| Reaction Details |
|---|
 | Report a problem with these data |
| Target | TRAF2 and NCK-interacting protein kinase |
|---|
| Ligand | BDBM579736 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ADP-Glo Kinase Assay |
|---|
| IC50 | 8.00±n/a nM |
|---|
| Citation |  Chang, SY; Lee, H; Kim, KY; Kim, BT; Kim, SS; Kim, SH; Lim, HJ; Heo, JN; Shin, SJ; Park, SY Compounds for inhibiting TNIK and medical uses thereof US Patent US11485711 Publication Date 11/1/2022 Chang, SY; Lee, H; Kim, KY; Kim, BT; Kim, SS; Kim, SH; Lim, HJ; Heo, JN; Shin, SJ; Park, SY Compounds for inhibiting TNIK and medical uses thereof US Patent US11485711 Publication Date 11/1/2022 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| TRAF2 and NCK-interacting protein kinase |
|---|
| Name: | TRAF2 and NCK-interacting protein kinase |
|---|
| Synonyms: | KIAA0551 | TNIK | TNIK_HUMAN | TRAF2 and NCK-interacting protein kinase (TNIK) | TRAF2- and NCK-interacting kinase |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 154954.98 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q9UKE5 |
|---|
| Residue: | 1360 |
|---|
| Sequence: | MASDSPARSLDEIDLSALRDPAGIFELVELVGNGTYGQVYKGRHVKTGQLAAIKVMDVTG
DEEEEIKQEINMLKKYSHHRNIATYYGAFIKKNPPGMDDQLWLVMEFCGAGSVTDLIKNT
KGNTLKEEWIAYICREILRGLSHLHQHKVIHRDIKGQNVLLTENAEVKLVDFGVSAQLDR
TVGRRNTFIGTPYWMAPEVIACDENPDATYDFKSDLWSLGITAIEMAEGAPPLCDMHPMR
ALFLIPRNPAPRLKSKKWSKKFQSFIESCLVKNHSQRPATEQLMKHPFIRDQPNERQVRI
QLKDHIDRTKKKRGEKDETEYEYSGSEEEEEENDSGEPSSILNLPGESTLRRDFLRLQLA
NKERSEALRRQQLEQQQRENEEHKRQLLAERQKRIEEQKEQRRRLEEQQRREKELRKQQE
REQRRHYEEQMRREEERRRAEHEQEYIRRQLEEEQRQLEILQQQLLHEQALLLEYKRKQL
EEQRQAERLQRQLKQERDYLVSLQHQRQEQRPVEKKPLYHYKEGMSPSEKPAWAKEVEER
SRLNRQSSPAMPHKVANRISDPNLPPRSESFSISGVQPARTPPMLRPVDPQIPHLVAVKS
QGPALTASQSVHEQPTKGLSGFQEALNVTSHRVEMPRQNSDPTSENPPLPTRIEKFDRSS
WLRQEEDIPPKVPQRTTSISPALARKNSPGNGSALGPRLGSQPIRASNPDLRRTEPILES
PLQRTSSGSSSSSSTPSSQPSSQGGSQPGSQAGSSERTRVRANSKSEGSPVLPHEPAKVK
PEESRDITRPSRPASYKKAIDEDLTALAKELRELRIEETNRPMKKVTDYSSSSEESESSE
EEEEDGESETHDGTVAVSDIPRLIPTGAPGSNEQYNVGMVGTHGLETSHADSFSGSISRE
GTLMIRETSGEKKRSGHSDSNGFAGHINLPDLVQQSHSPAGTPTEGLGRVSTHSQEMDSG
TEYGMGSSTKASFTPFVDPRVYQTSPTDEDEEDEESSAAALFTSELLRQEQAKLNEARKI
SVVNVNPTNIRPHSDTPEIRKYKKRFNSEILCAALWGVNLLVGTENGLMLLDRSGQGKVY
NLINRRRFQQMDVLEGLNVLVTISGKKNKLRVYYLSWLRNRILHNDPEVEKKQGWITVGD
LEGCIHYKVVKYERIKFLVIALKNAVEIYAWAPKPYHKFMAFKSFADLQHKPLLVDLTVE
EGQRLKVIFGSHTGFHVIDVDSGNSYDIYIPSHIQGNITPHAIVILPKTDGMEMLVCYED
EGVYVNTYGRITKDVVLQWGEMPTSVAYIHSNQIMGWGEKAIEIRSVETGHLDGVFMHKR
AQRLKFLCERNDKVFFASVRSGGSSQVFFMTLNRNSMMNW
|
|
|
|---|
| BDBM579736 |
|---|
| n/a |
|---|
| Name | BDBM579736 |
|---|
| Synonyms: | 4-((5-(4-(1H-imidazol-1- yl)phenyl)-1H-pyrazol-3- yl)amino)-3-chlorophenol | US11485711, Compound 176 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H14ClN5O |
|---|
| Mol. Mass. | 351.79 |
|---|
| SMILES | Oc1ccc(Nc2cc([nH]n2)-c2ccc(cc2)-n2ccnc2)c(Cl)c1 |
|---|
| Structure | 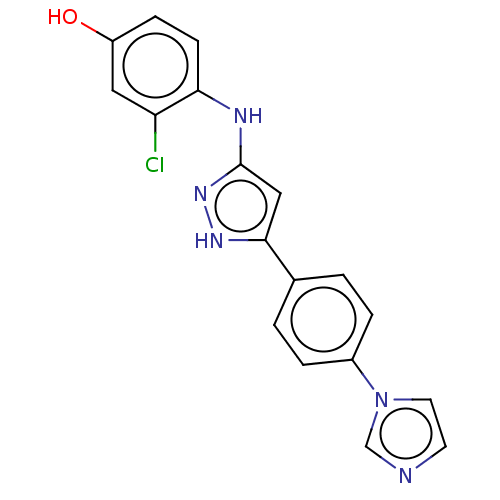
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Chang, SY; Lee, H; Kim, KY; Kim, BT; Kim, SS; Kim, SH; Lim, HJ; Heo, JN; Shin, SJ; Park, SY Compounds for inhibiting TNIK and medical uses thereof US Patent US11485711 Publication Date 11/1/2022
Chang, SY; Lee, H; Kim, KY; Kim, BT; Kim, SS; Kim, SH; Lim, HJ; Heo, JN; Shin, SJ; Park, SY Compounds for inhibiting TNIK and medical uses thereof US Patent US11485711 Publication Date 11/1/2022