

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50206821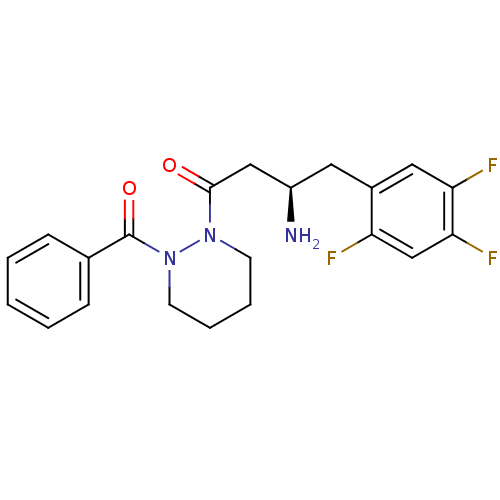 ((R)-3-amino-1-(2-benzoylpiperazin-1-yl)-4-(2,4,5-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of pig kidney DPP4 | Bioorg Med Chem Lett 17: 2622-8 (2007) Article DOI: 10.1016/j.bmcl.2007.01.111 BindingDB Entry DOI: 10.7270/Q21V5FS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50206820 ((R)-3-amino-1-(2-benzoyl-1,2-diazepan-1-yl)-4-(2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 56.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of pig kidney DPP4 | Bioorg Med Chem Lett 17: 2622-8 (2007) Article DOI: 10.1016/j.bmcl.2007.01.111 BindingDB Entry DOI: 10.7270/Q21V5FS4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579738 (N-(4-((5-(4-(1H-pyrazol-1- yl)phenyl)-1H-pyrazol-3...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM571620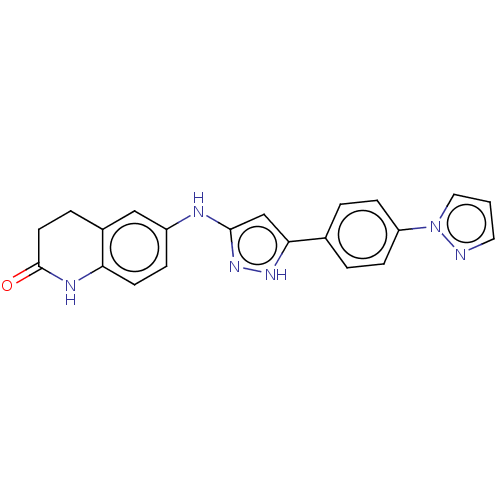 (6-((5-(4-(1H-pyrazol-1- yl)phenyl)-1H-pyrazol-3- y...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11447469-20220920-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24X5C2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM571666 (5-((5-(3-fluoro-4- hydroxyphenyl)-1H- pyrazol-3- y...