

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM571620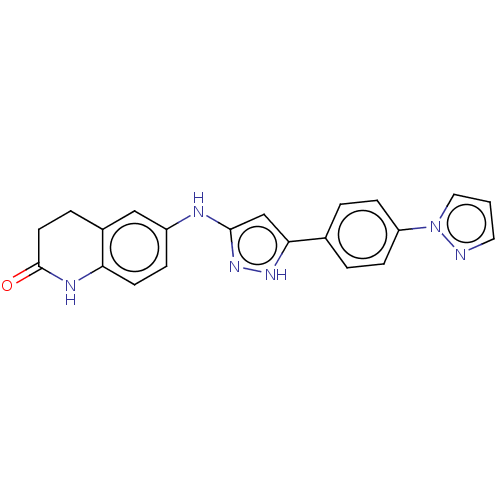 (6-((5-(4-(1H-pyrazol-1- yl)phenyl)-1H-pyrazol-3- y...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11447469-20220920-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24X5C2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579709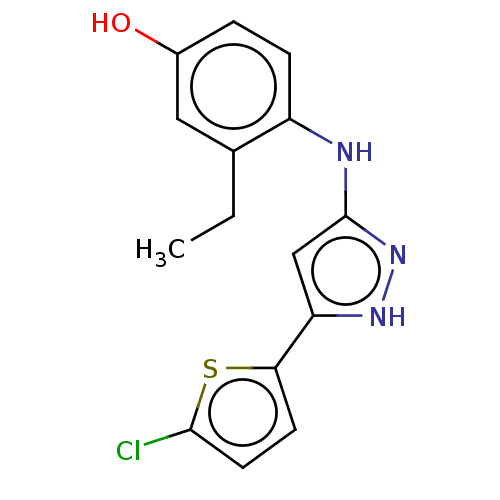 (4-((5-(5-chlorothiophen-2- yl)-1H-pyrazol-3-yl)ami...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM571666 (5-((5-(3-fluoro-4- hydroxyphenyl)-1H- pyrazol-3- y...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11447469-20220920-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24X5C2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579718 (3-ethyl-4-((5-(4-iodophenyl)- 1H-pyrazol-3-yl)amin...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM571674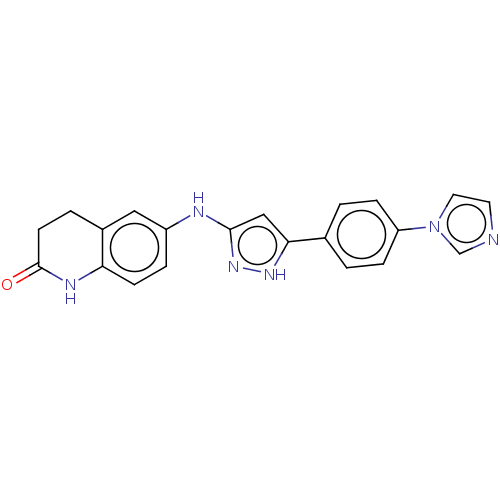 (6-((5-(4-(1H-imidazol-1- yl)phenyl)-1H-pyrazol-3- ...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11447469-20220920-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24X5C2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579738 (N-(4-((5-(4-(1H-pyrazol-1- yl)phenyl)-1H-pyrazol-3...