 Found 21 hits with Last Name = 'othman' and Initial = 'm'
Found 21 hits with Last Name = 'othman' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50182486
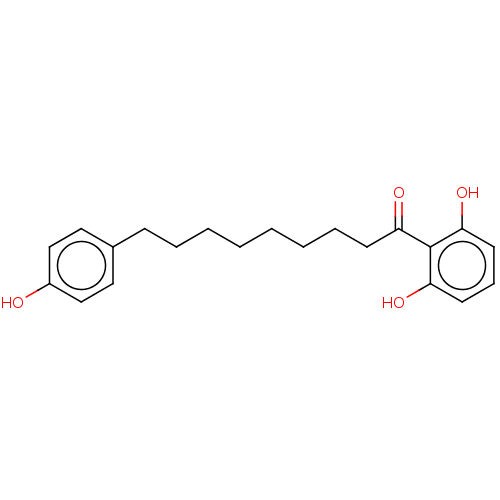
(MALABARICONE B)Show InChI InChI=1S/C21H26O4/c22-17-14-12-16(13-15-17)8-5-3-1-2-4-6-9-18(23)21-19(24)10-7-11-20(21)25/h7,10-15,22,24-25H,1-6,8-9H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of SMS2 (unknown origin) stably expressed in mouse ZS cells using 5 to 50 uM C6-NBD-ceramide as substrate preincubated for ... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50182487

(Malabaricone A)Show InChI InChI=1S/C21H26O3/c22-18(21-19(23)15-10-16-20(21)24)14-9-4-2-1-3-6-11-17-12-7-5-8-13-17/h5,7-8,10,12-13,15-16,23-24H,1-4,6,9,11,14H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of SMS2 (unknown origin) stably expressed in mouse ZS cells using 5 to 50 uM C6-NBD-ceramide as substrate preincubated for ... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50182491

(CHEBI:69015 | Malabaricone C)Show InChI InChI=1S/C21H26O5/c22-16-13-12-15(14-20(16)26)8-5-3-1-2-4-6-9-17(23)21-18(24)10-7-11-19(21)25/h7,10-14,22,24-26H,1-6,8-9H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of SMS2 (unknown origin) stably expressed in mouse ZS cells using 5 to 50 uM C6-NBD-ceramide as substrate preincubated for ... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Monastir
Curated by ChEMBL
| Assay Description
Inhibition of BchE in human plasma incubated for 30 mins using butyrylthiocholine substrate at 37 degC by DTNB dye based spectrophotometry |
Bioorg Med Chem Lett 25: 1665-70 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.028
BindingDB Entry DOI: 10.7270/Q20V8FFG |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50242188

(6-(8'Z-pentadecenyl)-salicylicacid | 6-[8'(Z)-pent...)Show InChI InChI=1S/C22H34O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-16-19-17-15-18-20(23)21(19)22(24)25/h7-8,15,17-18,23H,2-6,9-14,16H2,1H3,(H,24,25)/b8-7- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS2 (unknown origin) |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50182491

(CHEBI:69015 | Malabaricone C)Show InChI InChI=1S/C21H26O5/c22-16-13-12-15(14-20(16)26)8-5-3-1-2-4-6-9-17(23)21-18(24)10-7-11-19(21)25/h7,10-14,22,24-26H,1-6,8-9H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS2 (unknown origin) stably expressed in mouse ZS cells using C6-NBD-ceramide as substrate preincubated for 30 mins followed by substr... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50242188

(6-(8'Z-pentadecenyl)-salicylicacid | 6-[8'(Z)-pent...)Show InChI InChI=1S/C22H34O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-16-19-17-15-18-20(23)21(19)22(24)25/h7-8,15,17-18,23H,2-6,9-14,16H2,1H3,(H,24,25)/b8-7- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS1 (unknown origin) |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50182487

(Malabaricone A)Show InChI InChI=1S/C21H26O3/c22-18(21-19(23)15-10-16-20(21)24)14-9-4-2-1-3-6-11-17-12-7-5-8-13-17/h5,7-8,10,12-13,15-16,23-24H,1-4,6,9,11,14H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of SMS1 (unknown origin) stably expressed in mouse ZS cells using 5 to 50 uM C6-NBD-ceramide as substrate preincubated for ... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50182486