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11447469-20220920-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24X5C2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM571674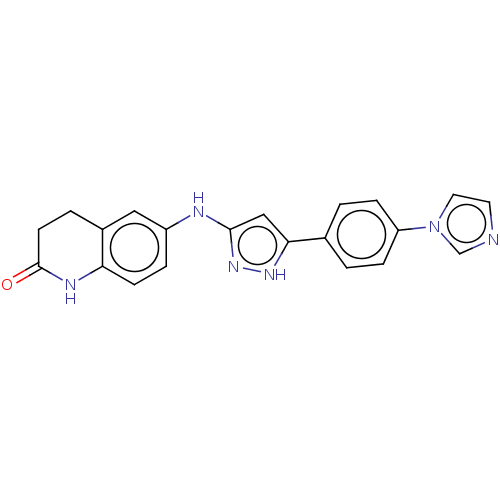 (6-((5-(4-(1H-imidazol-1- yl)phenyl)-1H-pyrazol-3- ...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11447469-20220920-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24X5C2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579704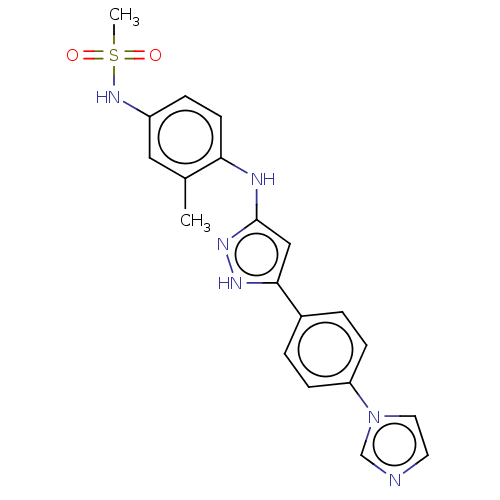 (N-(4-((5-(4-(1H-imidazol-1- yl)phenyl)-1H-pyrazol-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579709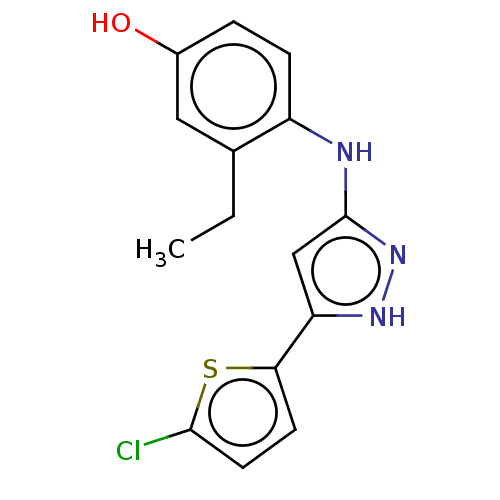 (4-((5-(5-chlorothiophen-2- yl)-1H-pyrazol-3-yl)ami...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579718 (3-ethyl-4-((5-(4-iodophenyl)- 1H-pyrazol-3-yl)amin...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337787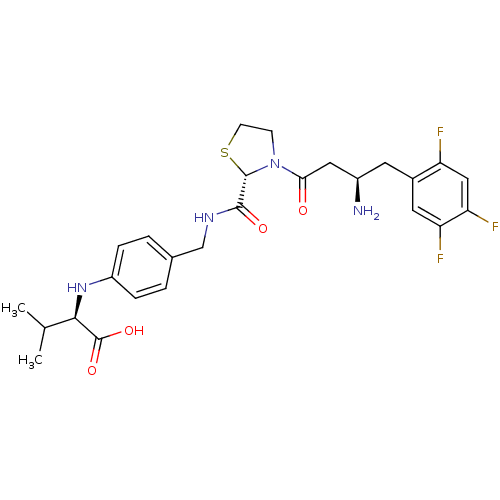 ((R)-2-(4-(((S)-3-((R)-3-amino-4-(2,4,5-trifluoroph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337789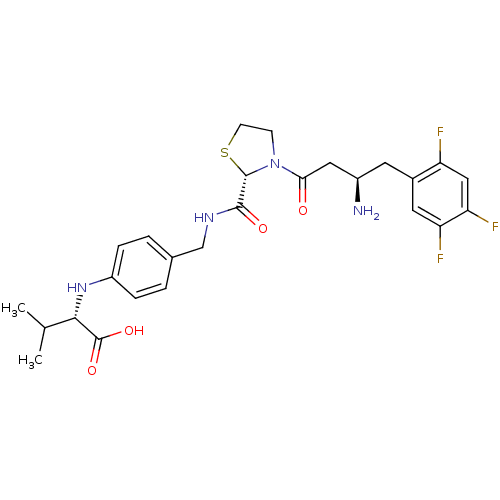 ((S)-2-(4-(((S)-3-((R)-3-amino-4-(2,4,5-trifluoroph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579739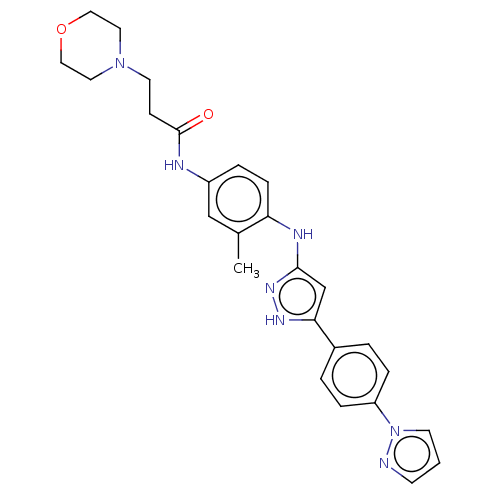 (N-(4-((5-(4-(1H-pyrazol-1- yl)phenyl)-1H-pyrazol-3...