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579704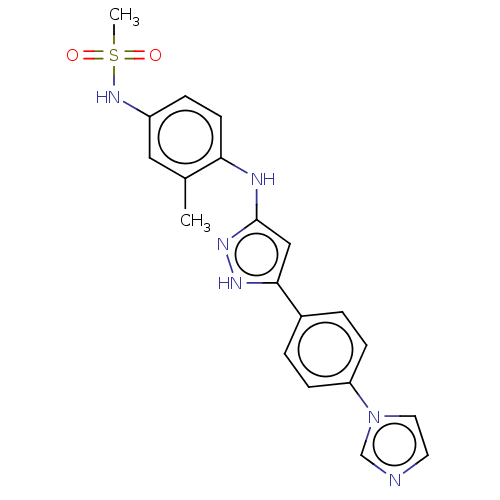 (N-(4-((5-(4-(1H-imidazol-1- yl)phenyl)-1H-pyrazol-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579739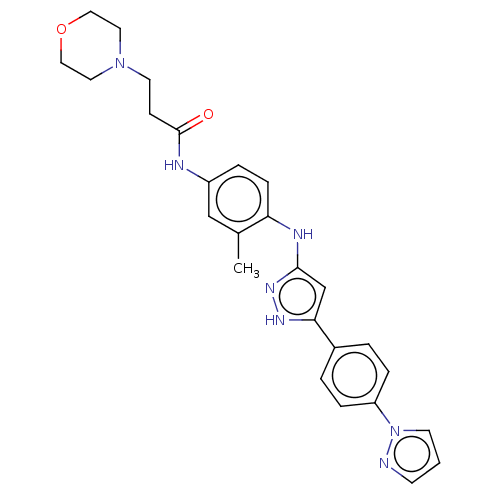 (N-(4-((5-(4-(1H-pyrazol-1- yl)phenyl)-1H-pyrazol-3...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579743 (N-(4-((5-(4-(1H-pyrazol-1- yl)phenyl)-1H-pyrazol-3...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579689 (N-(4-((5-(4-hydroxyphenyl)- 1H-pyrazol-3-yl)amino)...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579794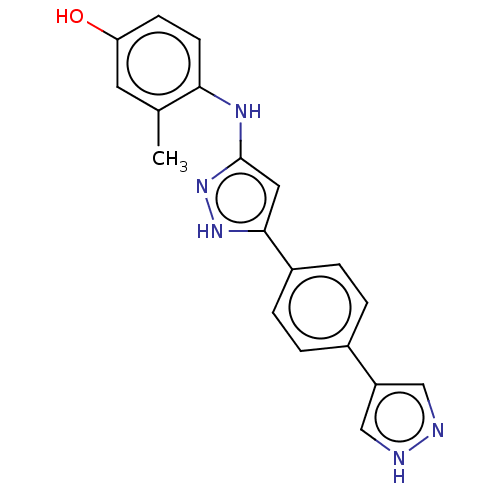 (4-((5-(4-(1H-pyrazol-4- yl)phenyl)-1H-pyrazol-3- y...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50464556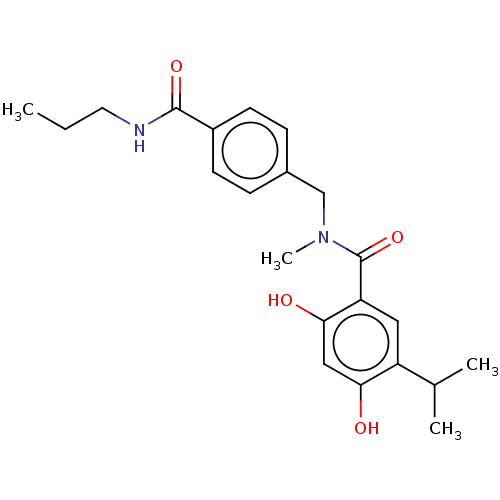 (CHEMBL4289811 | US10464907, Compound 21f | US10889...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Keimyung University Curated by ChEMBL | Assay Description Inhibition of FITC-geldanamycin binding to N-terminal domain of recombinant full-length HSP90alpha (unknown origin) after 16 hrs by fluorescence pola... | Eur J Med Chem 143: 390-401 (2018) Article DOI: 10.1016/j.ejmech.2017.11.054 BindingDB Entry DOI: 10.7270/Q29C713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579761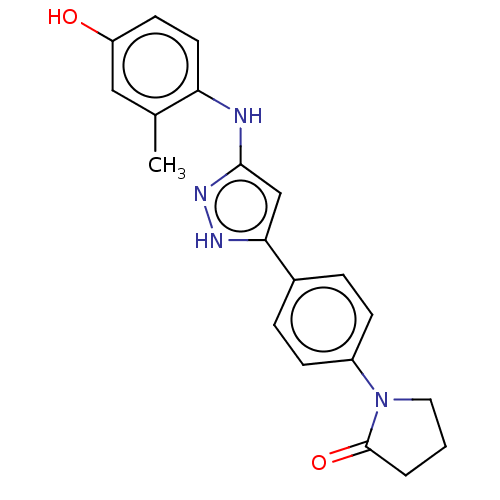 (1-(4-(3-((4-hydroxy-2- methylphenyl)amino)-1H- pyr...