

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50406868 (BIBW2992 | CHEMBL2347958) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University Curated by ChEMBL | Assay Description Inhibition of EGFR (unknown origin) | Eur J Med Chem 154: 29-43 (2018) Article DOI: 10.1016/j.ejmech.2018.05.006 BindingDB Entry DOI: 10.7270/Q2S46VJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50464538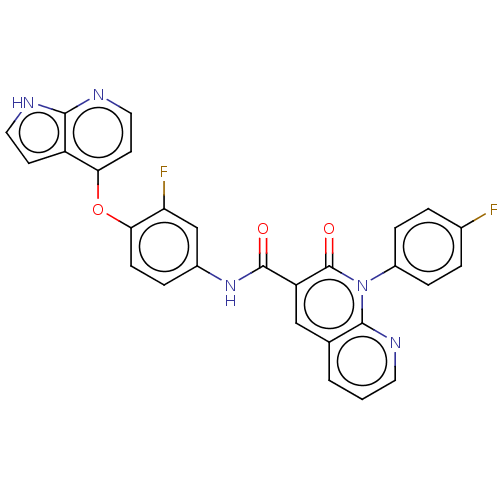 (CHEMBL4289577) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University Curated by ChEMBL | Assay Description Inhibition of Flt-3 (unknown origin) using poly(Glu, Tyr) 4:1 as substrate after 30 mins by HTRF assay | Eur J Med Chem 143: 266-275 (2018) Article DOI: 10.1016/j.ejmech.2017.11.034 BindingDB Entry DOI: 10.7270/Q2JW8HJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50399540 (FORETINIB | US10464902, Foretinib | US10882853, Co...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University Curated by ChEMBL | Assay Description Inhibition of cMET (unknown origin) using poly(Glu,Tyr) 4:1 substrate after 30 mins by HTFR analysis | Eur J Med Chem 143: 266-275 (2018) Article DOI: 10.1016/j.ejmech.2017.11.034 BindingDB Entry DOI: 10.7270/Q2JW8HJZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50322823 ((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science& Technology Normal University Curated by ChEMBL | Assay Description Inhibition of EGFR (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by addition of substrate by mobility shi... | Bioorg Med Chem 24: 1495-503 (2016) Article DOI: 10.1016/j.bmc.2016.02.017 BindingDB Entry DOI: 10.7270/Q2KS6TCZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50464538 (CHEMBL4289577) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University Curated by ChEMBL | Assay Description Inhibition of PDGFRbeta (unknown origin) using poly(Glu, Tyr) 4:1 as substrate after 30 mins by HTRF assay | Eur J Med Chem 143: 266-275 (2018) Article DOI: 10.1016/j.ejmech.2017.11.034 BindingDB Entry DOI: 10.7270/Q2JW8HJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50464538 (CHEMBL4289577) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University Curated by ChEMBL | Assay Description Inhibition of cMET (unknown origin) using poly(Glu,Tyr) 4:1 substrate after 30 mins by HTFR analysis | Eur J Med Chem 143: 266-275 (2018) Article DOI: 10.1016/j.ejmech.2017.11.034 BindingDB Entry DOI: 10.7270/Q2JW8HJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50464544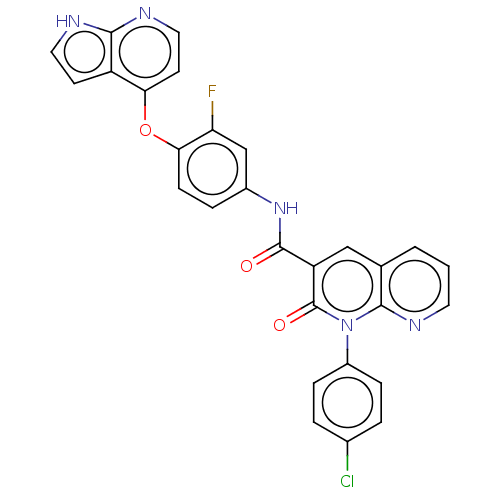 (CHEMBL4281698) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University Curated by ChEMBL | Assay Description Inhibition of cMET (unknown origin) using poly(Glu,Tyr) 4:1 substrate after 30 mins by HTFR analysis | Eur J Med Chem 143: 266-275 (2018) Article DOI: 10.1016/j.ejmech.2017.11.034 BindingDB Entry DOI: 10.7270/Q2JW8HJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581093 (CHEMBL5077644) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM25028 (4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science& Technology Normal University Curated by ChEMBL | Assay Description Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate incubated for 60 mins by Kinase-Glo luminescent kinase assay | Eur J Med Chem 116: 27-35 (2016) Article DOI: 10.1016/j.ejmech.2016.03.033 BindingDB Entry DOI: 