

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50111110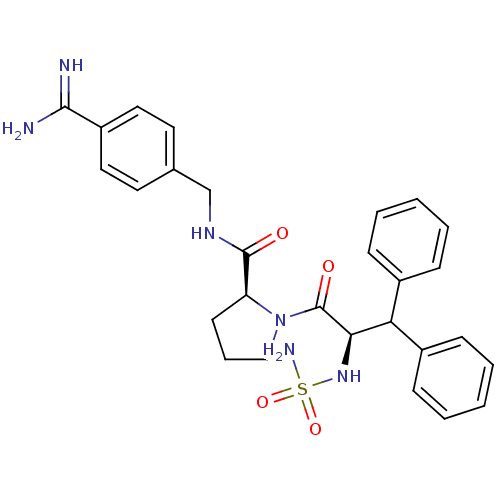 (2N-(4-Benzamidinemethyl)-1-[2-aminosulfonamido-3,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111110 (2N-(4-Benzamidinemethyl)-1-[2-aminosulfonamido-3,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111101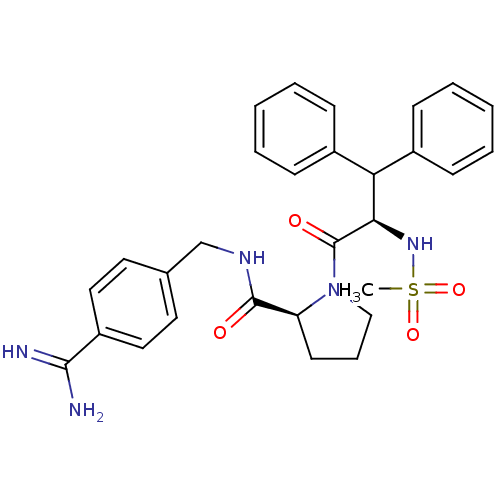 ((S)-1-((R)-2-Methanesulfonylamino-3,3-diphenyl-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111121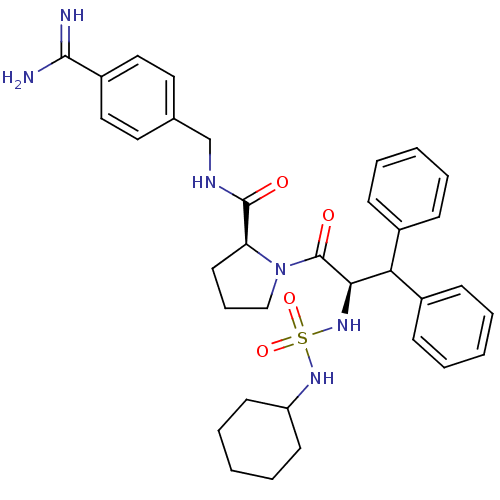 (2N-(4-Benzamidinemethyl)-1-[2-Cyclohexylaminosulfo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111101 ((S)-1-((R)-2-Methanesulfonylamino-3,3-diphenyl-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131789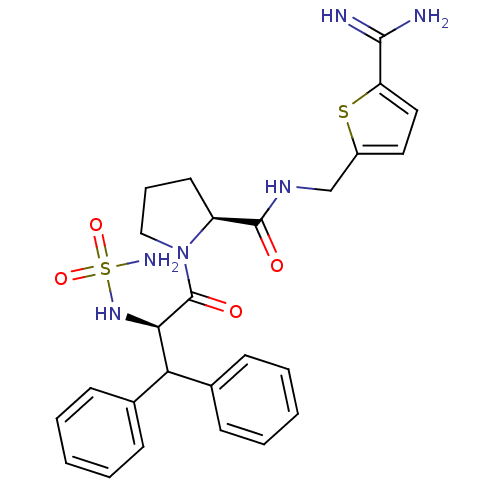 (1-(2-sulfonamide-amino-3,3-diphenyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111108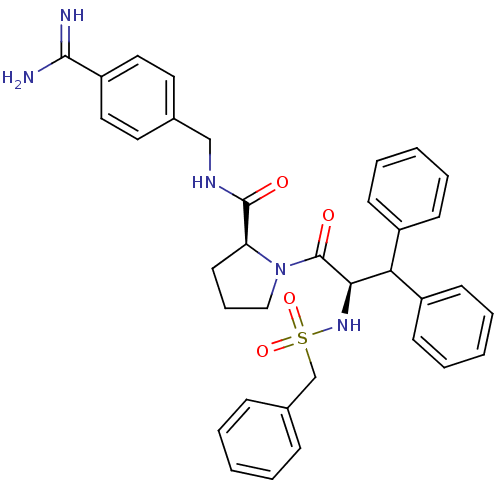 (2N-(4-Benzamidinemethyl)-1-[2-Benzylsulfonamido-3,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111105 ((S)-1-((R)-2-Methanesulfonylamino-3,3-diphenyl-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111105 ((S)-1-((R)-2-Methanesulfonylamino-3,3-diphenyl-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131790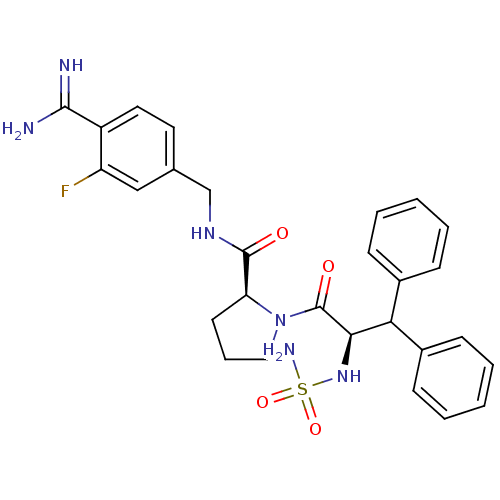 (1-(2-sulfonamideamino-3,3-diphenyl-propionyl)-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131778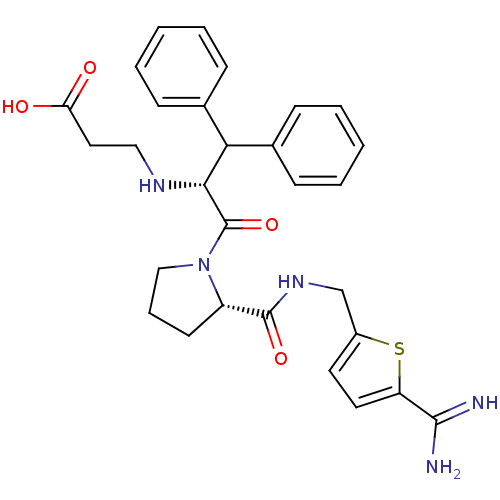 (3-(1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131795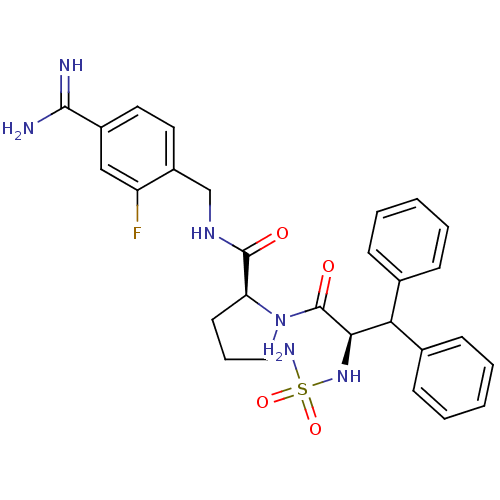 (1-(2-sulfonamideamino-3,3-diphenyl-propionyl)-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246899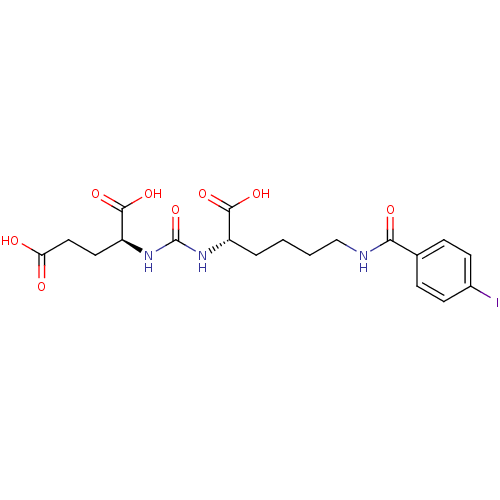 ((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University College of Medicine Curated by ChEMBL | Assay Description Inhibition of PSMA (unknown origin) | Bioorg Med Chem Lett 28: 572-576 (2018) Article DOI: 10.1016/j.bmcl.2018.01.047 BindingDB Entry DOI: 10.7270/Q2RN3BGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111120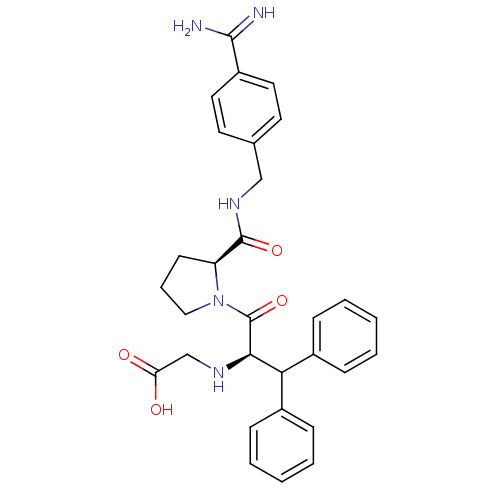 (2N-(4-Benzamidinemethyl)-1-[2-Aminoaceticacid-3,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131791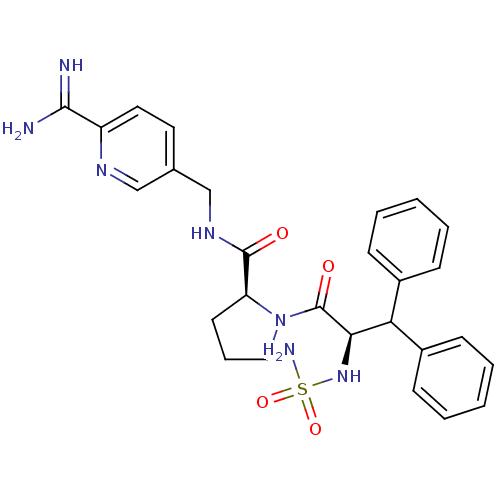 (1-(2-sulfonamideamino-3,3-diphenyl-propionyl)-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131780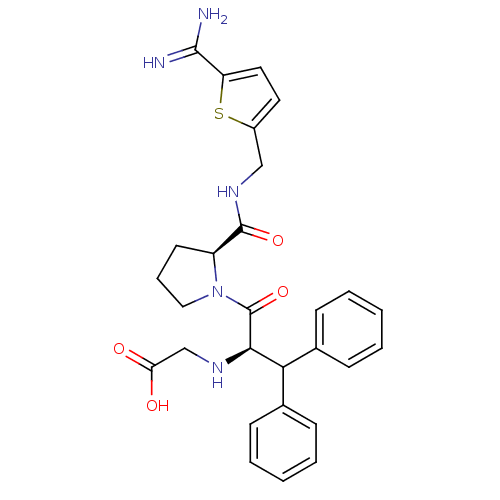 ((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131796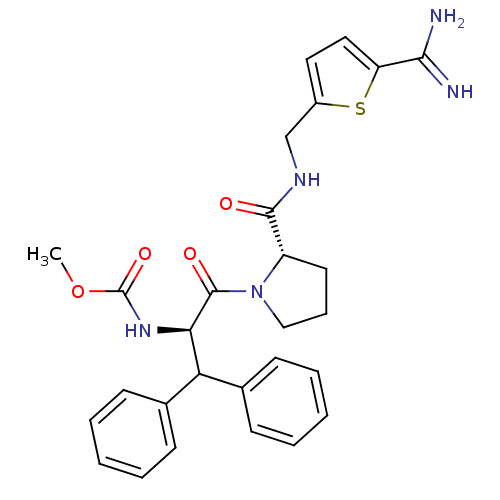 ((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111120 (2N-(4-Benzamidinemethyl)-1-[2-Aminoaceticacid-3,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131792 ((1-Benzhydryl-2-{2-[(6-carbamimidoyl-pyridin-3-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50061298 (CHEMBL3393837) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 assessed as inhibition of NADA-induced effect at 1 uM by FLIPR assay | Bioorg Med Chem Lett 25: 803-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.086 BindingDB Entry DOI: 10.7270/Q2JD4ZG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131782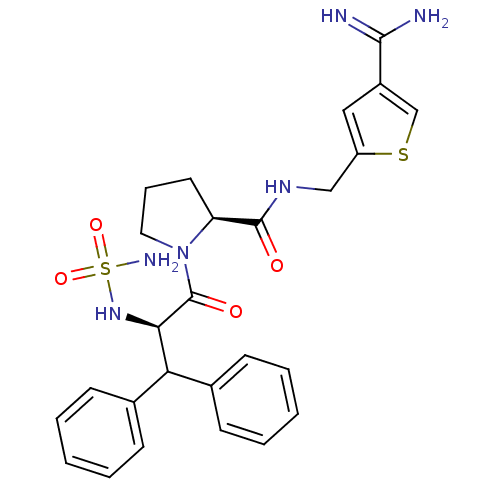 (1-(2-sulfonamide-amino-3,3-diphenyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131798 (1-(2-Methylamino-3,3-diphenyl-propionyl)-pyrrolidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111102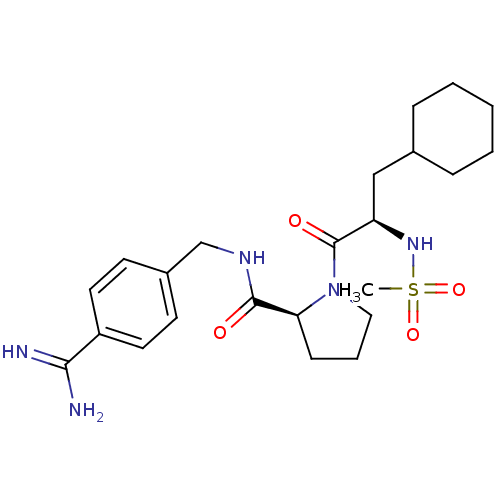 ((S)-1-((R)-3-Cyclohexyl-2-methanesulfonylamino-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50484591 (CHEMBL1939308) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from nAChR alpha4beta2 in rat brain membrane | J Med Chem 54: 7678-92 (2011) Article DOI: 10.1021/jm201045m BindingDB Entry DOI: 10.7270/Q20Z7646 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50484591 (CHEMBL1939308) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from