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579714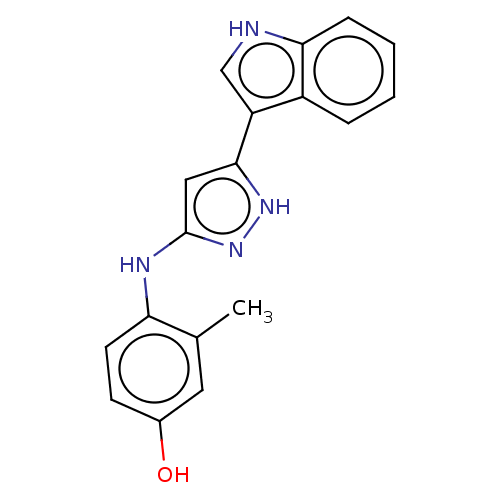 (4-((5-(1H-indol-3-yl)-1H- pyrazol-3-yl)amino)-3- m...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50464557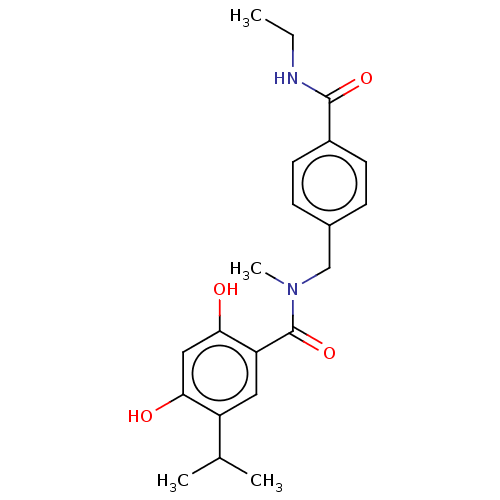 (CHEMBL4279132 | US10464907, Compound 21e | US10889...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Keimyung University Curated by ChEMBL | Assay Description Inhibition of FITC-geldanamycin binding to N-terminal domain of recombinant full-length HSP90alpha (unknown origin) after 16 hrs by fluorescence pola... | Eur J Med Chem 143: 390-401 (2018) Article DOI: 10.1016/j.ejmech.2017.11.054 BindingDB Entry DOI: 10.7270/Q29C713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579740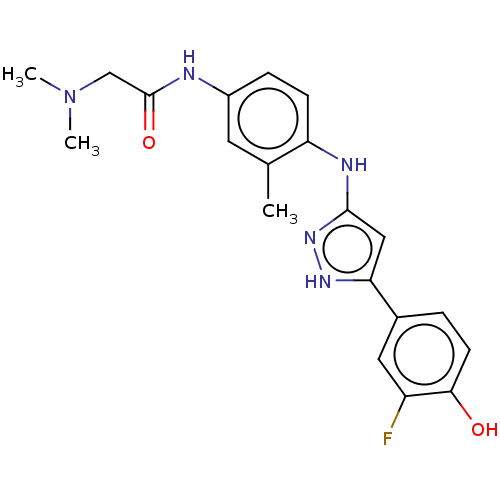 (2-(dimethylamino)-N-(4-((5- (3-fluoro-4-hydroxyphe...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579764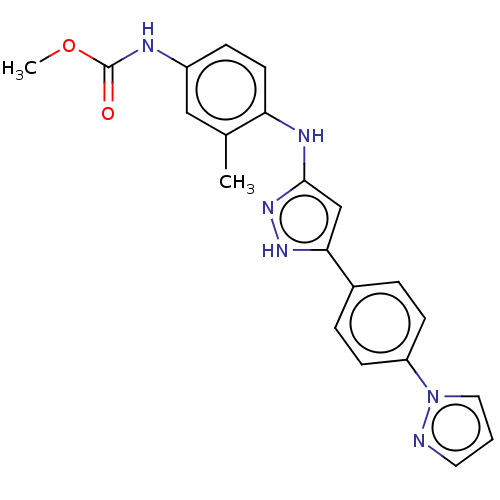 (US11485711, Compound 208 | methyl (4-((5-(4-(1H-py...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM571724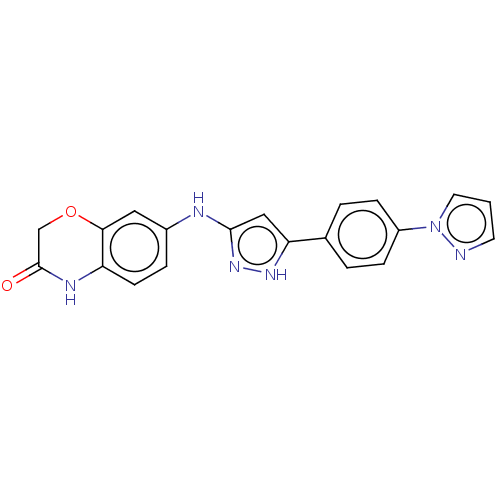 (7-((5-(4-(1H-pyrazol-1- yl)phenyl)-1H-pyrazol-3- y...