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579743 (N-(4-((5-(4-(1H-pyrazol-1- yl)phenyl)-1H-pyrazol-3...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579689 (N-(4-((5-(4-hydroxyphenyl)- 1H-pyrazol-3-yl)amino)...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337785 (2-(4-((3-((R)-3-amino-4-(2,4,5-trifluorophenyl)but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579794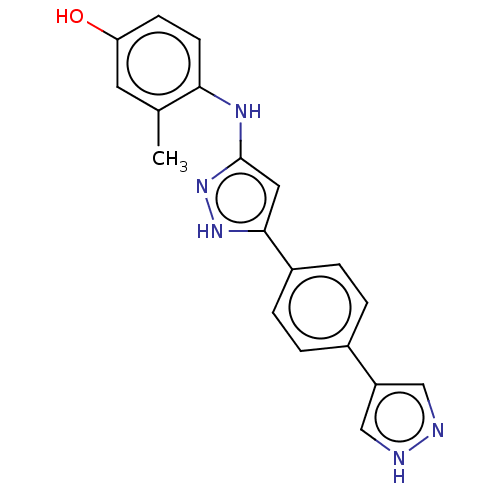 (4-((5-(4-(1H-pyrazol-4- yl)phenyl)-1H-pyrazol-3- y...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50500613 (CHEMBL3752642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University Curated by ChEMBL | Assay Description Inhibition of Cyclophilin A peptidyl-prolyl cis-trans isomerase activity (unknown origin) using Succ-Ala-Leu-Pro-Phe-p-nitroaniline as substrate by I... | J Med Chem 58: 9546-61 (2015) Article DOI: 10.1021/acs.jmedchem.5b01064 BindingDB Entry DOI: 10.7270/Q2RF5Z1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579761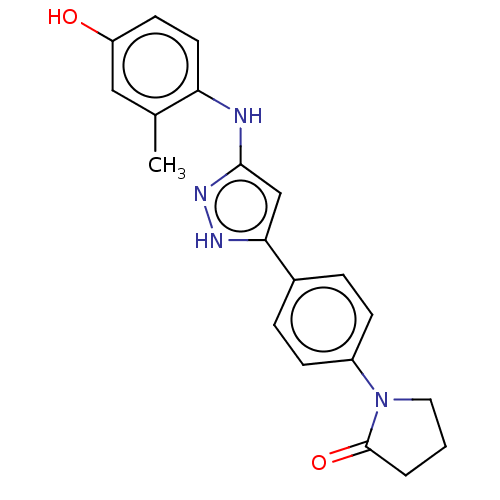 (1-(4-(3-((4-hydroxy-2- methylphenyl)amino)-1H- pyr...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579714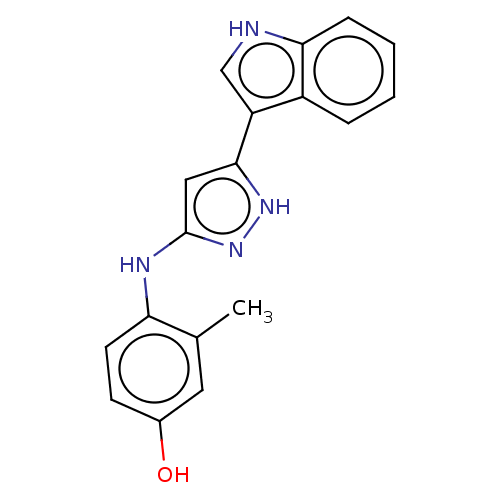 (4-((5-(1H-indol-3-yl)-1H- pyrazol-3-yl)amino)-3- m...