10.7270/Q2V40X38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50485254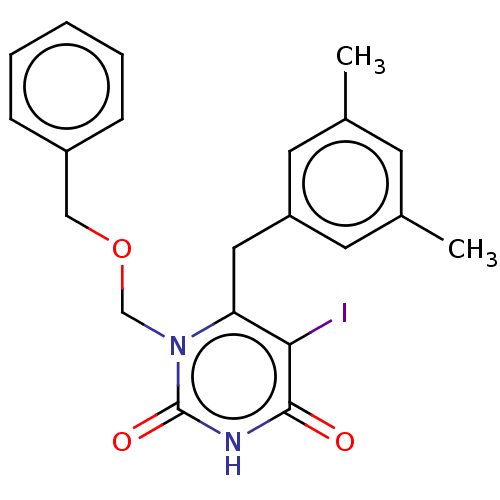 (CHEMBL2041783) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(ra)/oligo(dT)15 homopolymer template as substrate after 1 hr | J Med Chem 55: 2242-50 (2012) Article DOI: 10.1021/jm201506e BindingDB Entry DOI: 10.7270/Q22N554J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490787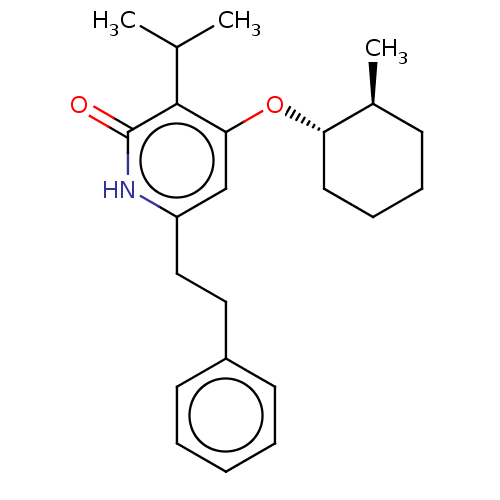 (CHEMBL2337416) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase after 1 hr by colorimetric assay | J Med Chem 56: 3593-608 (2013) Article DOI: 10.1021/jm400102x BindingDB Entry DOI: 10.7270/Q2N019FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50406869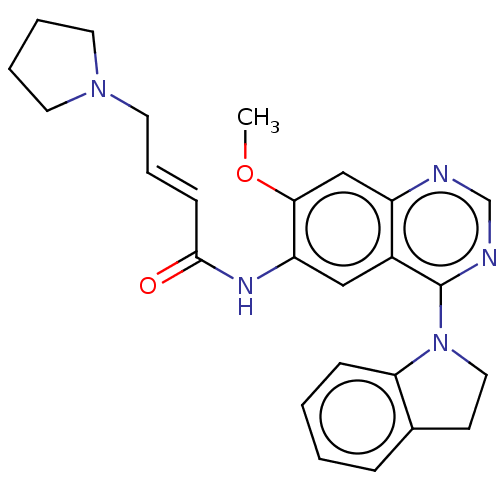 (CHEMBL4165894) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University Curated by ChEMBL | Assay Description Inhibition of wild type EGFR (unknown origin) using FAM-labeled peptide substrate after 10 mins by mobility shift assay | Eur J Med Chem 154: 29-43 (2018) Article DOI: 10.1016/j.ejmech.2018.05.006 BindingDB Entry DOI: 10.7270/Q2S46VJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50148026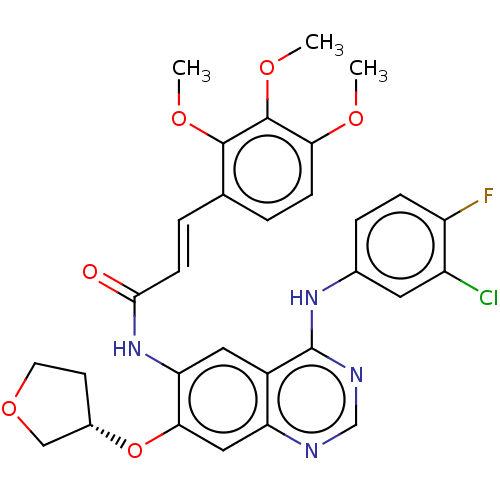 (CHEMBL3763790) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science& Technology Normal University Curated by ChEMBL | Assay Description Inhibition of EGFR (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by addition of substrate by mobility shi... | Bioorg Med Chem 24: 1495-503 (2016) Article DOI: 10.1016/j.bmc.2016.02.017 BindingDB Entry DOI: 10.7270/Q2KS6TCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581098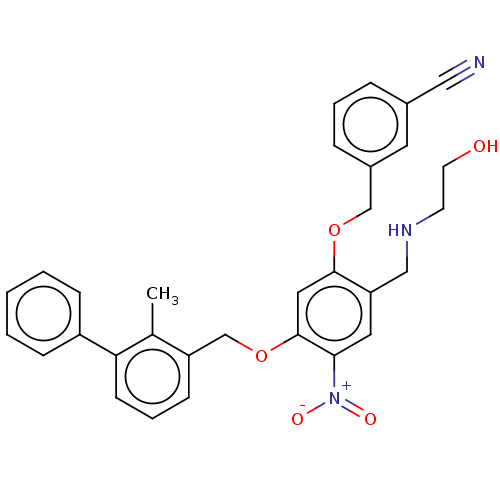 (CHEMBL5075026) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50464541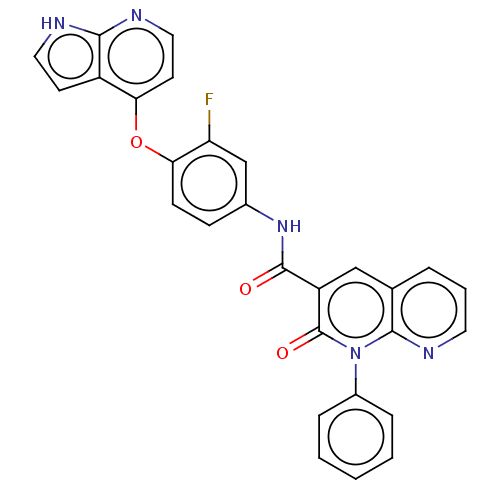 (CHEMBL4278233) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University Curated by ChEMBL | Assay Description Inhibition of cMET (unknown origin) using poly(Glu,Tyr) 4:1 substrate after 30 mins by HTFR analysis | Eur J Med Chem 143: 266-275 (2018) Article DOI: 10.1016/j.ejmech.2017.11.034 BindingDB Entry DOI: 10.7270/Q2JW8HJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50406874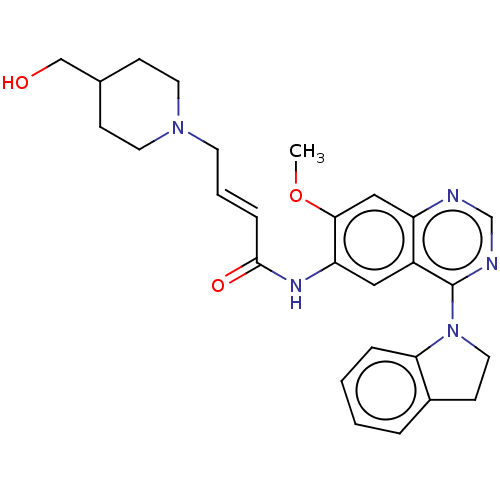 (CHEMBL4172718) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University Curated by ChEMBL | Assay Description Inhibition of wild type EGFR (unknown origin) using FAM-labeled peptide substrate after 10 mins by mobility shift assay | Eur J Med Chem 154: 29-43 (2018) Article DOI: 10.1016/j.ejmech.2018.05.006 BindingDB Entry DOI: 10.7270/Q2S46VJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581108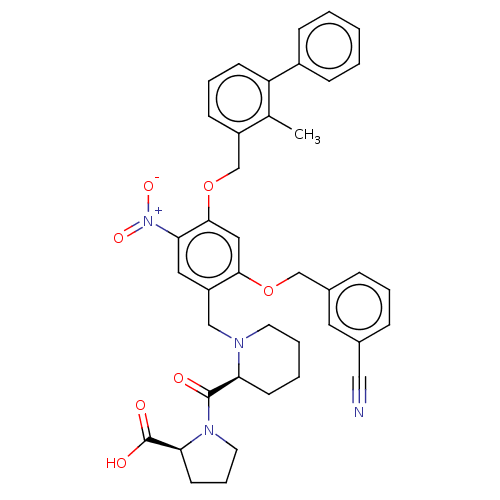 (CHEMBL5088674) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581106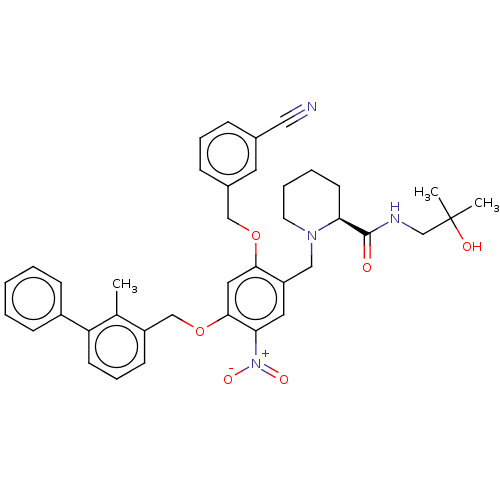 (CHEMBL5082629) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50406868 (BIBW2992 | CHEMBL2347958) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University Curated by ChEMBL | Assay Description Inhibition of wild type EGFR (unknown origin) using FAM-labeled peptide substrate after 10 mins by mobility shift assay | Eur J Med Chem 154: 29-43 (2018) Article DOI: 10.1016/j.ejmech.2018.05.006 BindingDB Entry DOI: 10.7270/Q2S46VJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50406870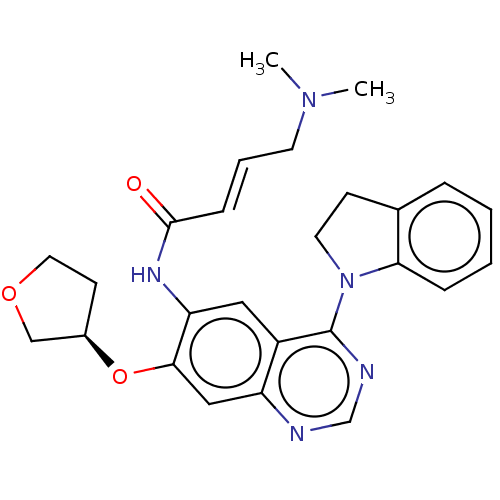 (CHEMBL4168254) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University Curated