nAChR alpha4beta2 in rat brain membrane | J Med Chem 54: 7678-92 (2011) Article DOI: 10.1021/jm201045m BindingDB Entry DOI: 10.7270/Q20Z7646 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111122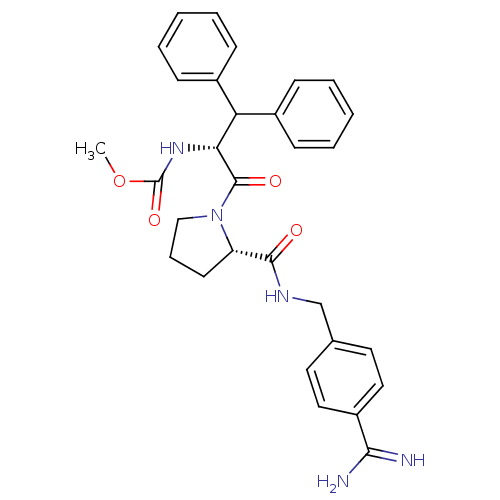 (2N-(4-Benzamidinemethyl)-1-[2-Carbamicacidmethyles...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111104 (2N-(4-Benzamidinemethyl)-1-[2-Amino-3,3-diphenyl-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111122 (2N-(4-Benzamidinemethyl)-1-[2-Carbamicacidmethyles...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50484594 (CHEMBL1939314) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0389 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from nAChR alpha4beta2 in rat brain membrane | J Med Chem 54: 7678-92 (2011) Article DOI: 10.1021/jm201045m BindingDB Entry DOI: 10.7270/Q20Z7646 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50417287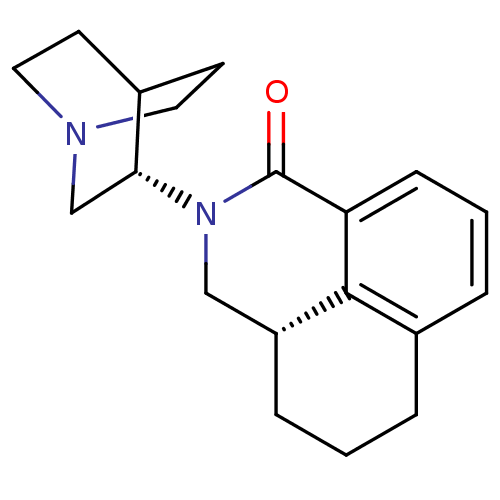 (Aloxi | Aurothioglucose | PALONOSETRON | PALONOSET...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50484594 (CHEMBL1939314) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from nAChR alpha4beta2 in rat brain membrane | J Med Chem 54: 7678-92 (2011) Article DOI: 