

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50103243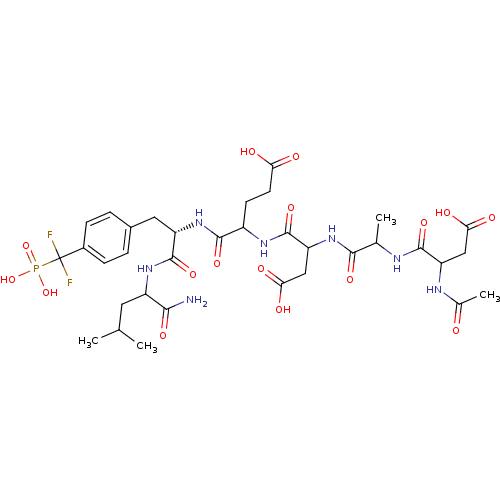 (4-{2-[2-(2-Acetylamino-3-carboxy-propionylamino)-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory activity against human Protein-tyrosinephosphatase 1B (PTP1B) | J Med Chem 44: 2869-78 (2001) BindingDB Entry DOI: 10.7270/Q237781C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50103235 (5-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory activity against human Protein-tyrosinephosphatase 1B (PTP1B) | J Med Chem 44: 2869-78 (2001) BindingDB Entry DOI: 10.7270/Q237781C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50103240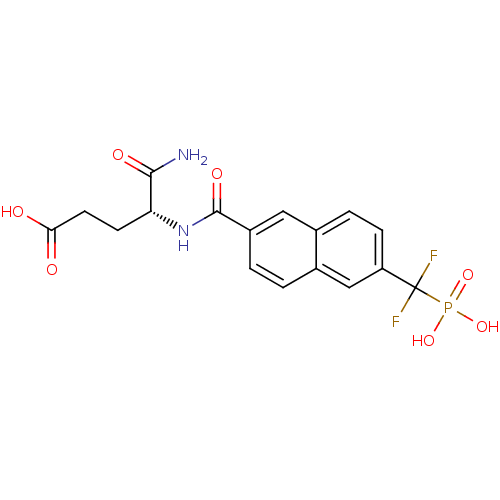 (4-Carbamoyl-4-{[6-(difluoro-phosphono-methyl)-naph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory activity against human Protein-tyrosinephosphatase 1B (PTP1B) | J Med Chem 44: 2869-78 (2001) BindingDB Entry DOI: 10.7270/Q237781C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50103227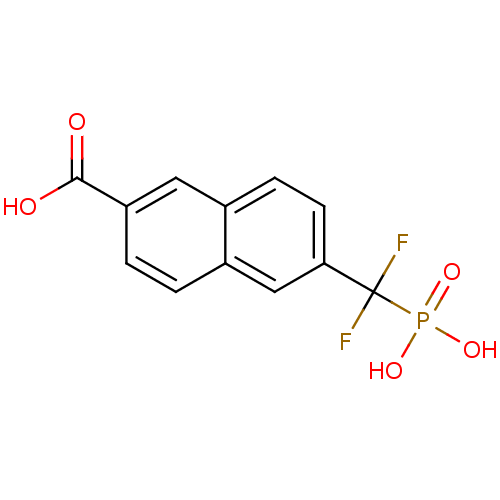 (6-(Difluoro-phosphono-methyl)-naphthalene-2-carbox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory activity against human Protein-tyrosinephosphatase 1B (PTP1B) | J Med Chem 44: 2869-78 (2001) BindingDB Entry DOI: 10.7270/Q237781C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50103238 (3-Carboxymethoxy-naphthalene-2,7-dicarboxylic acid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory activity against human Protein-tyrosinephosphatase 1B (PTP1B) | J Med Chem 44: 2869-78 (2001) BindingDB