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11447469-20220920-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24X5C2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579763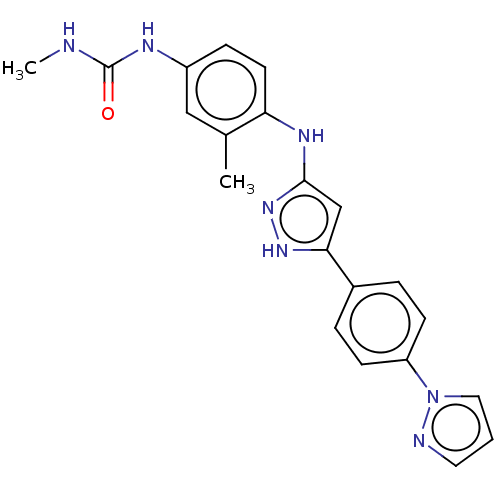 (1-(4-((5-(4-(1H-pyrazol-1- yl)phenyl)-1H-pyrazol-3...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579799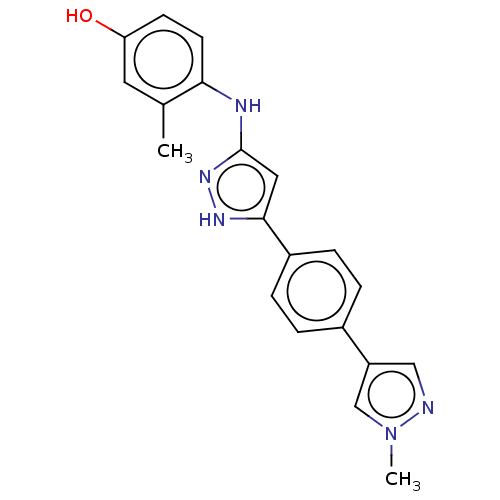 (3-methyl-4-((5-(4-(1-methyl-1H- pyrazol-4-yl)pheny...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579736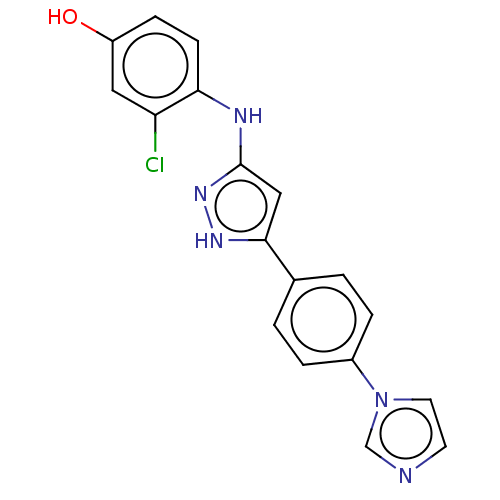 (4-((5-(4-(1H-imidazol-1- yl)phenyl)-1H-pyrazol-3- ...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579705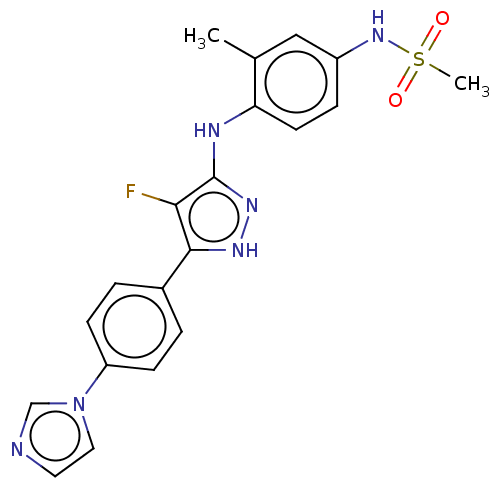 (N-(4-((5-(4-(1H-imidazol-1- yl)phenyl)-4-fluoro-1H...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579801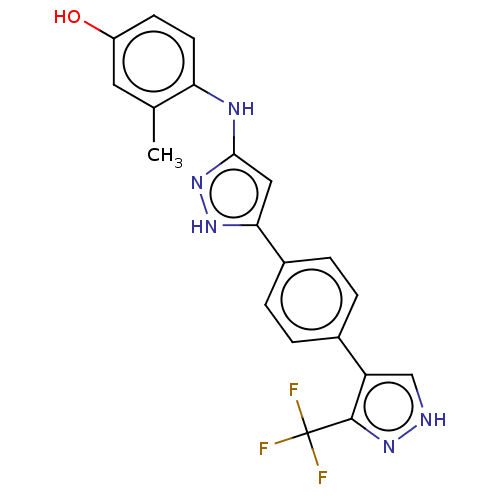 (3-methyl-4-((5-(4-(3- (trifluoromethyl)-1H-pyrazol...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579765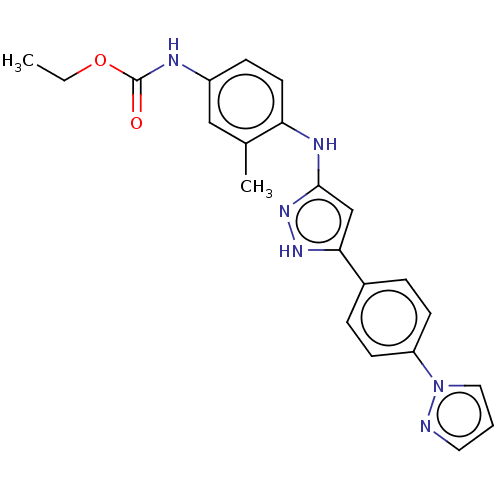 (US11485711, Compound 209 | ethyl (4-((5-(4-(1H-pyr...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579741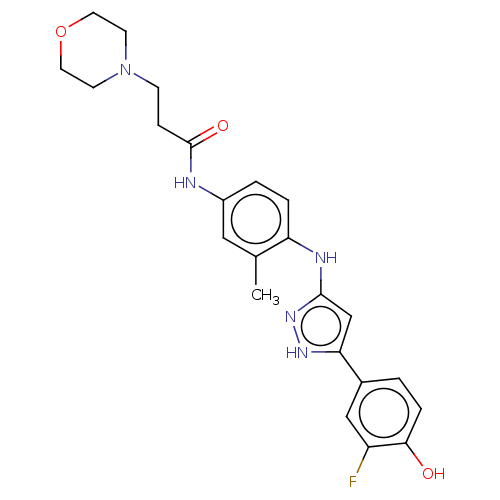 (N-(4-((5-(3-fluoro-4- hydroxyphenyl-1H-pyrazol-3- ...