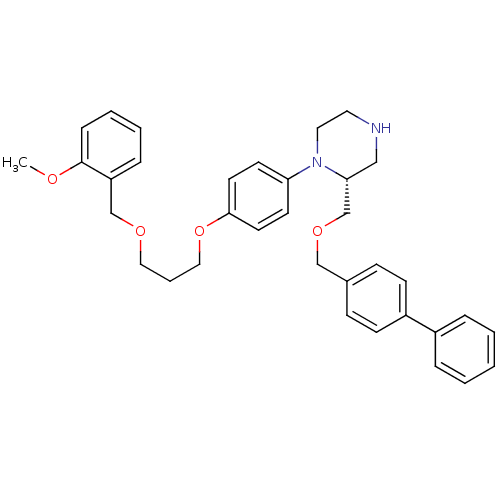
((2R)-1-(4-{3-[(2-methoxyphenyl)methoxy]propoxy}phe...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1CCNC[C@@H]1COCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C35H40N2O4/c1-38-35-11-6-5-10-31(35)26-39-22-7-23-41-34-18-16-32(17-19-34)37-21-20-36-24-33(37)27-40-25-28-12-14-30(15-13-28)29-8-3-2-4-9-29/h2-6,8-19,33,36H,7,20-27H2,1H3/t33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 13: 2657-64 (2005)
Article DOI: 10.1016/j.bmc.2005.01.048
BindingDB Entry DOI: 10.7270/Q2KP80D6 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17962
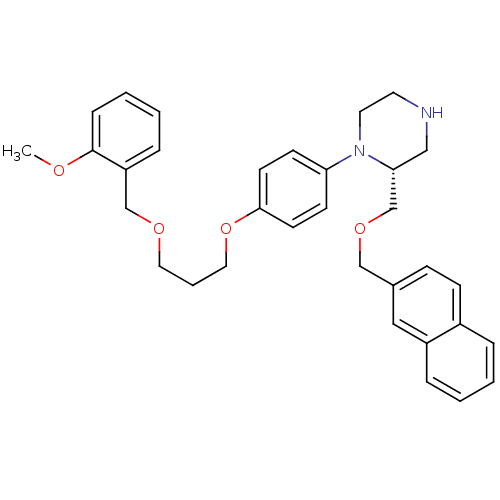
((2R)-1-(4-{3-[(2-methoxyphenyl)methoxy]propoxy}phe...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1CCNC[C@@H]1COCc1ccc2ccccc2c1 |r| Show InChI InChI=1S/C33H38N2O4/c1-36-33-10-5-4-9-29(33)24-37-19-6-20-39-32-15-13-30(14-16-32)35-18-17-34-22-31(35)25-38-23-26-11-12-27-7-2-3-8-28(27)21-26/h2-5,7-16,21,31,34H,6,17-20,22-25H2,1H3/t31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 13: 2657-64 (2005)
Article DOI: 10.1016/j.bmc.2005.01.048
BindingDB Entry DOI: 10.7270/Q2KP80D6 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17957
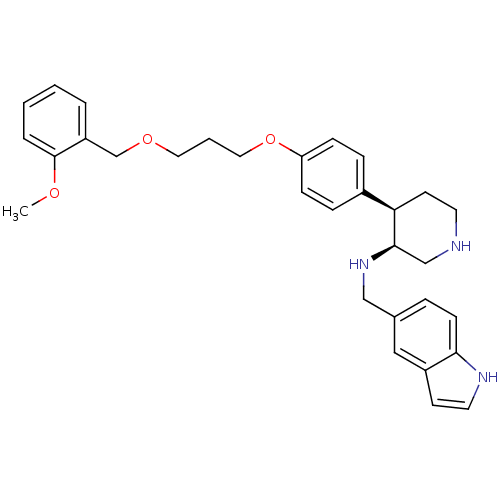
((3S,4R)-N-(1H-indol-5-ylmethyl)-4-(4-{3-[(2-methox...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)[C@H]1CCNC[C@H]1NCc1ccc2[nH]ccc2c1 |r| Show InChI InChI=1S/C31H37N3O3/c1-35-31-6-3-2-5-26(31)22-36-17-4-18-37-27-10-8-24(9-11-27)28-14-15-32-21-30(28)34-20-23-7-12-29-25(19-23)13-16-33-29/h2-3,5-13,16,19,28,30,32-34H,4,14-15,17-18,20-22H2,1H3/t28-,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.28E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 13: 59-68 (2005)
Article DOI: 10.1016/j.bmc.2004.09.056
BindingDB Entry DOI: 10.7270/Q2QC01SN |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17958
(6-({[(3S,4R)-4-(4-{3-[(2-methoxyphenyl)methoxy]pro...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)[C@H]1CCNC[C@H]1NCc1ccc2c(cccc2c1)C(O)=O |r| Show InChI InChI=1S/C34H38N2O5/c1-39-33-9-3-2-6-27(33)23-40-18-5-19-41-28-13-11-25(12-14-28)30-16-17-35-22-32(30)36-21-24-10-15-29-26(20-24)7-4-8-31(29)34(37)38/h2-4,6-15,20,30,32,35-36H,5,16-19,21-23H2,1H3,(H,37,38)/t30-,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 13: 59-68 (2005)
Article DOI: 10.1016/j.bmc.2004.09.056
BindingDB Entry DOI: 10.7270/Q2QC01SN |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17959
(N-[(3S,4R)-4-(4-{3-[(2-methoxyphenyl)methoxy]propo...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)[C@H]1CCNC[C@H]1NC(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C33H36N2O4/c1-37-32-10-5-4-9-28(32)23-38-19-6-20-39-29-15-13-25(14-16-29)30-17-18-34-22-31(30)35-33(36)27-12-11-24-7-2-3-8-26(24)21-27/h2-5,7-16,21,30-31,34H,6,17-20,22-23H2,1H3,(H,35,36)/t30-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 13: 59-68 (2005)
Article DOI: 10.1016/j.bmc.2004.09.056
BindingDB Entry DOI: 10.7270/Q2QC01SN |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17960
((3S,4R)-4-(4-{3-[(2-methoxyphenyl)methoxy]propoxy}...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)[C@H]1CCNC[C@H]1NCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C35H40N2O3/c1-38-35-11-6-5-10-31(35)26-39-22-7-23-40-32-18-16-30(17-19-32)33-20-21-36-25-34(33)37-24-27-12-14-29(15-13-27)28-8-3-2-4-9-28/h2-6,8-19,33-34,36-37H,7,20-26H2,1H3/t33-,34-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 13: 59-68 (2005)
Article DOI: 10.1016/j.bmc.2004.09.056
BindingDB Entry DOI: 10.7270/Q2QC01SN |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50009718