Entry DOI: 10.7270/Q237781C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50103234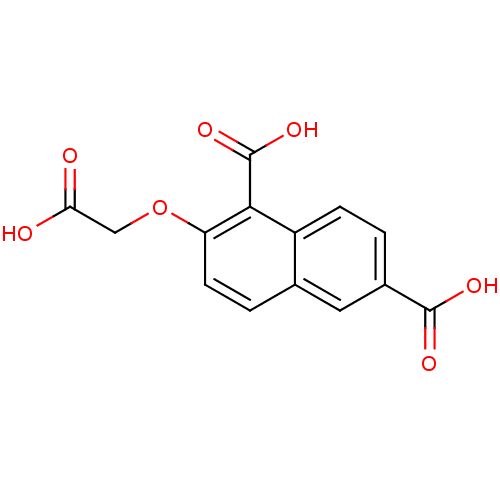 (2-Carboxymethoxy-naphthalene-1,6-dicarboxylic acid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory activity against human Protein-tyrosinephosphatase 1B (PTP1B) | J Med Chem 44: 2869-78 (2001) BindingDB Entry DOI: 10.7270/Q237781C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50103231 (CHEMBL98615 | Naphthalene-2,7-dicarboxylic acid) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >3.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory activity against human Protein-tyrosinephosphatase 1B (PTP1B) | J Med Chem 44: 2869-78 (2001) BindingDB Entry DOI: 10.7270/Q237781C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50103242 (CHEMBL316894 | Naphthalene trisulfonate (NTS) | na...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | PubMed | >4.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory activity against human Protein-tyrosinephosphatase 1B (PTP1B) | J Med Chem 44: 2869-78 (2001) BindingDB Entry DOI: 10.7270/Q237781C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50125697 (4-(3,4-Bis-azidomethyl-phenyl)-2,4-dioxo-butyric a...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Recombinant HIV-1 integrase (Standard transfer reaction) | Bioorg Med Chem Lett 13: 1215-9 (2003) BindingDB Entry DOI: 10.7270/Q23J3CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50125697 (4-(3,4-Bis-azidomethyl-phenyl)-2,4-dioxo-butyric a...