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50117298 (CHEMBL3613597) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of Tyk2 (unknown origin) | Bioorg Med Chem Lett 25: 3947-52 (2015) Article DOI: 10.1016/j.bmcl.2015.07.037 BindingDB Entry DOI: 10.7270/Q2Z039ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579708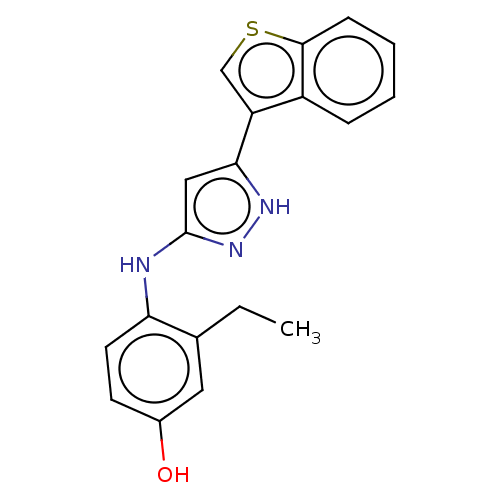 (4-((5-(benzo[b]thiophen-3- yl)-1H-pyrazol-3-yl)ami...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579800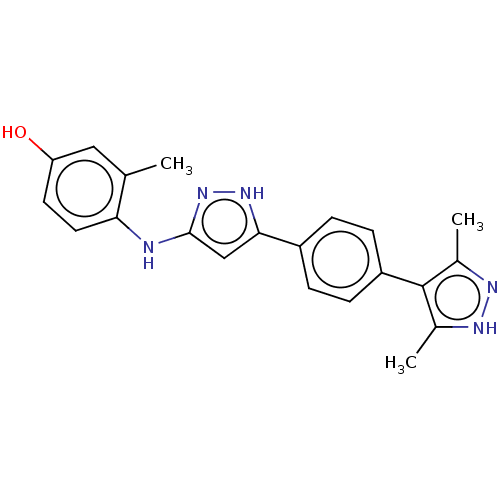 (4-((5-(4-(3,5-dimethyl-1H-pyrazol- 4-yl)phenyl)-1H...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50464562 (CHEMBL4293186 | US10464907, Compound 21d | US10889...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Keimyung University Curated by ChEMBL | Assay Description Inhibition of FITC-geldanamycin binding to N-terminal domain of recombinant full-length HSP90alpha (unknown origin) after 16 hrs by fluorescence pola... | Eur J Med Chem 143: 390-401 (2018) Article DOI: 10.1016/j.ejmech.2017.11.054 BindingDB Entry DOI: 10.7270/Q29C713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579693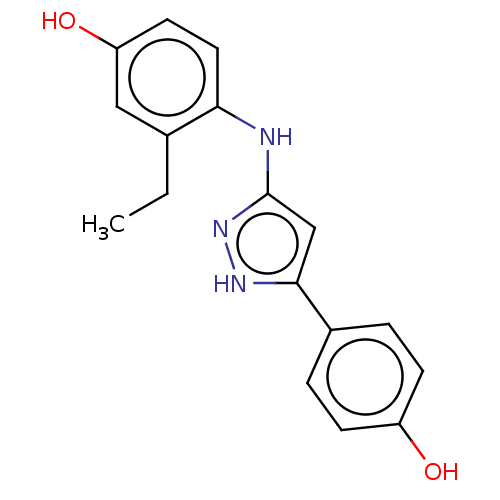 (3-ethyl-4-((5-(4- hydroxyphenyl)-1H-pyrazol-3- yl)...