

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50129748 (N,N-Dimethylaminoethyl 18-methoxycoronaridinate) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Vermont Curated by ChEMBL | Assay Description Binding affinity was determined against Opioid receptor kappa 1 from guinea pig brain membranes | J Med Chem 46: 2716-30 (2003) Article DOI: 10.1021/jm020562o BindingDB Entry DOI: 10.7270/Q23X87CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Homo sapiens (Human)) | BDBM50129749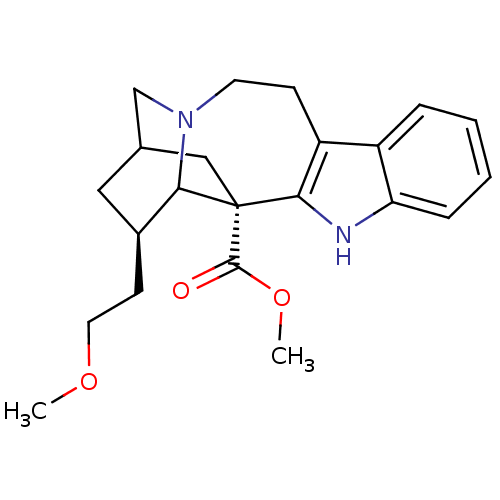 (18-methoxycoronaridine) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Vermont Curated by ChEMBL | Assay Description Binding affinity towards Nicotinic acetylcholine receptor alpha3-beta4 determined from kinetic measures of koff/kon | J Med Chem 46: 2716-30 (2003) Article DOI: 10.1021/jm020562o BindingDB Entry DOI: 10.7270/Q23X87CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50129749 (18-methoxycoronaridine) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Vermont Curated by ChEMBL | Assay Description Binding affinity was determined against Opioid receptor mu 1 from guinea pig brain membranes | J Med Chem 46: 2716-30 (2003) Article DOI: 10.1021/jm020562o BindingDB Entry DOI: 10.7270/Q23X87CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50129749 (18-methoxycoronaridine) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Vermont Curated by ChEMBL | Assay Description Binding affinity was determined against Opioid receptor delta 1 from guinea pig brain membranes | J Med Chem 46: 2716-30 (2003) Article DOI: 10.1021/jm020562o BindingDB Entry DOI: 10.7270/Q23X87CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50129749 (18-methoxycoronaridine) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Vermont Curated by ChEMBL | Assay Description Binding affinity was determined against Opioid receptor kappa 1 from guinea pig brain membranes | J Med Chem 46: 2716-30 (2003) Article DOI: 