(2-Ethyl-5,7-dimethyl-3-[2'-(1H-tetrazol-5-yl)-biph...)Show SMILES CCc1nc2c(C)cc(C)nc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H23N7/c1-4-21-26-22-15(2)13-16(3)25-24(22)31(21)14-17-9-11-18(12-10-17)19-7-5-6-8-20(19)23-27-29-30-28-23/h5-13H,4,14H2,1-3H3,(H,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50347575

(CHEMBL1801736)Show SMILES CCc1nc2c(C)c(C)c(C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C27H27N7/c1-5-24-29-25-16(3)15(2)17(4)28-27(25)34(24)23-13-11-19-14-18(10-12-21(19)23)20-8-6-7-9-22(20)26-30-32-33-31-26/h6-10,12,14,23H,5,11,13H2,1-4H3,(H,30,31,32,33)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.82E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50347576

(CHEMBL1801713)Show SMILES CCc1nc2c(C)cc(C)nc2n1[C@@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C26H25N7/c1-4-23-28-24-15(2)13-16(3)27-26(24)33(23)22-12-10-18-14-17(9-11-20(18)22)19-7-5-6-8-21(19)25-29-31-32-30-25/h5-9,11,13-14,22H,4,10,12H2,1-3H3,(H,29,30,31,32)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.62E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50282478

(2-Butyl-5,7-dimethyl-3-{5-[2-(1H-tetrazol-5-yl)-ph...)Show SMILES CCCCc1nc2c(C)cc(C)nc2n1C1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C28H29N7/c1-4-5-10-25-30-26-17(2)15-18(3)29-28(26)35(25)24-14-12-20-16-19(11-13-22(20)24)21-8-6-7-9-23(21)27-31-33-34-32-27/h6-9,11,13,15-16,24H,4-5,10,12,14H2,1-3H3,(H,31,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50043280