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Recombinant HIV-1 integrase (3'processing) | Bioorg Med Chem Lett 13: 1215-9 (2003) BindingDB Entry DOI: 10.7270/Q23J3CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50125701 (4-(5,8-Diazido-5,6,7,8-tetrahydro-naphthalen-2-yl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Recombinant HIV-1 integrase (Standard transfer reaction) | Bioorg Med Chem Lett 13: 1215-9 (2003) BindingDB Entry DOI: 10.7270/Q23J3CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50115572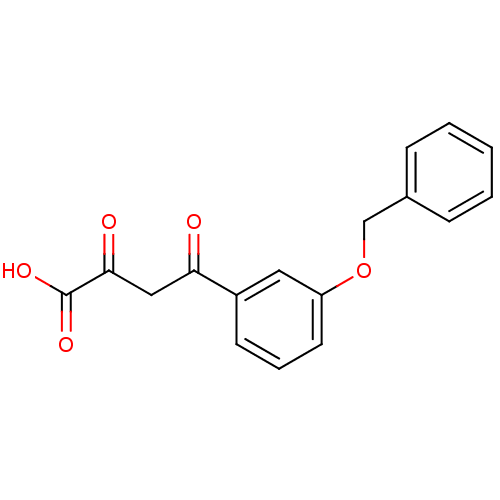 ((Z)-4-(3-Benzyloxy-phenyl)-2-hydroxy-4-oxo-but-2-e...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute/NIH Curated by ChEMBL | Assay Description Inhibitory effect on strand transfer of proviral DNA into host DNA in an extracellular HIV-1 integrase assay | J Med Chem 45: 3184-94 (2002) BindingDB Entry DOI: 10.7270/Q26D5TPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50115572 ((Z)-4-(3-Benzyloxy-phenyl)-2-hydroxy-4-oxo-but-2-e...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Recombinant HIV-1 integrase (Standard transfer reaction) | Bioorg Med Chem Lett 13: 1215-9 (2003) BindingDB Entry DOI: 10.7270/Q23J3CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50125699 (4-(1,3-Diazido-indan-5-yl)-2,4-dioxo-butyric acid ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Recombinant HIV-1 integrase (Standard transfer reaction) | Bioorg Med Chem Lett 13: 1215-9 (2003) BindingDB Entry DOI: 10.7270/Q23J3CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM107681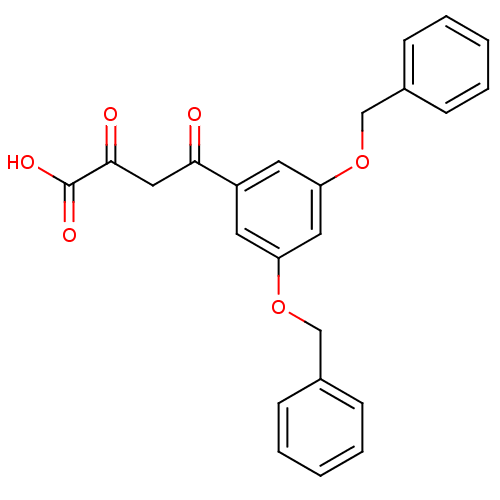 ((2Z)-4-[3,5-bis(benzyloxy)phenyl]-2-hydroxy-4- oxo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Recombinant HIV-1 integrase (Standard transfer reaction) | Bioorg Med Chem Lett 13: 1215-9 (2003) BindingDB Entry DOI: 10.7270/Q23J3CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM107681 ((2Z)-4-[3,5-bis(benzyloxy)phenyl]-2-hydroxy-4- oxo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute/NIH Curated by ChEMBL | Assay Description Inhibitory effect on strand transfer of proviral DNA into host DNA in an extracellular HIV-1 integrase assay | J Med Chem 45: 3184-94 (2002) BindingDB Entry DOI: 10.7270/Q26D5TPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM107693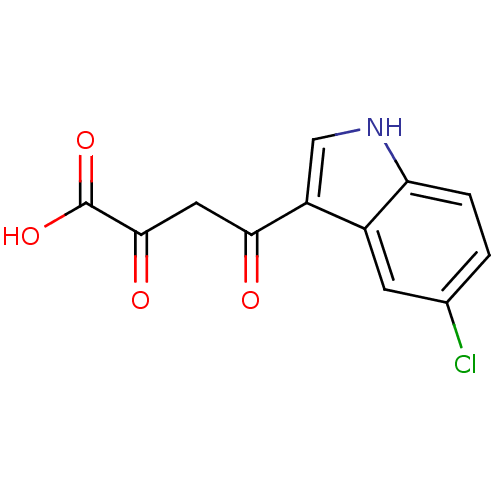 ((2Z)-4-(5-chloro-1H-indol-3-yl)-2-hydroxy-4-oxobut...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute/NIH Curated by ChEMBL | Assay Description Inhibitory effect on strand transfer of proviral DNA into host DNA in an extracellular HIV-1 integrase assay | J Med Chem 45: 3184-94 (2002) BindingDB Entry DOI: 10.7270/Q26D5TPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50093435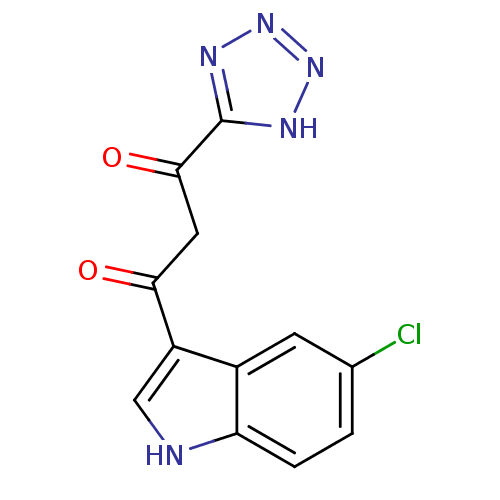 ((Z)-1-(5-Chloro-1H-indol-3-yl)-3-hydroxy-3-(1H-tet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | MMDB PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute/NIH Curated by ChEMBL | Assay Description Inhibitory effect on strand transfer of proviral DNA into host DNA in an extracellular HIV-1 integrase assay | J Med Chem 45: 3184-94 (2002) BindingDB Entry DOI: 