10.1021/jm020562o BindingDB Entry DOI: 10.7270/Q23X87CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18224 (6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of recombinant human DHFR assessed as reduction in consumption of NADPH using 9 uM DHFA as substrate | Bioorg Med Chem Lett 29: 1874-1880 (2019) Article DOI: 10.1016/j.bmcl.2019.06.004 BindingDB Entry DOI: 10.7270/Q2P84GBC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18224 (6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of human recombinant DHFR expressed in Escherichia coli Rosetta Gami B (DE3) competent cells using DHFA as substrate and NADPH | Bioorg Med Chem 26: 2640-2650 (2018) Article DOI: 10.1016/j.bmc.2018.04.032 BindingDB Entry DOI: 10.7270/Q2QJ7KXF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM31777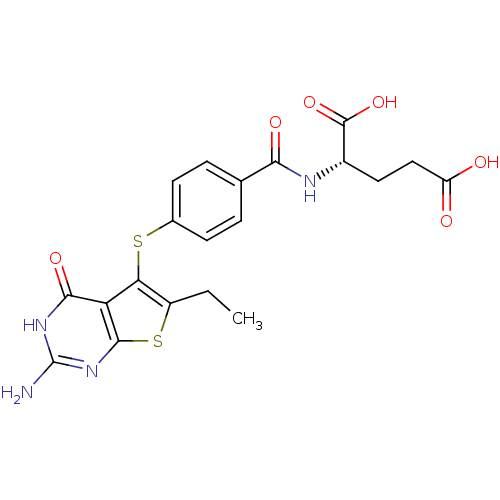 (thieno[2,3-d]pyrimidine deriv., 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18268 (5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of recombinant human DHFR assessed as reduction in consumption of NADPH using 18 uM DHFA as substrate | Bioorg Med Chem Lett 29: 1874-1880 (2019) Article DOI: 10.1016/j.bmcl.2019.06.004 BindingDB Entry DOI: 10.7270/Q2P84GBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18224 (6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of recombinant human DHFR assessed as reduction in consumption of NADPH using 18 uM DHFA as substrate | Bioorg Med Chem Lett 29: 1874-1880 (2019) Article DOI: 10.1016/j.bmcl.2019.06.004 BindingDB Entry DOI: 10.7270/Q2P84GBC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM31776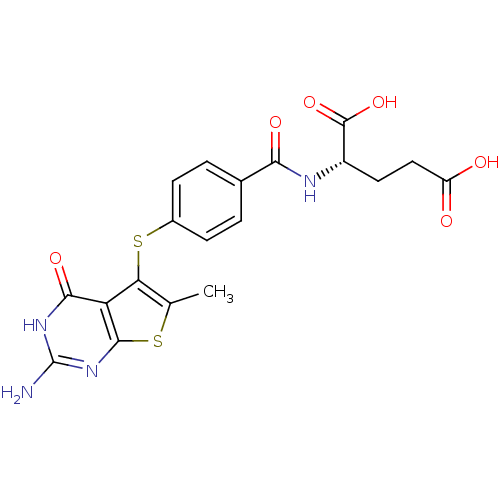 (thieno[2,3-d]pyrimidine deriv., 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM31782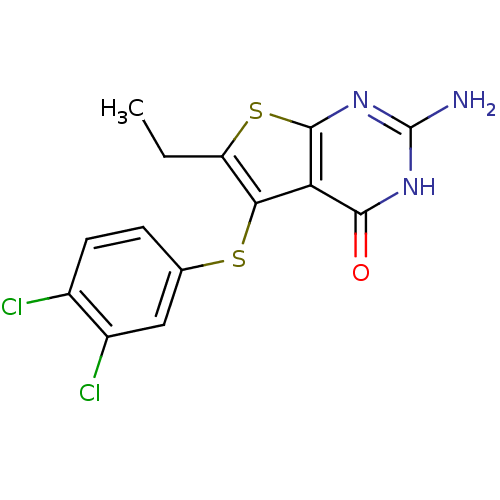 (thieno[2,3-d]pyrimidine deriv., 2e) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM31784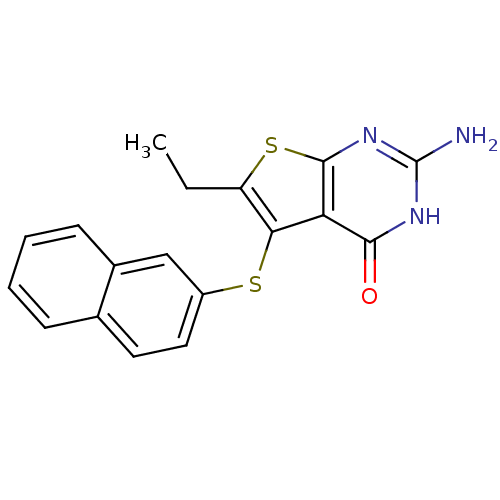 (thieno[2,3-d]pyrimidine deriv., 