by ChEMBL | Assay Description Inhibition of wild type EGFR (unknown origin) using FAM-labeled peptide substrate after 10 mins by mobility shift assay | Eur J Med Chem 154: 29-43 (2018) Article DOI: 10.1016/j.ejmech.2018.05.006 BindingDB Entry DOI: 10.7270/Q2S46VJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581095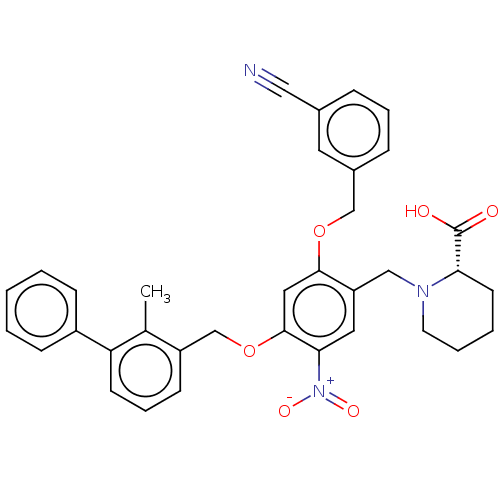 (CHEMBL5078895) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50406868 (BIBW2992 | CHEMBL2347958) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University Curated by ChEMBL | Assay Description Inhibition of wild type EGFR T790M mutant (unknown origin) using FAM-labeled peptide substrate after 10 mins by mobility shift assay | Eur J Med Chem 154: 29-43 (2018) Article DOI: 10.1016/j.ejmech.2018.05.006 BindingDB Entry DOI: 10.7270/Q2S46VJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4779 (CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University Curated by ChEMBL | Assay Description Inhibition of EGFR (unknown origin) | Eur J Med Chem 154: 29-43 (2018) Article DOI: 10.1016/j.ejmech.2018.05.006 BindingDB Entry DOI: 10.7270/Q2S46VJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581099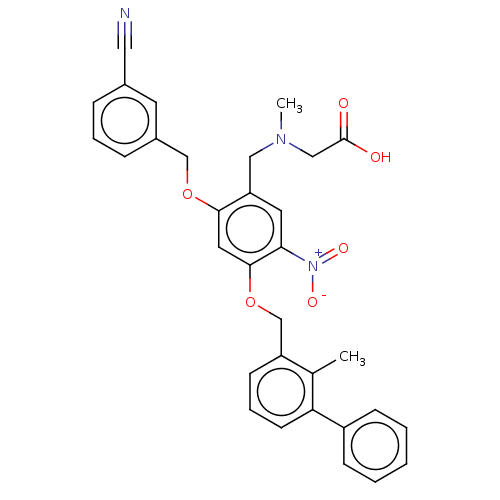 (CHEMBL5093703) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581097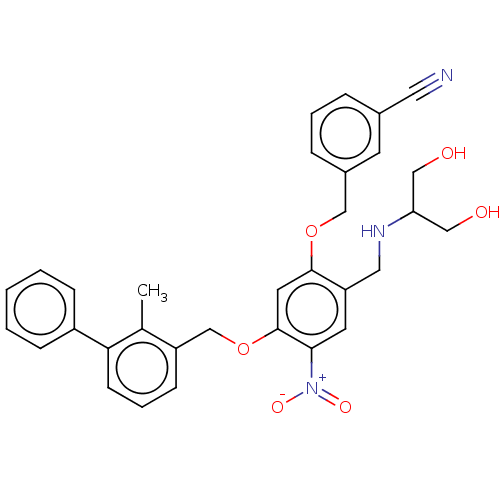 (CHEMBL5070574) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581107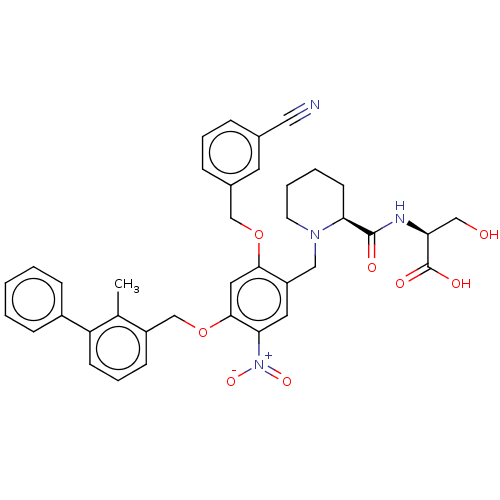 (CHEMBL5093193) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581096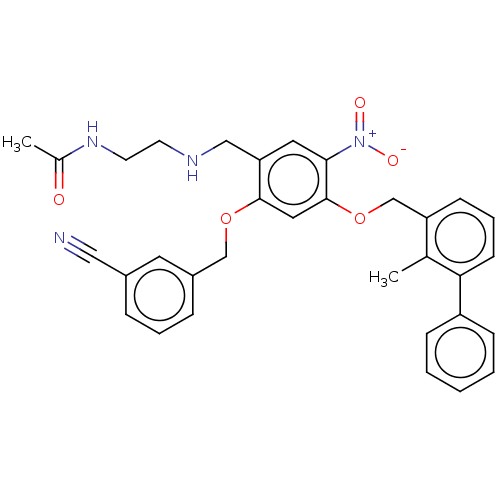 (CHEMBL5078627) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50464542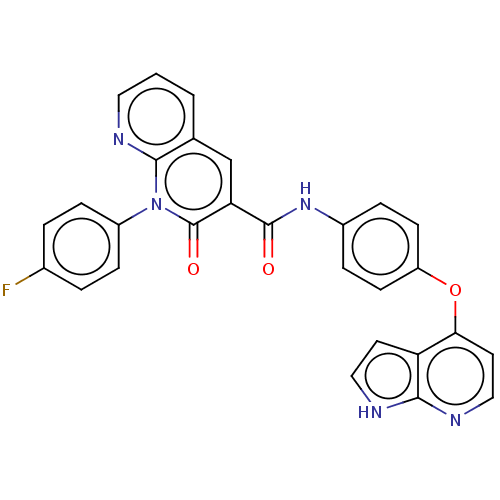 (CHEMBL4280004) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University Curated by ChEMBL | Assay Description Inhibition of cMET (unknown origin) using poly(Glu,Tyr) 4:1 substrate after 30 mins by HTFR analysis | Eur J Med Chem 143: 266-275 (2018) Article DOI: 10.1016/j.ejmech.2017.11.034 BindingDB Entry DOI: 10.7270/Q2JW8HJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581102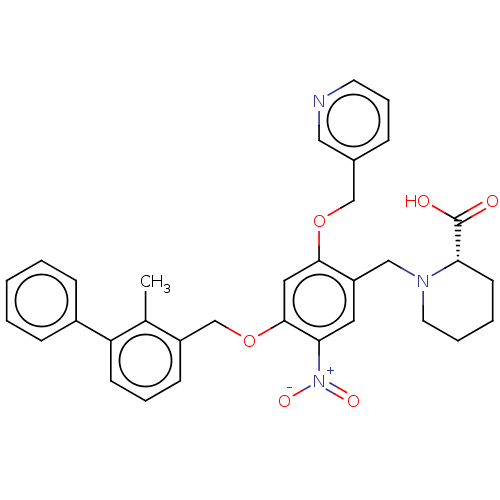 (CHEMBL5090961) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581073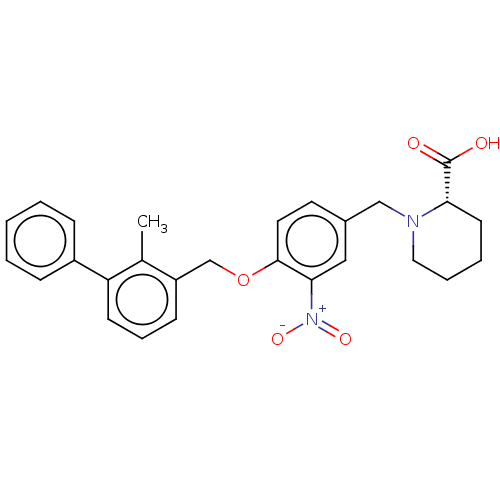 (CHEMBL5086947) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581064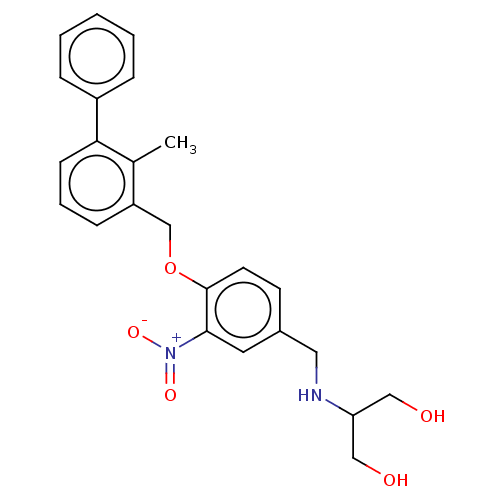 (CHEMBL5088877) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50148025 (CHEMBL3763360) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science& Technology Normal University Curated by ChEMBL | Assay Description Inhibition of EGFR (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by addition of substrate by mobility shi... | Bioorg Med Chem 24: 1495-503 (2016) Article DOI: 10.1016/j.bmc.2016.02.017 BindingDB Entry DOI: 10.7270/Q2KS6TCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50464543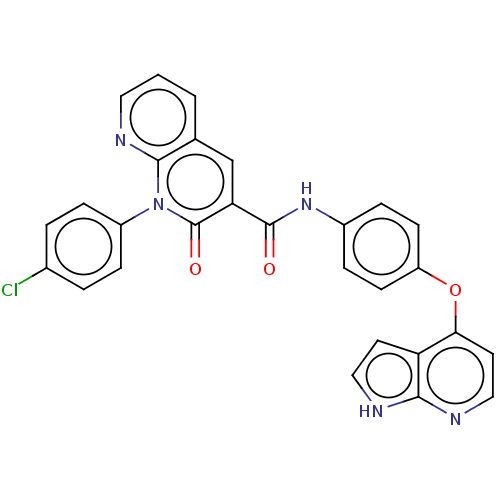 (CHEMBL4287907) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University Curated by ChEMBL | Assay Description Inhibition of cMET (unknown origin) using poly(Glu,Tyr) 4:1 substrate after 30 mins by HTFR analysis | Eur J Med Chem 143: 266-275 (2018) Article DOI: 10.1016/j.ejmech.2017.11.034 BindingDB Entry DOI: 10.7270/Q2JW8HJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581103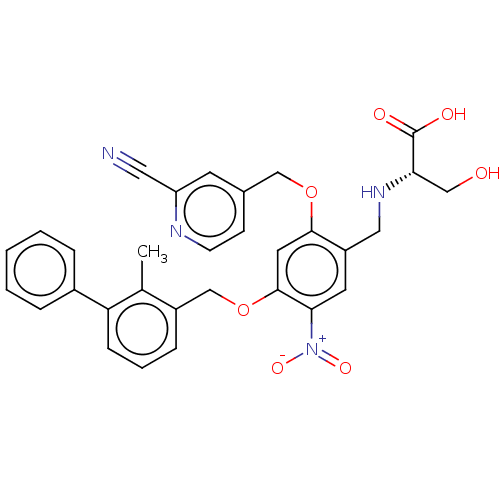 (CHEMBL5086842) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581104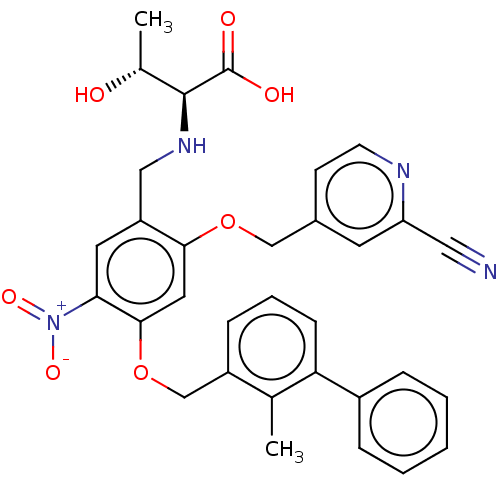 (CHEMBL5090423) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581105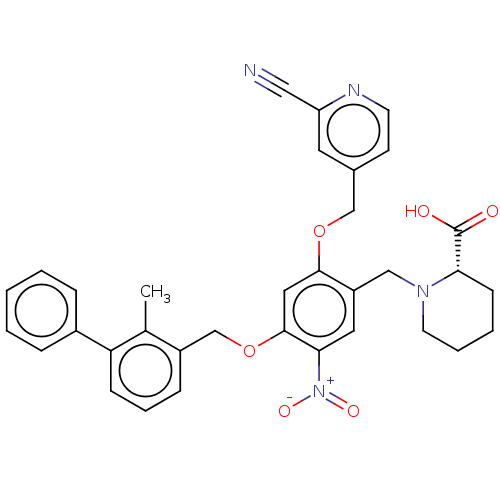 (CHEMBL5085480) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581084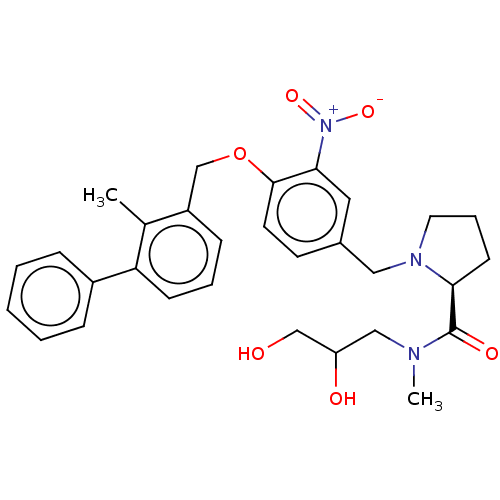 (CHEMBL5091096) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50490782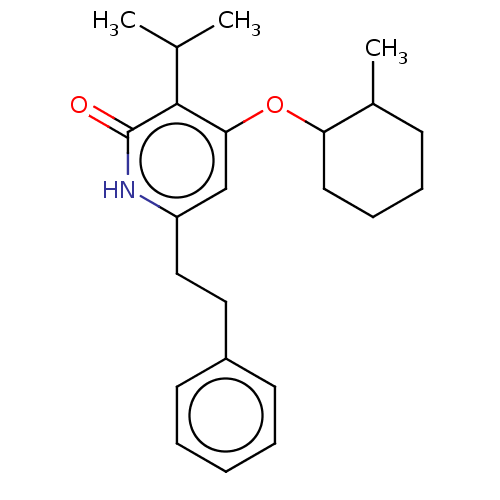 (CHEMBL2337437) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase after 1 hr by colorimetric assay | J Med Chem 56: 3593-608 (2013) Article DOI: 10.1021/jm400102x BindingDB Entry DOI: 10.7270/Q2N019FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50485259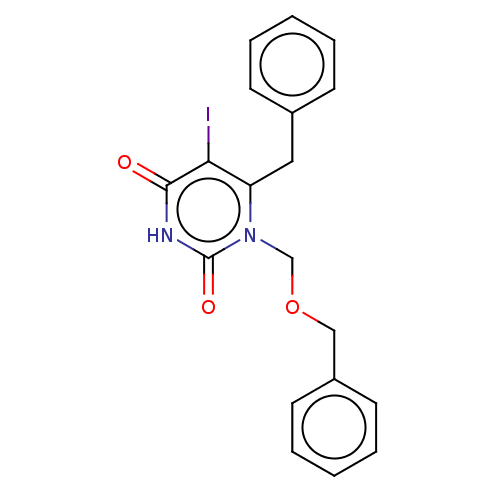 (CHEMBL2041782) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(ra)/oligo(dT)15 homopolymer template as substrate after 1 hr | J Med Chem 55: 2242-50 (2012) Article DOI: 10.1021/jm201506e BindingDB Entry DOI: 10.7270/Q22N554J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM25045 (3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science& Technology Normal University Curated by ChEMBL | Assay Description Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate incubated for 60 mins by Kinase-Glo luminescent kinase assay | Eur J Med Chem 116: 27-35 (2016) Article DOI: 10.1016/j.ejmech.2016.03.033 BindingDB Entry DOI: 10.7270/Q2V40X38 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581062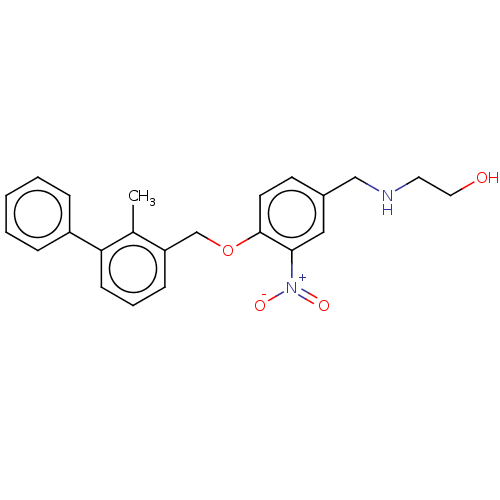 (CHEMBL5070280) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581087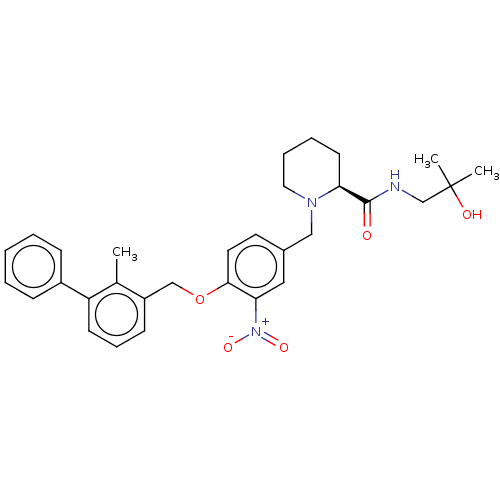 (CHEMBL5079965) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50485255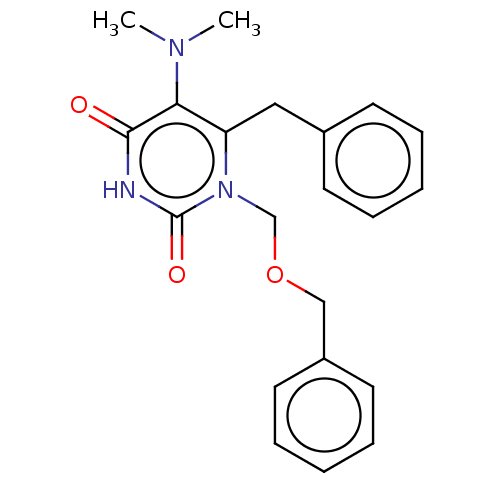 (CHEMBL2041791) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(ra)/oligo(dT)15 homopolymer template as substrate after 1 hr | J Med Chem 55: 2242-50 (2012) Article DOI: 10.1021/jm201506e BindingDB Entry DOI: 10.7270/Q22N554J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581089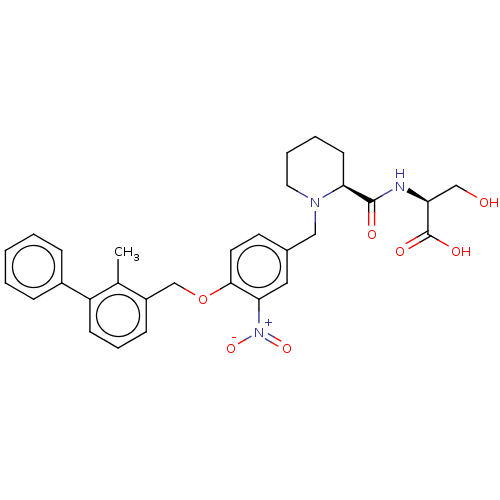 (CHEMBL5079769) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581092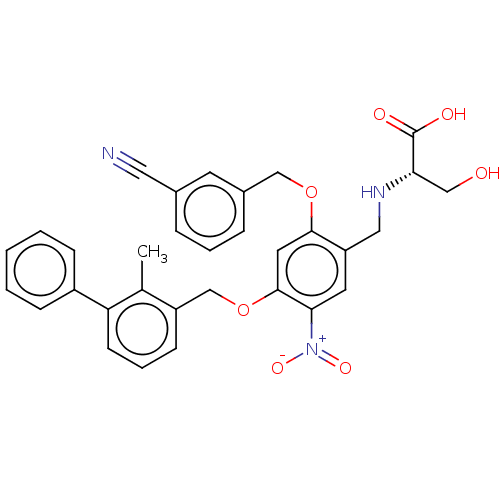 (CHEMBL5076672) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581088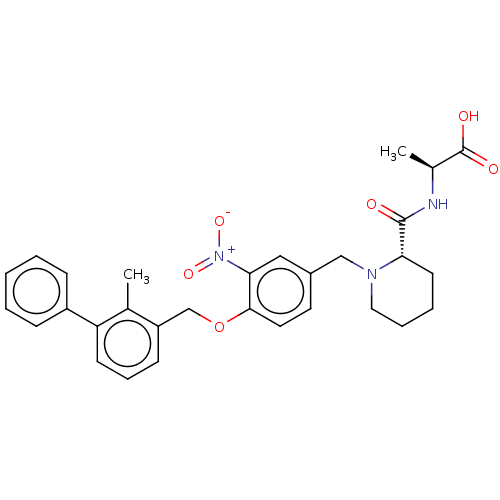 (CHEMBL5089603) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581061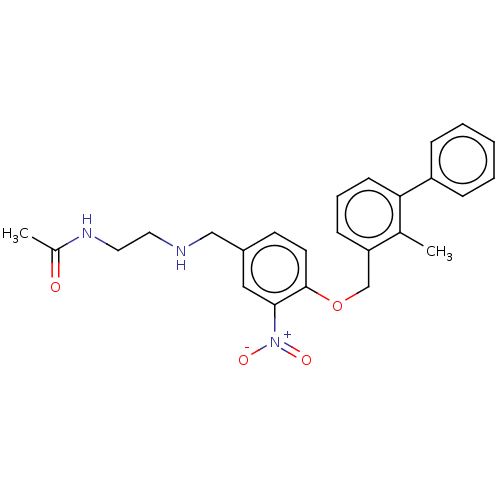 (CHEMBL5088075) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50485250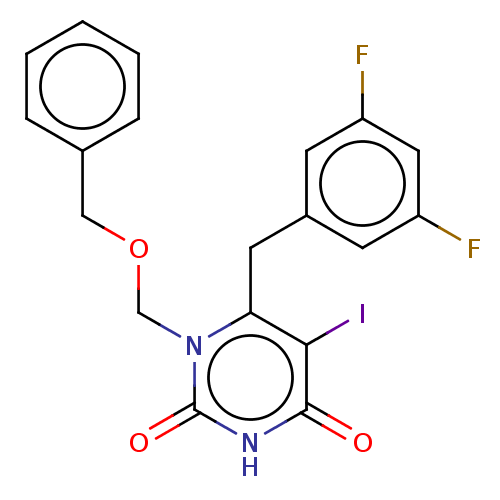 (CHEMBL2041784) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(ra)/oligo(dT)15 homopolymer template as substrate after 1 hr | J Med Chem 55: 2242-50 (2012) Article DOI: 10.1021/jm201506e BindingDB Entry DOI: 10.7270/Q22N554J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581091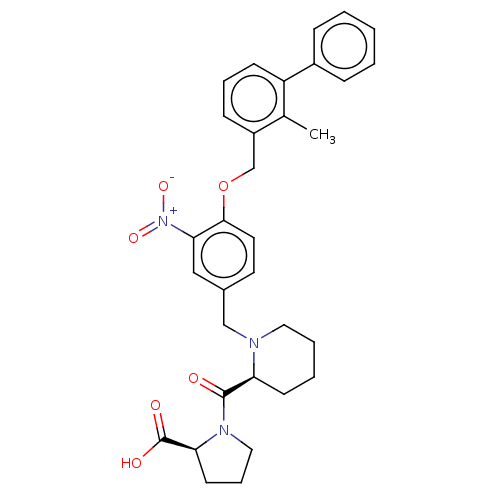 (CHEMBL5075125) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death 1 ligand/protein 1 (Homo sapiens-Homo sapiens (Human)) | BDBM50581101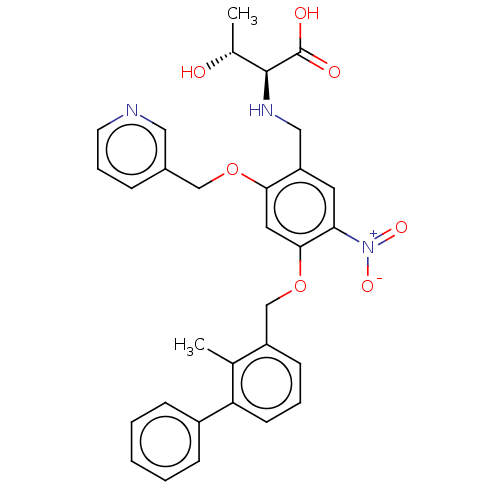 (CHEMBL5078434) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PD-1/PD-L1 interaction assessed as disruption of PD-1/PD-L1 complex incubated for 2 hrs by Tag-1 Eu3+ and anti-Tag2-XL665 based H... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00370 BindingDB Entry DOI: 10.7270/Q2K93CD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 216 total ) | Next | Last >> |