(4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)-c1nc2ccccc2n1C Show InChI InChI=1S/C33H30N4O2/c1-4-9-30-35-31-21(2)18-24(32-34-27-12-7-8-13-28(27)36(32)3)19-29(31)37(30)20-22-14-16-23(17-15-22)25-10-5-6-11-26(25)33(38)39/h5-8,10-19H,4,9,20H2,1-3H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50347572

(CHEMBL1801737)Show SMILES CCc1nc2c(C)c(CC)c(C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C28H29N7/c1-5-20-16(3)26-28(29-17(20)4)35(25(6-2)30-26)24-14-12-19-15-18(11-13-22(19)24)21-9-7-8-10-23(21)27-31-33-34-32-27/h7-11,13,15,24H,5-6,12,14H2,1-4H3,(H,31,32,33,34)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50347571

(CHEMBL1801714)Show SMILES CCCc1nc2c(C)cc(C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C27H27N7/c1-4-7-24-29-25-16(2)14-17(3)28-27(25)34(24)23-13-11-19-15-18(10-12-21(19)23)20-8-5-6-9-22(20)26-30-32-33-31-26/h5-6,8-10,12,14-15,23H,4,7,11,13H2,1-3H3,(H,30,31,32,33)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50049240
((+-)-5-((4-(2-(5-ethyl-2-pyridinyl)ethoxy)phenyl)m...)Show InChI InChI=1S/C19H20N2O3S/c1-2-13-3-6-15(20-12-13)9-10-24-16-7-4-14(5-8-16)11-17-18(22)21-19(23)25-17/h3-8,12,22H,2,9-11H2,1H3,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50347568

(CHEMBL1801735)Show SMILES Cc1cc(C)c2nc(C3CC3)n([C@H]3CCc4cc(ccc34)-c3ccccc3-c3nnn[nH]3)c2n1 |r| Show InChI InChI=1S/C27H25N7/c1-15-13-16(2)28-27-24(15)29-26(17-7-8-17)34(27)23-12-10-19-14-18(9-11-21(19)23)20-5-3-4-6-22(20)25-30-32-33-31-25/h3-6,9,11,13-14,17,23H,7-8,10,12H2,1-2H3,(H,30,31,32,33)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 762 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50347567

(CHEMBL1801712)Show SMILES CCc1nc2c(C)cc(C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C26H25N7/c1-4-23-28-24-15(2)13-16(3)27-26(24)33(23)22-12-10-18-14-17(9-11-20(18)22)19-7-5-6-8-21(19)25-29-31-32-30-25/h5-9,11,13-14,22H,4,10,12H2,1-3H3,(H,29,30,31,32)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 591 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50282484

(2-Ethyl-5,7-dimethyl-3-{5-[2-(1H-tetrazol-5-yl)-ph...)Show SMILES CCc1nc2c(C)cc(C)nc2n1C1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C26H25N7/c1-4-23-28-24-15(2)13-16(3)27-26(24)33(23)22-12-10-18-14-17(9-11-20(18)22)19-7-5-6-8-21(19)25-29-31-32-30-25/h5-9,11,13-14,22H,4,10,12H2,1-3H3,(H,29,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 574 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50347566

(CHEMBL1801738)Show SMILES CCc1nc2c(C)cc(CC)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C27H27N7/c1-4-19-14-16(3)25-27(28-19)34(24(5-2)29-25)23-13-11-18-15-17(10-12-21(18)23)20-8-6-7-9-22(20)26-30-32-33-31-26/h6-10,12,14-15,23H,4-5,11,13H2,1-3H3,(H,30,31,32,33)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 494 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50347569

(CHEMBL1801734)Show SMILES CC(C)c1nc2c(C)cc(C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C27H27N7/c1-15(2)26-29-24-16(3)13-17(4)28-27(24)34(26)23-12-10-19-14-18(9-11-21(19)23)20-7-5-6-8-22(20)25-30-32-33-31-25/h5-9,11,13-15,23H,10,12H2,1-4H3,(H,30,31,32,33)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 295 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assay |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data