2g) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM31780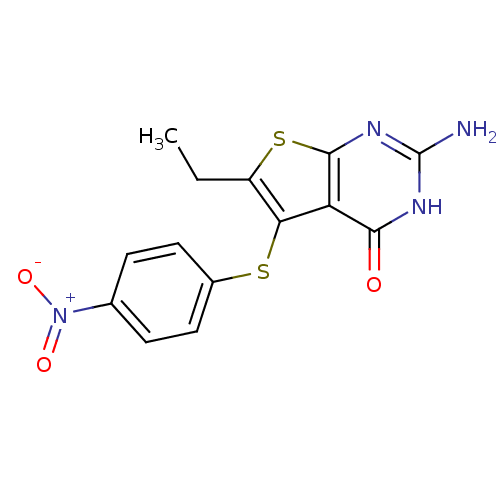 (thieno[2,3-d]pyrimidine deriv., 2c) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM31779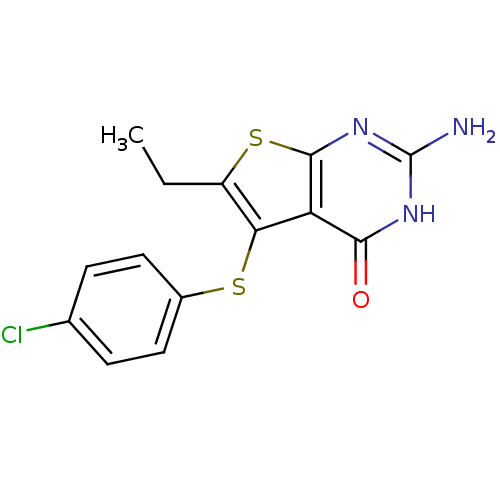 (thieno[2,3-d]pyrimidine deriv., 2b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50522394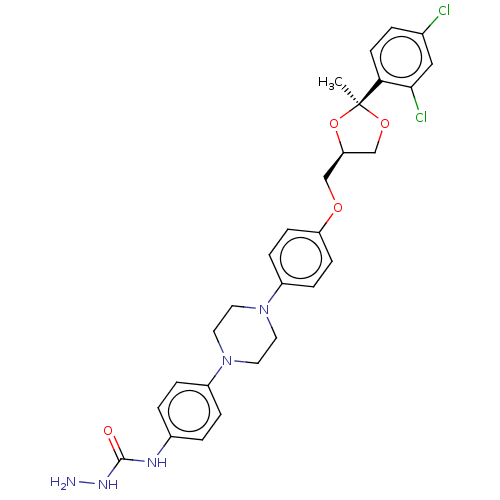 (CHEMBL4472296) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of smo-mediated hedgehog signaling pathway in mouse ASZ001 cells assessed as decrease in Gli1 mRNA expression after 48 hrs by qPCR method | J Med Chem 62: 3873-3885 (2019) Article DOI: 10.1021/acs.jmedchem.8b01283 BindingDB Entry DOI: 10.7270/Q2NC64M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM31788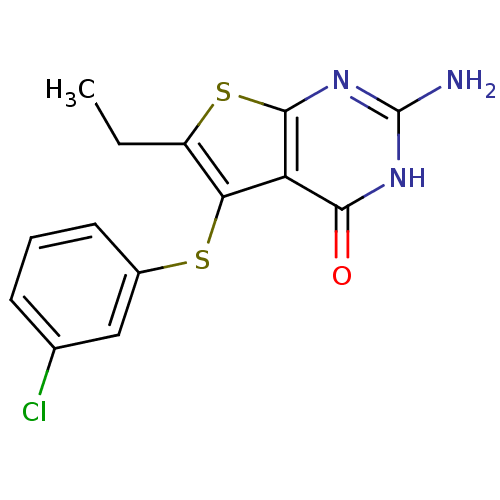 (thieno[2,3-d]pyrimidine deriv., 2k) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM31787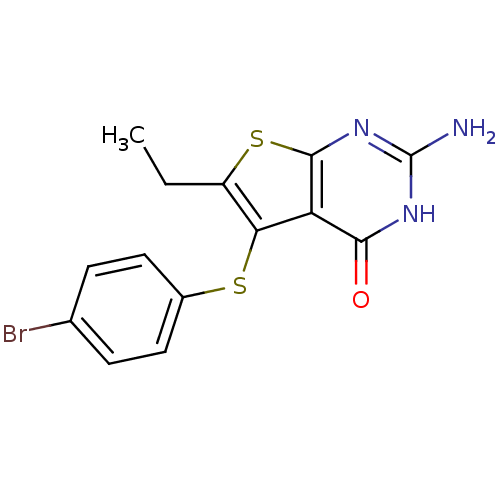 (thieno[2,3-d]pyrimidine deriv., 2j) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM31781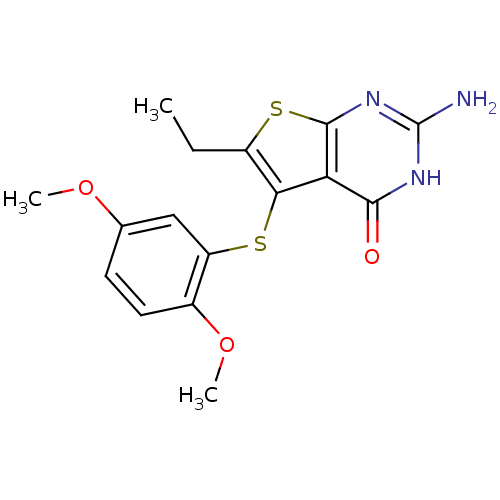 (thieno[2,3-d]pyrimidine deriv., 2d) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM31783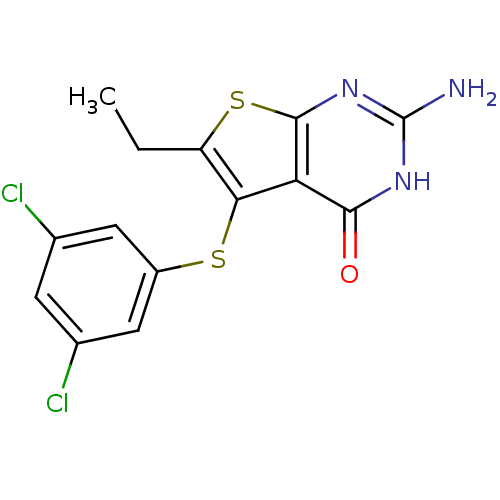 (thieno[2,3-d]pyrimidine deriv., 2f) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM31785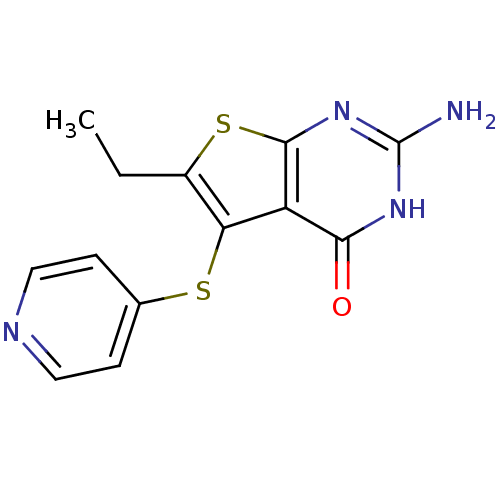 (thieno[2,3-d]pyrimidine deriv., 2h) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM31777 (thieno[2,3-d]pyrimidine deriv., 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Duquesne University | Assay Description TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM31777 (thieno[2,3-d]pyrimidine deriv., 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM18771 ((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM31776 (thieno[2,3-d]pyrimidine deriv., 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM31789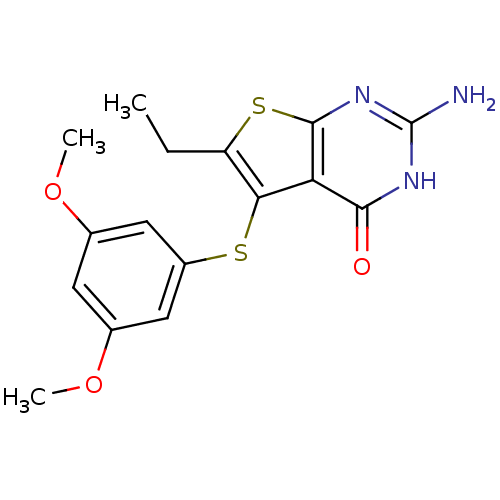 (thieno[2,3-d]pyrimidine deriv., 