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579703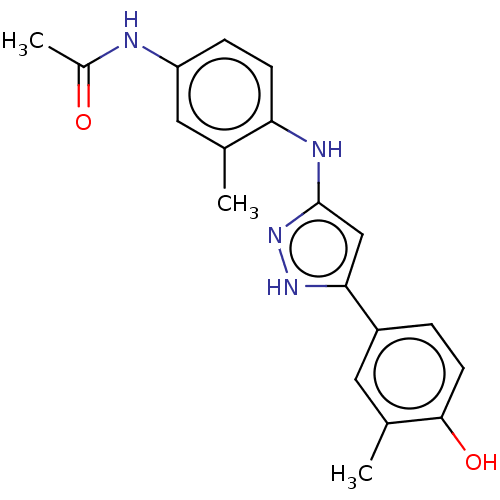 (N-(4-((5-(4-hydroxy-3- methylphenyl)-1H-pyrazol-3-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50008059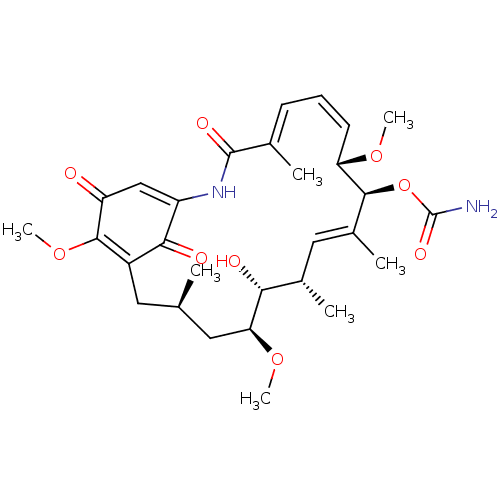 (CHEBI:5292 | GELDANAMYCIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Keimyung University Curated by ChEMBL | Assay Description Inhibition of fluorescein isothiocyanate labeled geldanamycin binding to N-terminal domain of full length human recombinant Hsp90alpha expressed in E... | Eur J Med Chem 124: 1069-1080 (2016) Article DOI: 10.1016/j.ejmech.2016.10.038 BindingDB Entry DOI: 10.7270/Q29G5PZZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50008059 (CHEBI:5292 | GELDANAMYCIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Keimyung University Curated by ChEMBL | Assay Description Inhibition of FITC-geldanamycin binding to N-terminal domain of recombinant full-length HSP90alpha (unknown origin) after 16 hrs by fluorescence pola... | Eur J Med Chem 143: 390-401 (2018) Article DOI: 10.1016/j.ejmech.2017.11.054 BindingDB Entry DOI: 10.7270/Q29C713M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579735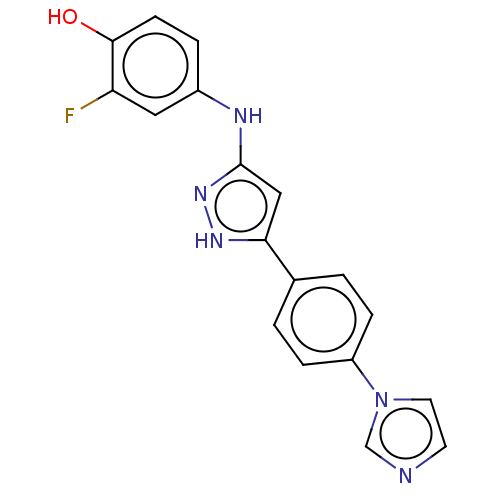 (4-((5-(4-(1H-inidazol-1- yl)phenyl)-1H-pyrazol-3- ...