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337779 (2-(4-((3-((R)-3-amino-4-(2,4,5-trifluorophenyl)but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579740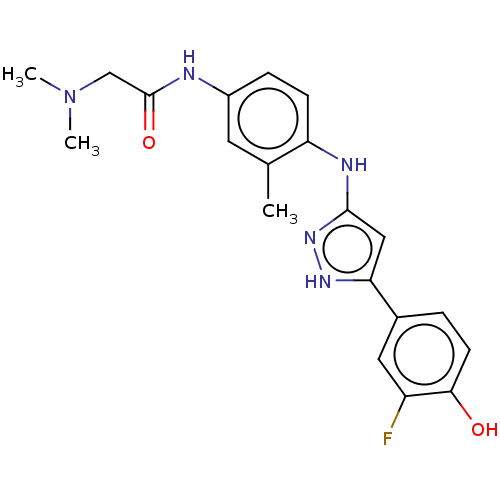 (2-(dimethylamino)-N-(4-((5- (3-fluoro-4-hydroxyphe...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579764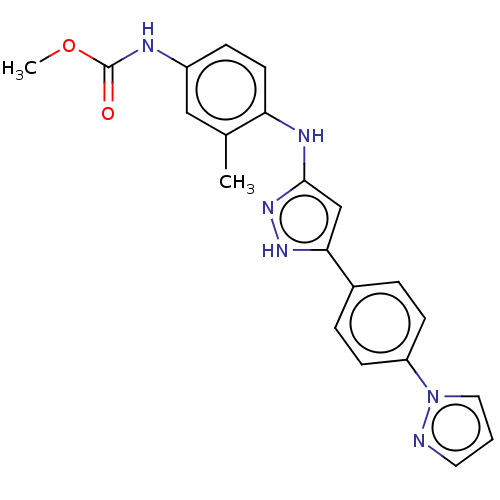 (US11485711, Compound 208 | methyl (4-((5-(4-(1H-py...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM571724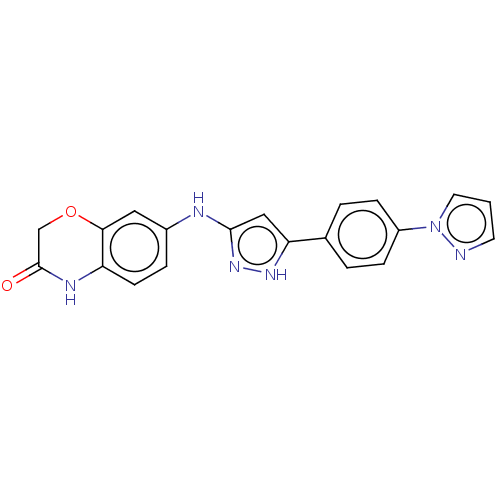 (7-((5-(4-(1H-pyrazol-1- yl)phenyl)-1H-pyrazol-3- y...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11447469-20220920-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24X5C2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University Curated by ChEMBL | Assay Description Inhibition of Cyclophilin A peptidyl-prolyl cis-trans isomerase activity (unknown origin) using Succ-Ala-Leu-Pro-Phe-p-nitroaniline as substrate by I... | J Med Chem 58: 9546-61 (2015) Article DOI: 10.1021/acs.jmedchem.5b01064 BindingDB Entry DOI: 10.7270/Q2RF5Z1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579799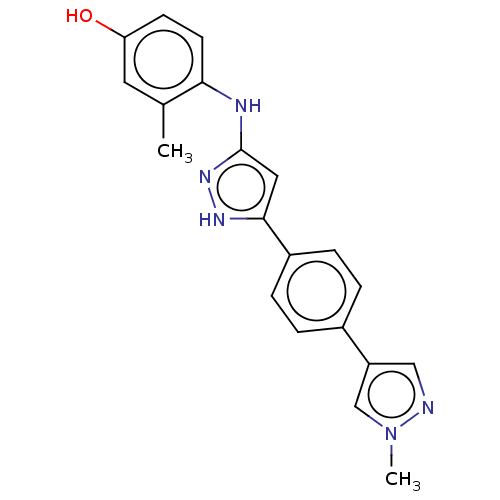 (3-methyl-4-((5-(4-(1-methyl-1H- pyrazol-4-yl)pheny...