2l) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM31786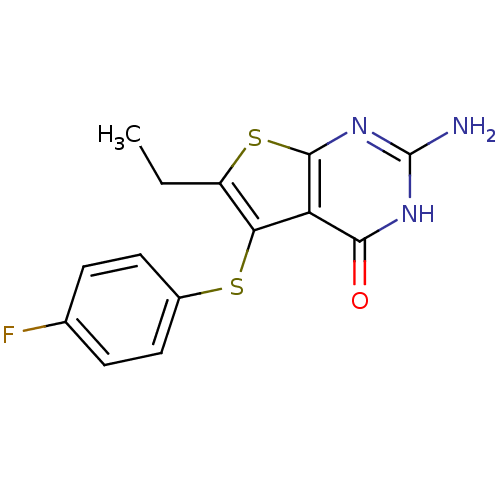 (thieno[2,3-d]pyrimidine deriv., 2i) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM31790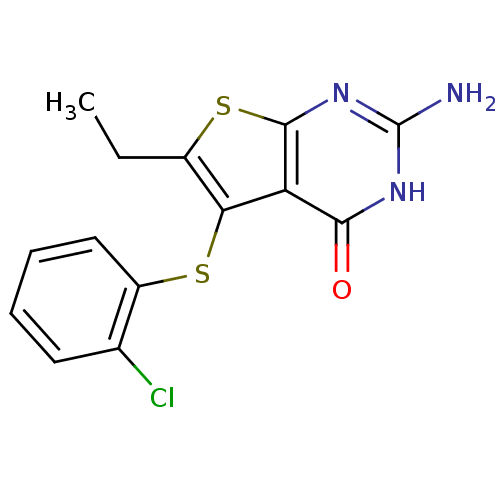 (thieno[2,3-d]pyrimidine deriv., 2m) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM31778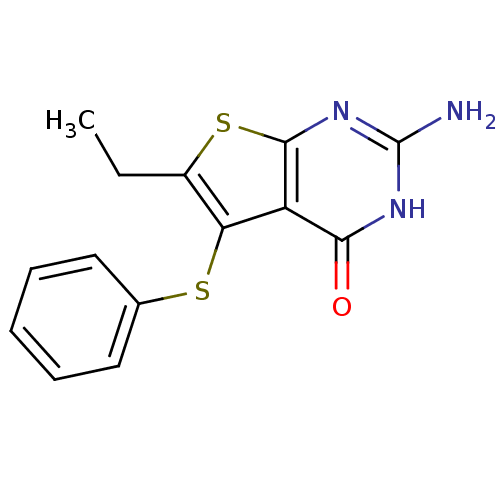 (thieno[2,3-d]pyrimidine deriv., 2a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis jirovecii) | BDBM50461744 (CHEMBL4227124) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of Pneumocystis jirovecii recombinant DHFR expressed in Escherichia coli Rosetta Gami B (DE3) competent cells using DHFA as substrate and ... | Bioorg Med Chem 26: 2640-2650 (2018) Article DOI: 10.1016/j.bmc.2018.04.032 BindingDB Entry DOI: 10.7270/Q2QJ7KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM31776 (thieno[2,3-d]pyrimidine deriv., 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM31776 (thieno[2,3-d]pyrimidine deriv., 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Duquesne University | Assay Description TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM31776 (thieno[2,3-d]pyrimidine deriv., 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Duquesne University | Assay Description TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis jirovecii) | BDBM18224 (6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of Pneumocystis jirovecii recombinant DHFR expressed in Escherichia coli Rosetta Gami B (DE3) competent cells using DHFA as substrate and ... | Bioorg Med Chem 26: 2640-2650 (2018) Article DOI: 10.1016/j.bmc.2018.04.032 BindingDB Entry DOI: 10.7270/Q2QJ7KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50011478 (ITRAZOLE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone as substrate after 10 mins by LC/MS/MS analysis | J Med Chem 59: 3635-49 (2016) Article DOI: 10.1021/acs.jmedchem.5b01718 BindingDB Entry DOI: 10.7270/Q2FX7CC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM31777 (thieno[2,3-d]pyrimidine deriv., 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Duquesne University | Assay Description TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis jirovecii) | BDBM50461735 (CHEMBL4228488) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of Pneumocystis jirovecii recombinant DHFR expressed in Escherichia coli Rosetta Gami B (DE3) competent cells using DHFA as substrate and ... | Bioorg Med Chem 26: 2640-2650 (2018) Article DOI: 10.1016/j.bmc.2018.04.032 BindingDB Entry DOI: 10.7270/Q2QJ7KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis jirovecii) | BDBM50461739 (CHEMBL4227384) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of Pneumocystis jirovecii recombinant DHFR expressed in Escherichia coli Rosetta Gami B (DE3) competent cells using DHFA as substrate and ... | Bioorg Med Chem 26: 2640-2650 (2018) Article DOI: 10.1016/j.bmc.2018.04.032 BindingDB Entry DOI: 10.7270/Q2QJ7KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis jirovecii) | BDBM50461745 (CHEMBL4226262) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of Pneumocystis jirovecii recombinant DHFR expressed in Escherichia coli Rosetta Gami B (DE3) competent cells using DHFA as substrate and ... | Bioorg Med Chem 26: 2640-2650 (2018) Article DOI: 10.1016/j.bmc.2018.04.032 BindingDB Entry DOI: 10.7270/Q2QJ7KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis jirovecii) | BDBM50461738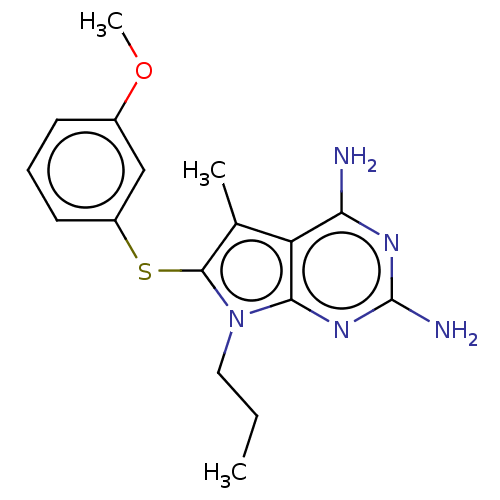 (CHEMBL4228228) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of Pneumocystis jirovecii recombinant DHFR expressed in Escherichia coli Rosetta Gami B (DE3) competent cells using DHFA as substrate and ... | Bioorg Med Chem 26: 2640-2650 (2018) Article DOI: 10.1016/j.bmc.2018.04.032 BindingDB Entry DOI: 10.7270/Q2QJ7KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM18771 ((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Duquesne University | Assay Description TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50522382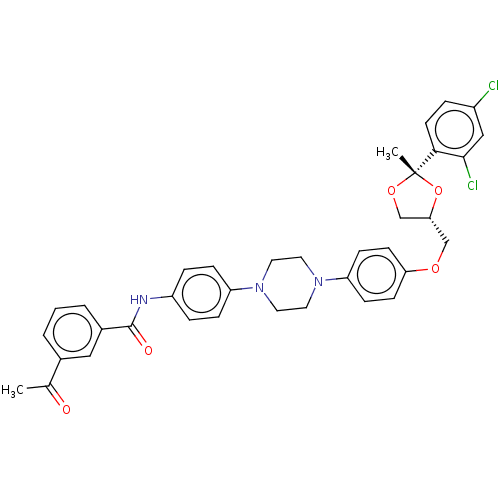 (CHEMBL4450586) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of smo-mediated hedgehog signaling pathway in mouse ASZ001 cells assessed as decrease in Gli1 mRNA expression after 48 hrs by qPCR method | J Med Chem 62: 3873-3885 (2019) Article DOI: 10.1021/acs.jmedchem.8b01283 BindingDB Entry DOI: 10.7270/Q2NC64M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM31777 (thieno[2,3-d]pyrimidine deriv., 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... | J Med Chem 52: 4892-902 (2009) Article DOI: 10.1021/jm900490a BindingDB Entry DOI: 10.7270/Q2CR5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis jirovecii) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of Pneumocystis jirovecii recombinant DHFR expressed in Escherichia coli Rosetta Gami B (DE3) competent cells using DHFA as substrate and ... | Bioorg Med Chem 26: 2640-2650 (2018) Article DOI: 10.1016/j.bmc.2018.04.032 BindingDB Entry DOI: 10.7270/Q2QJ7KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis jirovecii) | BDBM18262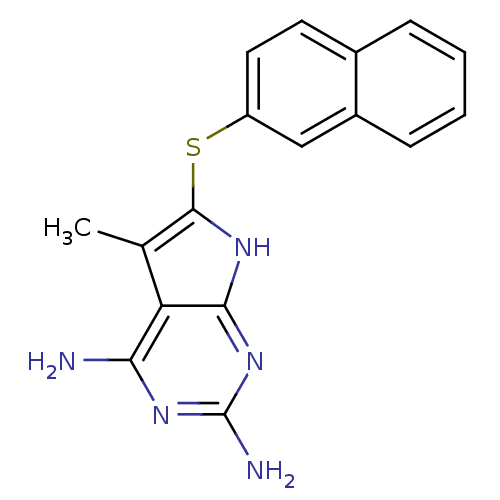 (5-methyl-6-(naphthalen-2-ylsulfanyl)-7H-pyrrolo[2,...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of Pneumocystis jirovecii recombinant DHFR expressed in Escherichia coli Rosetta Gami B (DE3) competent cells using DHFA as substrate and ... | Bioorg Med Chem 26: 2640-2650 (2018) Article DOI: 10.1016/j.bmc.2018.04.032 BindingDB Entry DOI: 10.7270/Q2QJ7KXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50522397 (CHEMBL4440172) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of smo-mediated hedgehog signaling pathway in mouse ASZ001 cells assessed as decrease in Gli1 mRNA expression after 48 hrs by qPCR method | J Med Chem 62: 3873-3885 (2019) Article DOI: 10.1021/acs.jmedchem.8b01283 BindingDB Entry DOI: 10.7270/Q2NC64M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 329 total ) | Next | Last >> |