10.7270/Q26D5TPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50330327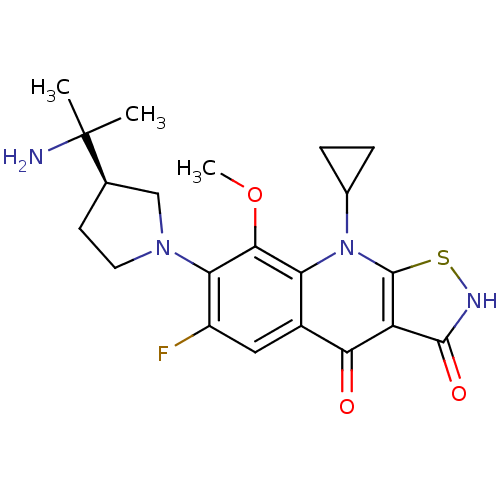 ((R)-7-(3-(2-aminopropan-2-yl)pyrrolidin-1-yl)-9-cy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of wild-type Staphylococcus aureus DNA gyrase assessed as inhibition of supercoiling of pBR322 DNA after 60 min by gel electrophoresis ass... | J Med Chem 54: 3268-82 (2011) Article DOI: 10.1021/jm101604v BindingDB Entry DOI: 10.7270/Q28S4SRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50483850 (CHEMBL1774147) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of wild-type Staphylococcus aureus DNA gyrase assessed as inhibition of supercoiling of pBR322 DNA after 60 min by gel electrophoresis ass... | J Med Chem 54: 3268-82 (2011) Article DOI: 10.1021/jm101604v BindingDB Entry DOI: 10.7270/Q28S4SRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50056891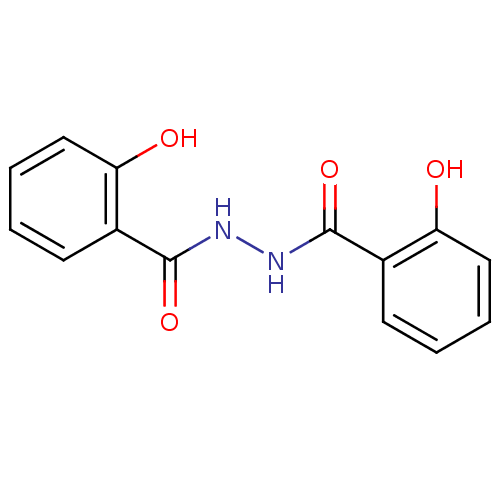 (2-Hydroxy-benzoic acid N'-(2-hydroxy-benzoyl)-hydr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 integrase (preincubated with Mn+2, and drug for 30 min followed by DNA for 1h) in strand transfer of preassemble | J Med Chem 45: 5661-70 (2002) BindingDB Entry DOI: 10.7270/Q2M61JMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50370120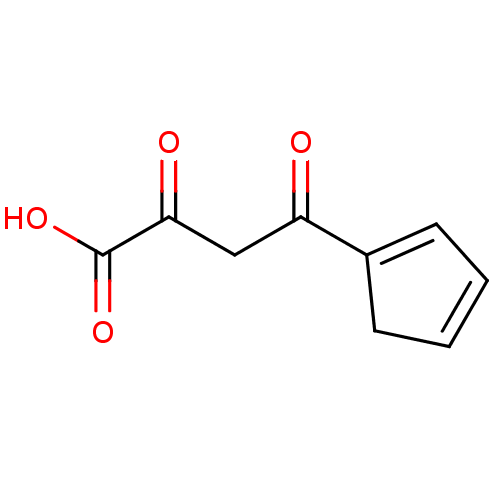 (CHEMBL611459) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute/NIH Curated by ChEMBL | Assay Description Inhibitory effect on strand transfer of proviral DNA into host DNA in an extracellular HIV-1 integrase assay | J Med Chem 45: 3184-94 (2002) BindingDB Entry DOI: 10.7270/Q26D5TPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109055 (US9695205, 1) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109056 (US9695205, 2) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109057 (US9695205, 3) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109058 (US9695205, 4) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109059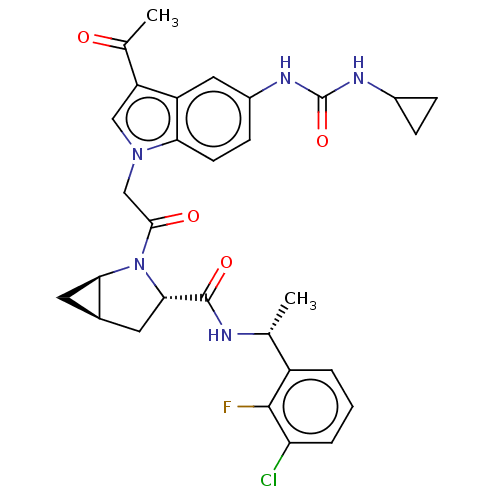 (US9695205, 5) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109061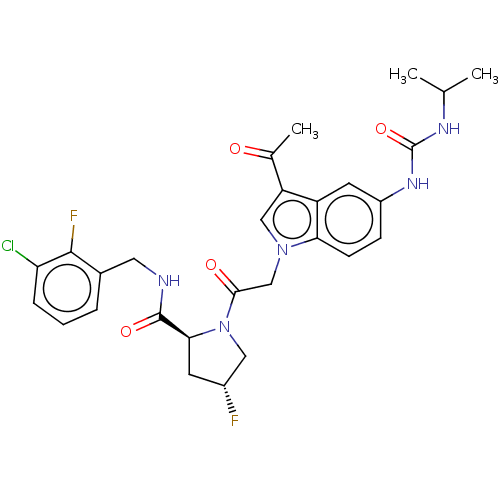 (US9695205, 6) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109091 (US9695205, 7) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109092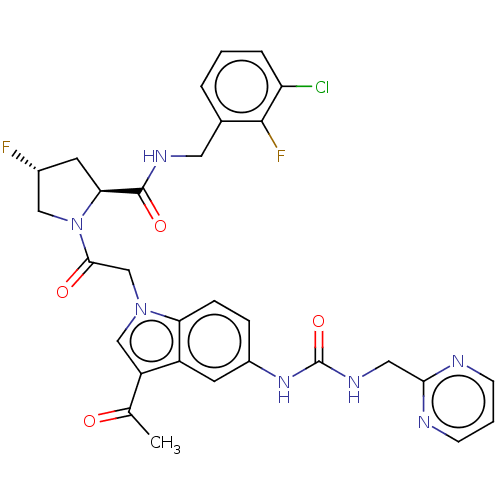 (US9695205, 8) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109093 (US9695205, 9) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109094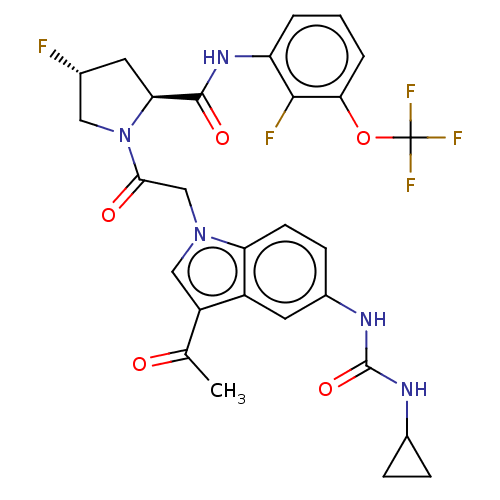 (US9695205, 10) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109103 (US9695205, 11) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109104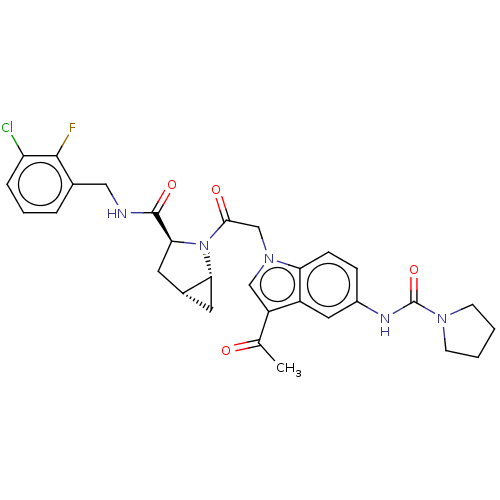 (US9695205, 12) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109178 (US9695205, 13) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109199 (US9695205, 14) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109358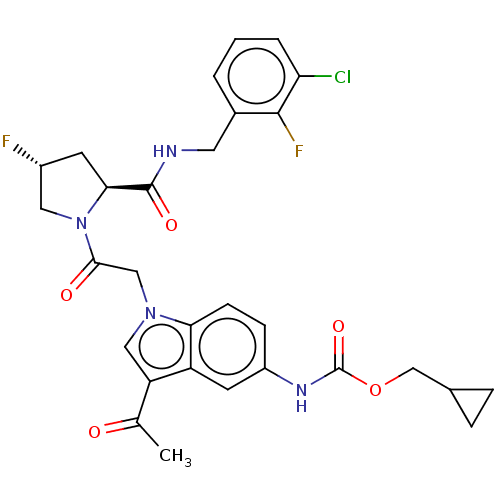 (US9695205, 15) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109385 (US9695205, 16) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109391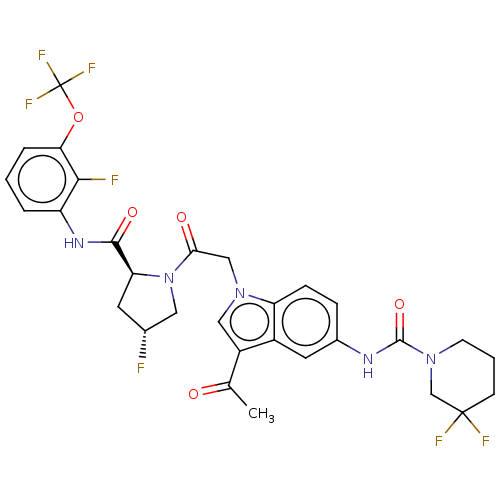 (US9695205, 17) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109393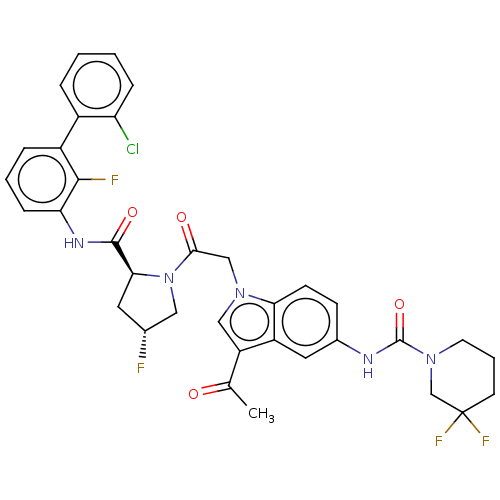 (US9695205, 18) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109466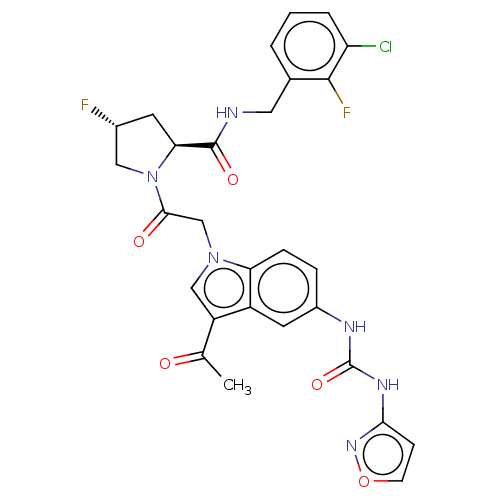 (US9695205, 19) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109468 (US9695205, 20) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109469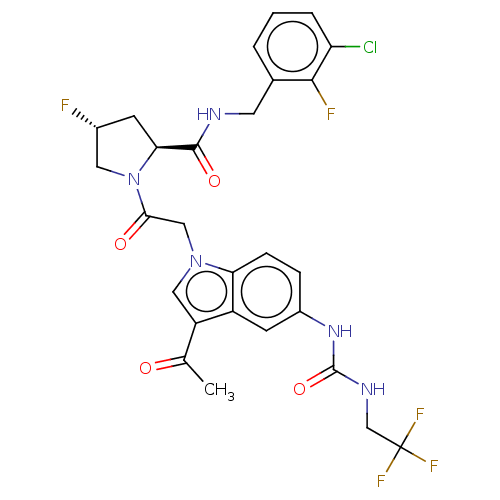 (US9695205, 21) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109544 (US9695205, 22) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109545 (US9695205, 23) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109940 (US9695205, 24) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM109944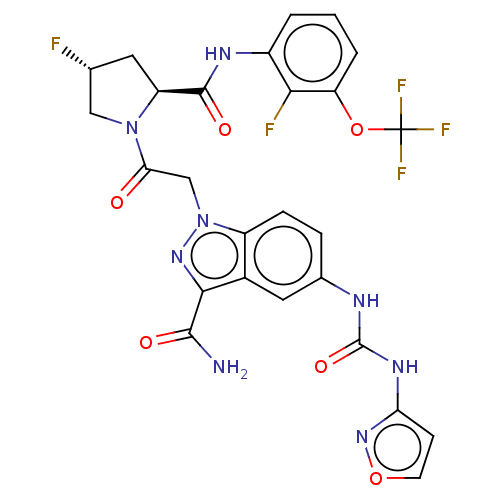 (US9695205, 25) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM110039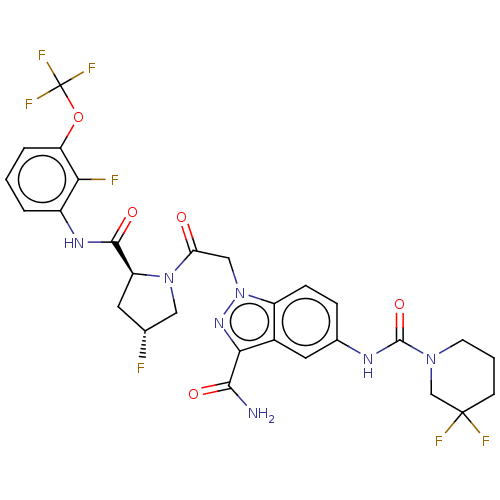 (US9695205, 26) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM110040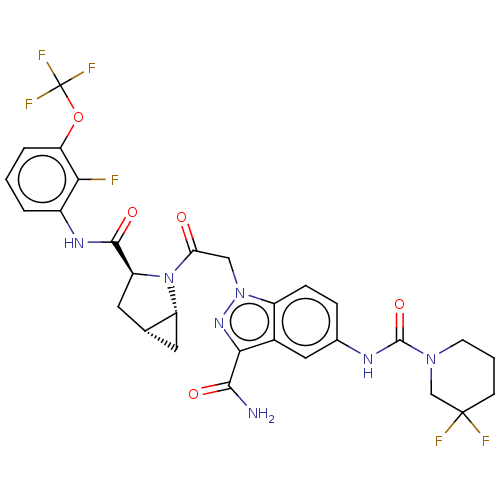 (US9695205, 27) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM110042 (US9695205, 28) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Achillion Pharmaceuticals, Inc. US Patent | Assay Description Human factor D (purified from human serum, Complement Technology, Inc.) at 80 nM final concentration is incubated with test compound at various conce... | US Patent US9695205 (2017) BindingDB Entry DOI: 10.7270/Q2VQ30TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3981 total ) | Next | Last >> |