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579736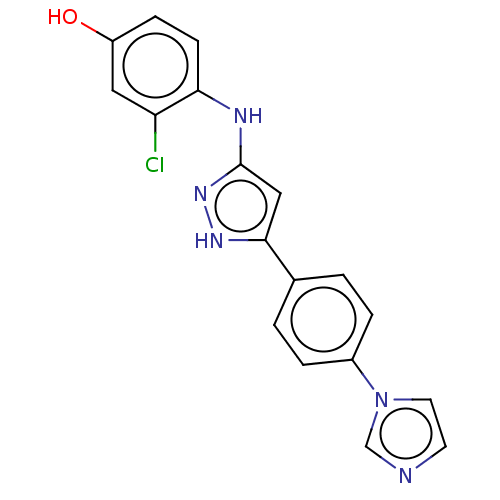 (4-((5-(4-(1H-imidazol-1- yl)phenyl)-1H-pyrazol-3- ...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579763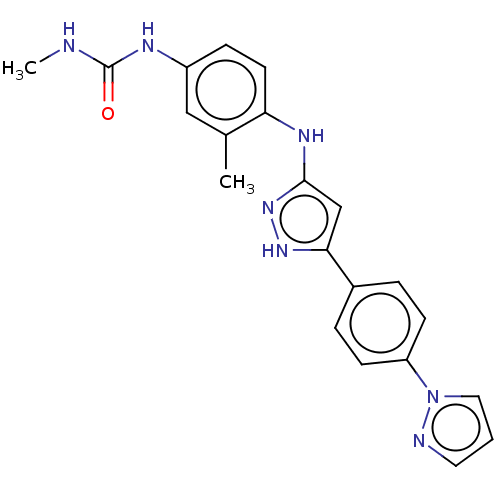 (1-(4-((5-(4-(1H-pyrazol-1- yl)phenyl)-1H-pyrazol-3...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579705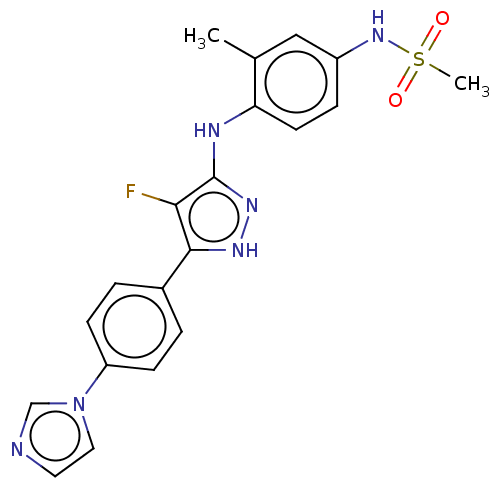 (N-(4-((5-(4-(1H-imidazol-1- yl)phenyl)-4-fluoro-1H...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50102161 (1-{2-[4-(3,4-Dichloro-phenyl)-piperazin-1-yl]-2-ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Laboratory of Lipid Metabolism and Atherosclerosis Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Lp-PLA2 | Bioorg Med Chem Lett 15: 1525-7 (2005) Article DOI: 10.1016/j.bmcl.2004.11.063 BindingDB Entry DOI: 10.7270/Q28915CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50102161 (1-{2-[4-(3,4-Dichloro-phenyl)-piperazin-1-yl]-2-ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Laboratory of Lipid Metabolism and Atherosclerosis Curated by ChEMBL | Assay Description In vitro inhibitory activity against human lipoprotein-associated phospholiphase A2 | Bioorg Med Chem Lett 15: 1525-7 (2005) Article DOI: 10.1016/j.bmcl.2004.11.063 BindingDB Entry DOI: 10.7270/Q28915CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579801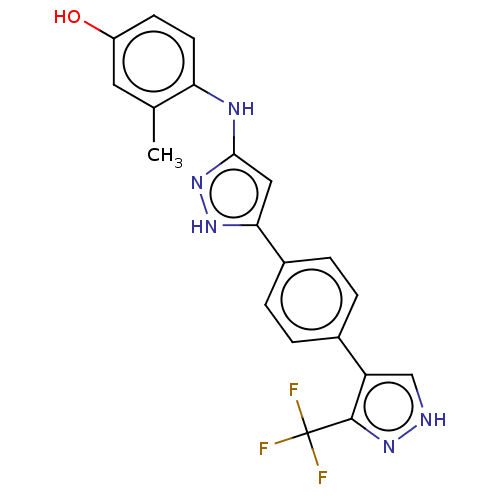 (3-methyl-4-((5-(4-(3- (trifluoromethyl)-1H-pyrazol...