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579730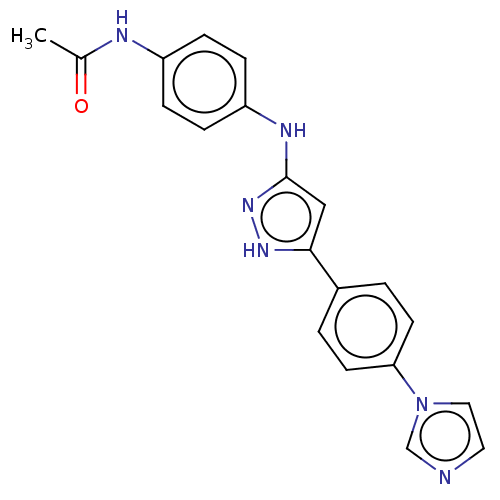 (N-(4-((5-(4-(1H-imidazol-1- yl)phenyl)-1H-pyrazol-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579731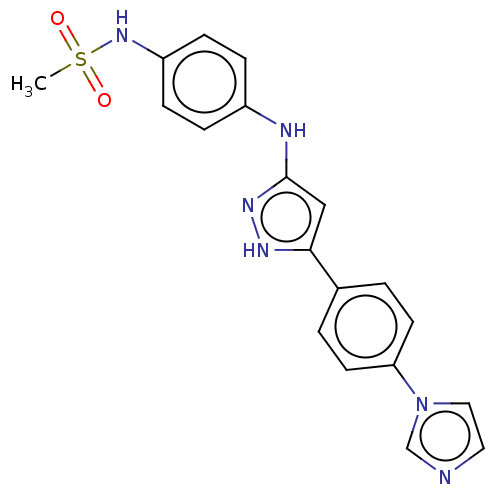 (N-(4-((5-(4-(1H-imidazol-1- yl)phenyl)-1H-pyrazol-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579710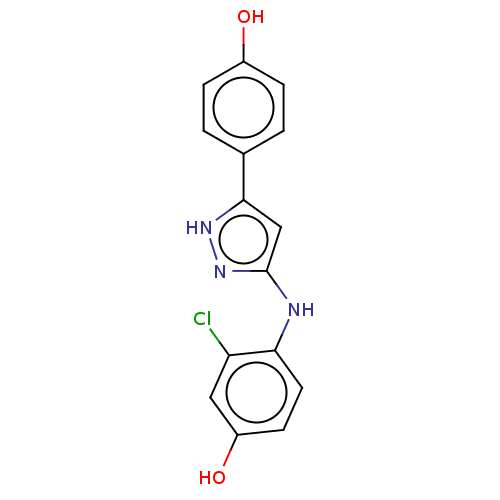 (3-chloro-4-((5-(4- hydroxyphenyl)-1H-pyrazol-3- yl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579798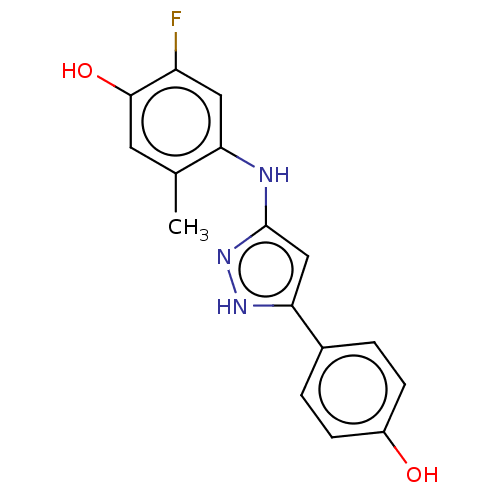 (2-fluoro-4-((5-(4-hydroxyphenyl)- 1H-pyrazol-3-yl)...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579797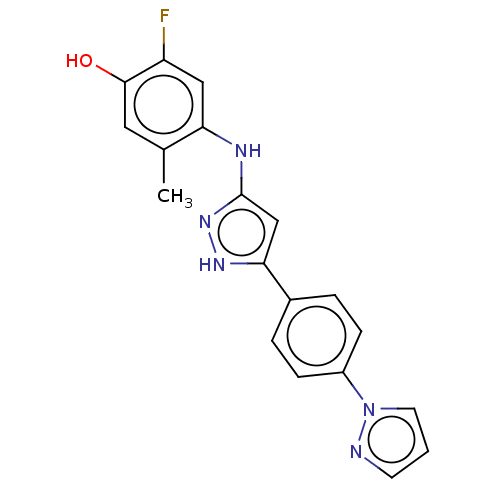 (4-((5-(4-(1H-pyrazol-1-yl)phenyl)- 1H-pyrazol-3-yl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579713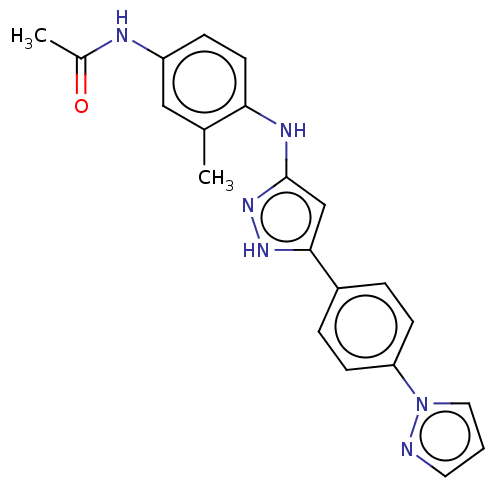 (N-(4-((5-(4-(1H-pyrazol-1- yl)phenyl)-1H-pyrazol-3...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579702 (4-(3-((4-hydroxy-2- methylphenyl)amino)-1H- pyrazo...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579712 (4-((5-(4-(1H-pyrazol-1- yl)phenyl)-1H-pyrazol-3- y...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579726 (N-(4-((5-(3-fluoro-4- hydroxyphenyl)-1H-pyrazol-3-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50592766 (CHEMBL5175785) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114582 BindingDB Entry DOI: 10.7270/Q28S4TW4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579770 (1-(4-(3-((4-hydroxy-2- methylphenyl)amino)-1H- pyr...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579804 (US11485711, Compound 281 | methyl (4-((5-(4-(1H-py...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579727 (4-(3-((2-ethyl-4- hydroxyphenyl)amino)-1H- pyrazol...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM571722 (4-(3-((1H-indazol-5- yl)amino)-1H-pyrazol-5- yl)ph...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11447469-20220920-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24X5C2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50592758 (CHEMBL5188138) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114582 BindingDB Entry DOI: 10.7270/Q28S4TW4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579733 (4-((5-(4-(1H-imidazol-1- yl)phenyl)-1H-pyrazol-3- ...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 841 total ) | Next | Last >> |