(MALABARICONE B)Show InChI InChI=1S/C21H26O4/c22-17-14-12-16(13-15-17)8-5-3-1-2-4-6-9-18(23)21-19(24)10-7-11-20(21)25/h7,10-15,22,24-25H,1-6,8-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of SMS1 (unknown origin) stably expressed in mouse ZS cells using 5 to 50 uM C6-NBD-ceramide as substrate preincubated for ... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50182491

(CHEBI:69015 | Malabaricone C)Show InChI InChI=1S/C21H26O5/c22-16-13-12-15(14-20(16)26)8-5-3-1-2-4-6-9-17(23)21-18(24)10-7-11-19(21)25/h7,10-14,22,24-26H,1-6,8-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of SMS1 (unknown origin) stably expressed in mouse ZS cells using 5 to 50 uM C6-NBD-ceramide as substrate preincubated for ... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50182486

(MALABARICONE B)Show InChI InChI=1S/C21H26O4/c22-17-14-12-16(13-15-17)8-5-3-1-2-4-6-9-18(23)21-19(24)10-7-11-20(21)25/h7,10-15,22,24-25H,1-6,8-9H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS2 (unknown origin) stably expressed in mouse ZS cells using C6-NBD-ceramide as substrate preincubated for 30 mins followed by substr... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50182491

(CHEBI:69015 | Malabaricone C)Show InChI InChI=1S/C21H26O5/c22-16-13-12-15(14-20(16)26)8-5-3-1-2-4-6-9-17(23)21-18(24)10-7-11-19(21)25/h7,10-14,22,24-26H,1-6,8-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS1 (unknown origin) stably expressed in mouse ZS cells using C6-NBD-ceramide as substrate preincubated for 30 mins followed by substr... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50182486

(MALABARICONE B)Show InChI InChI=1S/C21H26O4/c22-17-14-12-16(13-15-17)8-5-3-1-2-4-6-9-18(23)21-19(24)10-7-11-20(21)25/h7,10-15,22,24-25H,1-6,8-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS1 (unknown origin) stably expressed in mouse ZS cells using C6-NBD-ceramide as substrate preincubated for 30 mins followed by substr... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50182487

(Malabaricone A)Show InChI InChI=1S/C21H26O3/c22-18(21-19(23)15-10-16-20(21)24)14-9-4-2-1-3-6-11-17-12-7-5-8-13-17/h5,7-8,10,12-13,15-16,23-24H,1-4,6,9,11,14H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS1 (unknown origin) stably expressed in mouse ZS cells using C6-NBD-ceramide as substrate preincubated for 30 mins followed by substr... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50182487

(Malabaricone A)Show InChI InChI=1S/C21H26O3/c22-18(21-19(23)15-10-16-20(21)24)14-9-4-2-1-3-6-11-17-12-7-5-8-13-17/h5,7-8,10,12-13,15-16,23-24H,1-4,6,9,11,14H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS2 (unknown origin) stably expressed in mouse ZS cells using C6-NBD-ceramide as substrate preincubated for 30 mins followed by substr... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50182488

(CHEMBL3819036)Show InChI InChI=1S/C21H26O5/c22-16-11-9-15(10-12-16)7-5-3-1-2-4-6-8-18(24)21-19(25)13-17(23)14-20(21)26/h9-14,22-23,25-26H,1-8H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS2 (unknown origin) stably expressed in mouse ZS cells using C6-NBD-ceramide as substrate preincubated for 30 mins followed by substr... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Homo sapiens (Human)) | BDBM50182488

(CHEMBL3819036)Show InChI InChI=1S/C21H26O5/c22-16-11-9-15(10-12-16)7-5-3-1-2-4-6-8-18(24)21-19(25)13-17(23)14-20(21)26/h9-14,22-23,25-26H,1-8H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS1 (unknown origin) stably expressed in mouse ZS cells using C6-NBD-ceramide as substrate preincubated for 30 mins followed by substr... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Mus musculus) | BDBM50182491

(CHEBI:69015 | Malabaricone C)Show InChI InChI=1S/C21H26O5/c22-16-13-12-15(14-20(16)26)8-5-3-1-2-4-6-9-17(23)21-18(24)10-7-11-19(21)25/h7,10-14,22,24-26H,1-6,8-9H2 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS2 in MEF cells using C6-NBD-ceramide as substrate preincubated for 1 hr followed by substrate addition and measured after 1 hr by HP... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 1
(Mus musculus) | BDBM50182491