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579765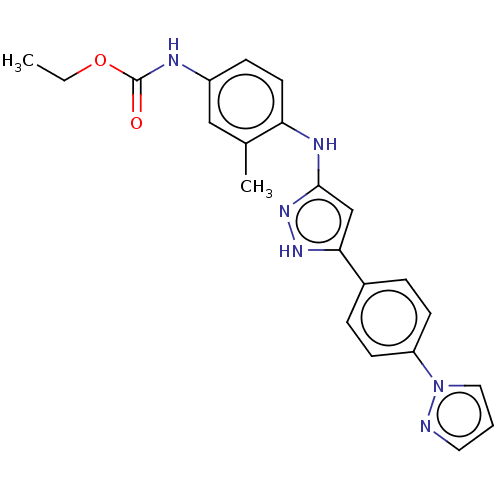 (US11485711, Compound 209 | ethyl (4-((5-(4-(1H-pyr...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579741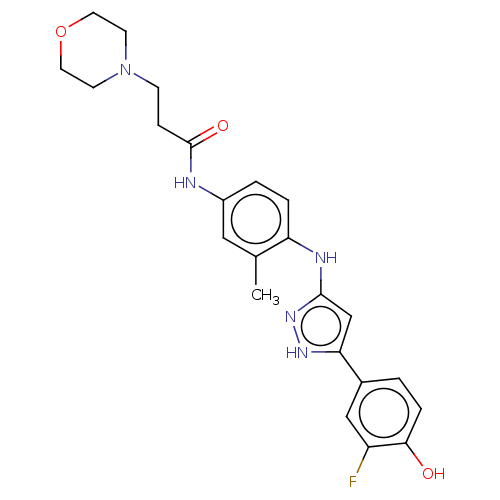 (N-(4-((5-(3-fluoro-4- hydroxyphenyl-1H-pyrazol-3- ...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579708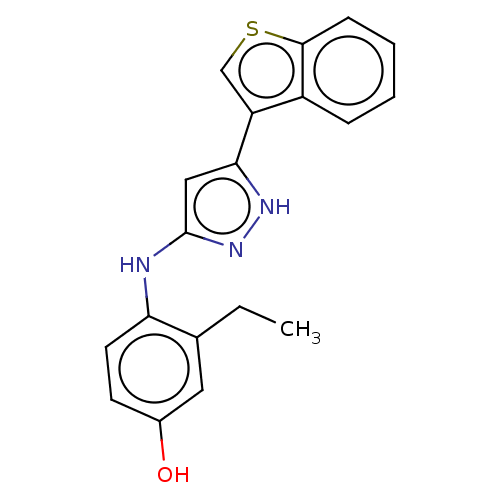 (4-((5-(benzo[b]thiophen-3- yl)-1H-pyrazol-3-yl)ami...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337791 ((R)-2-(4-(((R)-3-((R)-3-amino-4-(2,4,5-trifluoroph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579800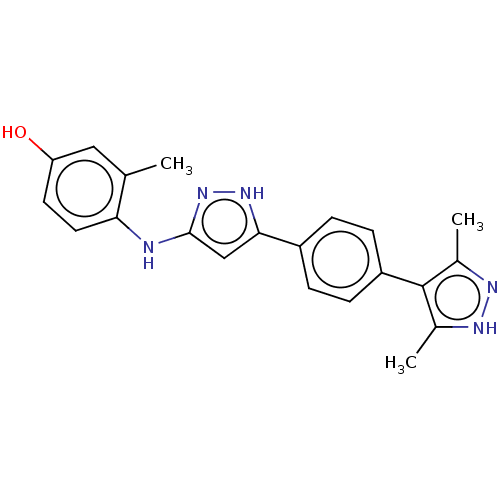 (4-((5-(4-(3,5-dimethyl-1H-pyrazol- 4-yl)phenyl)-1H...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50500614 (CHEMBL3754544) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University Curated by ChEMBL | Assay Description Inhibition of Cyclophilin A peptidyl-prolyl cis-trans isomerase activity (unknown origin) using Succ-Ala-Leu-Pro-Phe-p-nitroaniline as substrate by I... | J Med Chem 58: 9546-61 (2015) Article DOI: 10.1021/acs.jmedchem.5b01064 BindingDB Entry DOI: 10.7270/Q2RF5Z1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579693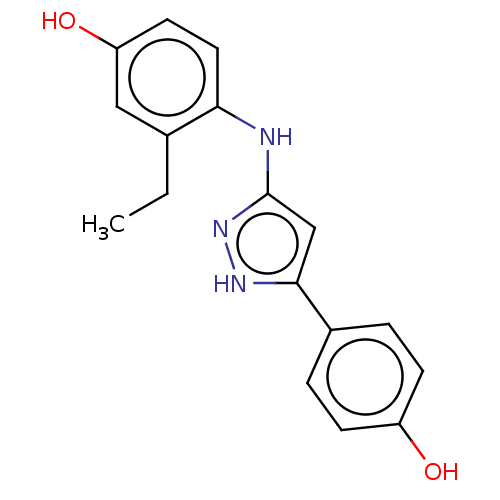 (3-ethyl-4-((5-(4- hydroxyphenyl)-1H-pyrazol-3- yl)...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579703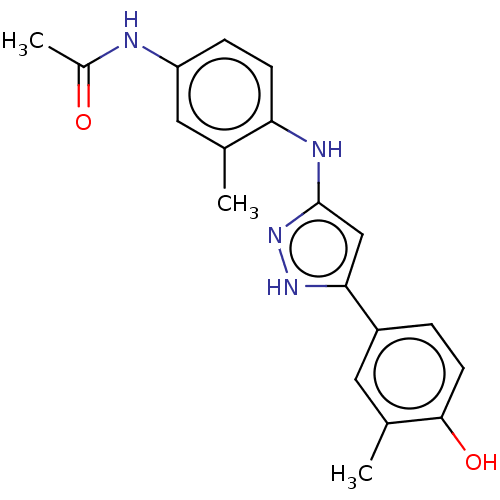 (N-(4-((5-(4-hydroxy-3- methylphenyl)-1H-pyrazol-3-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337783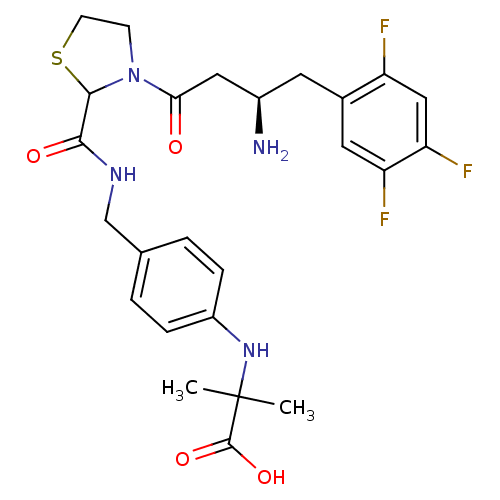 (2-(4-((3-((R)-3-amino-4-(2,4,5-trifluorophenyl)but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337793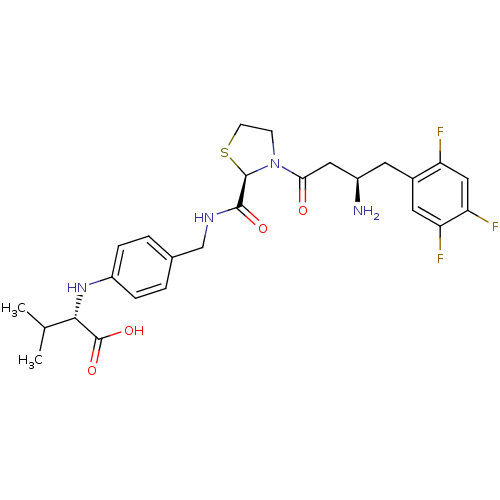 ((S)-2-(4-(((R)-3-((R)-3-amino-4-(2,4,5-trifluoroph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579735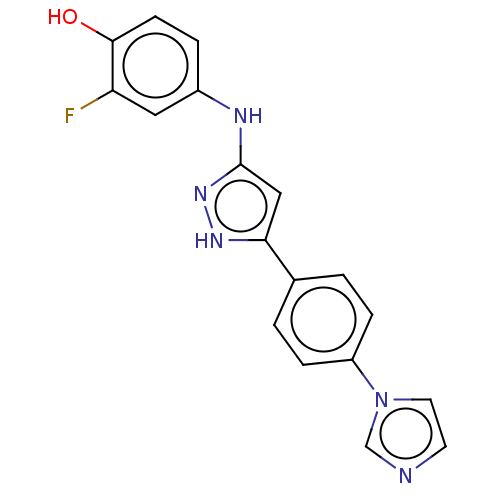 (4-((5-(4-(1H-inidazol-1- yl)phenyl)-1H-pyrazol-3- ...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579730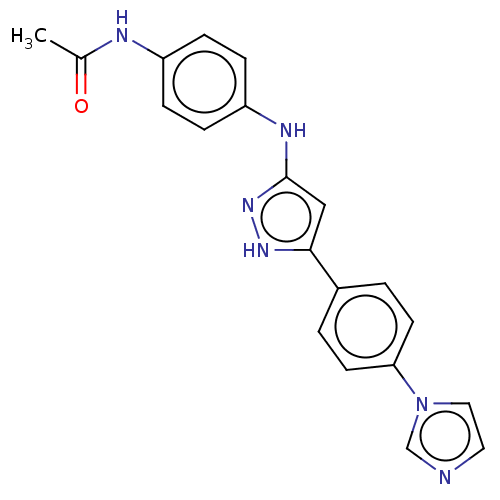 (N-(4-((5-(4-(1H-imidazol-1- yl)phenyl)-1H-pyrazol-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579731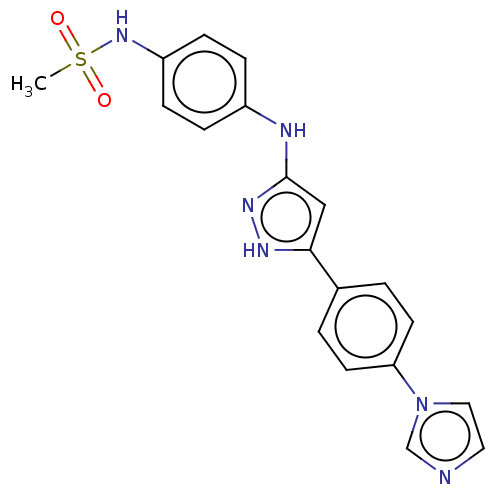 (N-(4-((5-(4-(1H-imidazol-1- yl)phenyl)-1H-pyrazol-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579710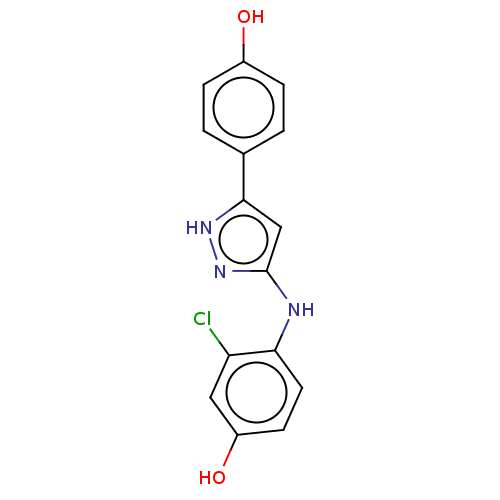 (3-chloro-4-((5-(4- hydroxyphenyl)-1H-pyrazol-3- yl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50337788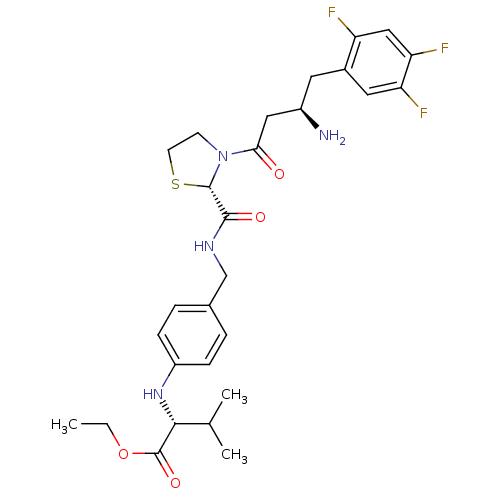 ((R)-ethyl 2-(4-(((S)-3-((R)-3-amino-4-(2,4,5-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579798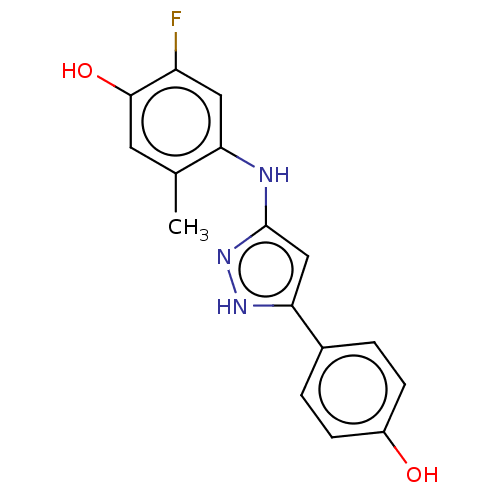 (2-fluoro-4-((5-(4-hydroxyphenyl)- 1H-pyrazol-3-yl)...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579797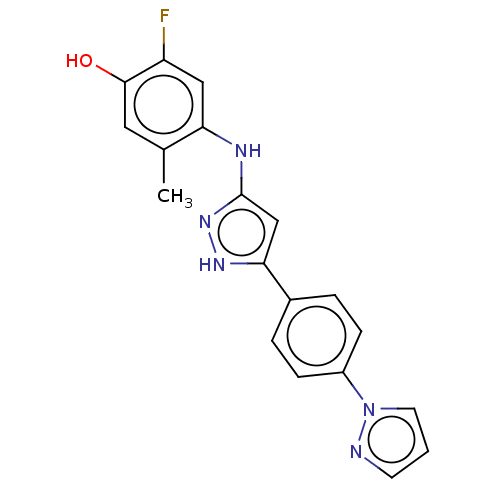 (4-((5-(4-(1H-pyrazol-1-yl)phenyl)- 1H-pyrazol-3-yl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP4 after 60 mins by fluorescence plate reader | Bioorg Med Chem Lett 21: 1366-70 (2011) Article DOI: 10.1016/j.bmcl.2011.01.041 BindingDB Entry DOI: 10.7270/Q25B02R9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Anoctamin-1 (Homo sapiens (Human)) | BDBM50503445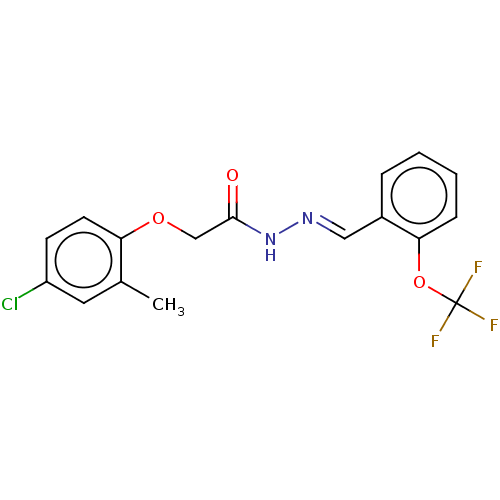 (CHEMBL4445544 | US11274074, Example 1 [Chemical Fo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Inhibition of YFP-fused ANO1 (unknown origin) expressed in FRT cells after 20 mins by fluorescence quenching method | Eur J Med Chem 160: 245-255 (2018) Article DOI: 10.1016/j.ejmech.2018.10.002 BindingDB Entry DOI: 10.7270/Q24T6NMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 571 total ) | Next | Last >> |