(CHEBI:69015 | Malabaricone C)Show InChI InChI=1S/C21H26O5/c22-16-13-12-15(14-20(16)26)8-5-3-1-2-4-6-9-17(23)21-18(24)10-7-11-19(21)25/h7,10-14,22,24-26H,1-6,8-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of SMS1 in MEF cells using C6-NBD-ceramide as substrate preincubated for 1 hr followed by substrate addition and measured after 1 hr by HP... |
ACS Med Chem Lett 10: 1154-1158 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00171
BindingDB Entry DOI: 10.7270/Q2WS8XPS |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50067211
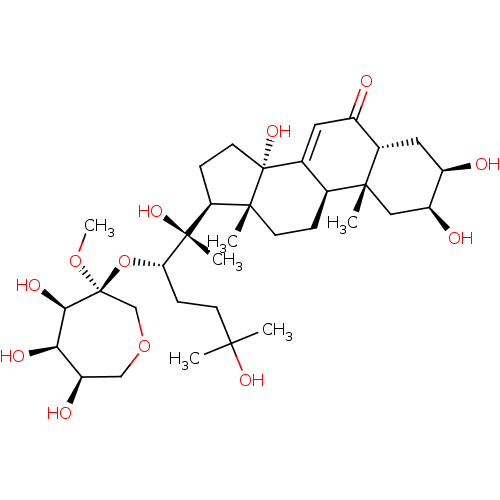
(CHEMBL3400571)Show SMILES [H][C@@]1(CC[C@@]2(O)C3=CC(=O)[C@]4([H])C[C@@H](O)[C@@H](O)C[C@]4(C)[C@@]3([H])CC[C@]12C)[C@@](C)(O)[C@H](CCC(C)(C)O)O[C@]1(COC[C@@H](O)[C@@H](O)[C@H]1O)OC |r,t:6| Show InChI InChI=1S/C34H56O12/c1-29(2,41)10-9-26(46-34(44-6)17-45-16-24(38)27(39)28(34)40)32(5,42)25-8-12-33(43)19-13-21(35)20-14-22(36)23(37)15-30(20,3)18(19)7-11-31(25,33)4/h13,18,20,22-28,36-43H,7-12,14-17H2,1-6H3/t18-,20-,22+,23-,24+,25-,26-,27+,28+,30+,31+,32+,33+,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.37E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Monastir
Curated by ChEMBL
| Assay Description
Inhibition of BchE in human plasma incubated for 30 mins using butyrylthiocholine substrate at 37 degC by DTNB dye based spectrophotometry |
Bioorg Med Chem Lett 25: 1665-70 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.028
BindingDB Entry DOI: 10.7270/Q20V8FFG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50326777
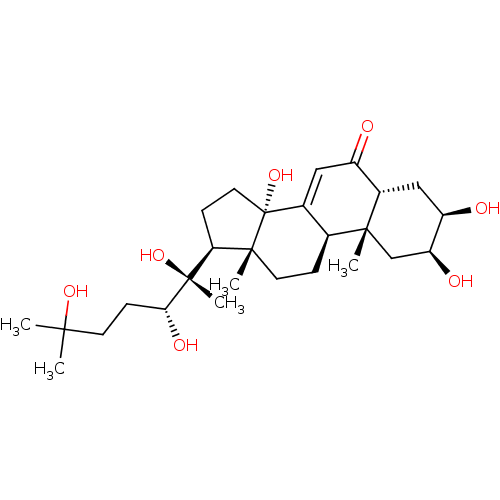
((2beta,3beta,5beta,22R)-2,3,14,20,22,25-hexahydrox...)Show SMILES CC(C)(O)CC[C@@H](O)[C@](C)(O)[C@H]1CC[C@@]2(O)C3=CC(=O)[C@@H]4C[C@@H](O)[C@@H](O)C[C@]4(C)[C@H]3CC[C@]12C |r,t:16| Show InChI InChI=1S/C27H44O7/c1-23(2,32)9-8-22(31)26(5,33)21-7-11-27(34)16-12-18(28)17-13-19(29)20(30)14-24(17,3)15(16)6-10-25(21,27)4/h12,15,17,19-22,29-34H,6-11,13-14H2,1-5H3/t15-,17-,19+,20-,21-,22+,24+,25+,26+,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.05E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Monastir
Curated by ChEMBL
| Assay Description
Inhibition of BchE in human plasma incubated for 30 mins using butyrylthiocholine substrate at 37 degC by DTNB dye based spectrophotometry |
Bioorg Med Chem Lett 25: 1665-70 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.028
BindingDB Entry DOI: 10.7270/Q20V8FFG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data