10.1021/jm201045m BindingDB Entry DOI: 10.7270/Q20Z7646 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111109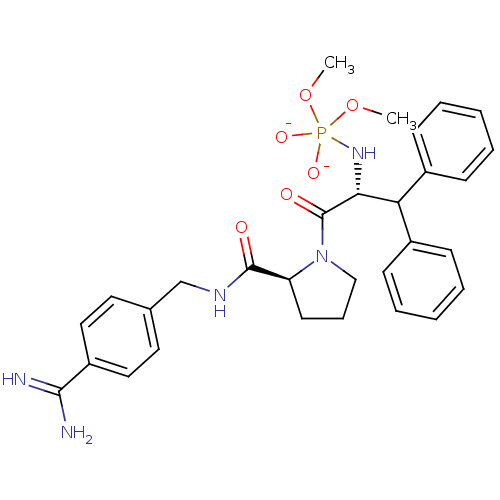 ({(R)-1-Benzhydryl-2-[(S)-2-(4-carbamimidoyl-benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111098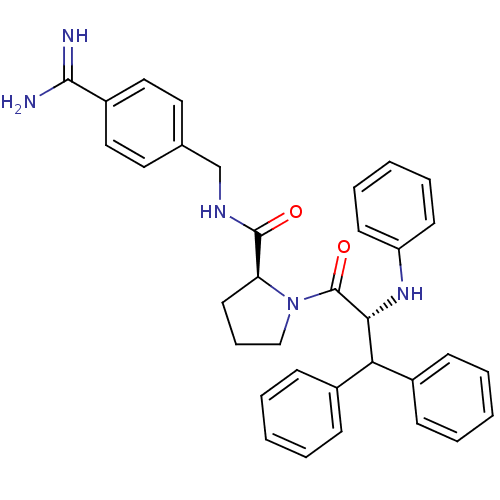 (2N-(4-Benzamidinemethyl)-1-[2-Phenylamino-3,3-diph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111103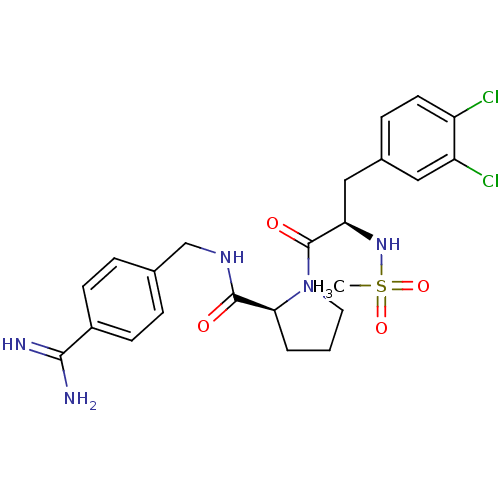 ((S)-1-[(R)-3-(3,4-Dichloro-phenyl)-2-methanesulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50071654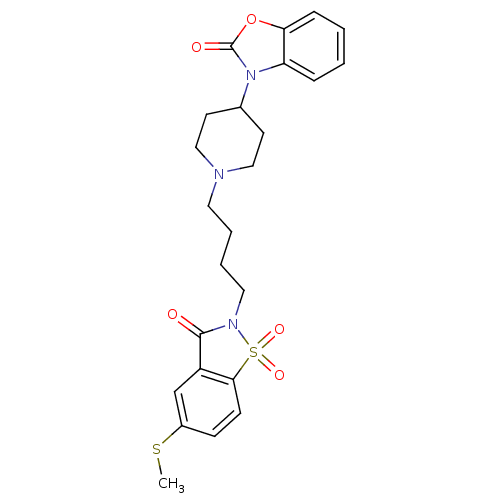 (3-{1-[4-(5-Methylsulfanyl-1,1,3-trioxo-1,3-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Ability to displace beta ([125I]-iodo-4-hydroxyphenyl)-ethylaminomethyl tetralone from human cloned Alpha-1a adrenergic receptor stably expressed in ... | Bioorg Med Chem Lett 8: 2467-72 (1999) BindingDB Entry DOI: 10.7270/Q2NP23K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080882 (Benzamidrazone analogue | CHEMBL312244) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human thrombin was determined in vitro. | Bioorg Med Chem Lett 9: 2483-6 (1999) BindingDB Entry DOI: 10.7270/Q2CC0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50071655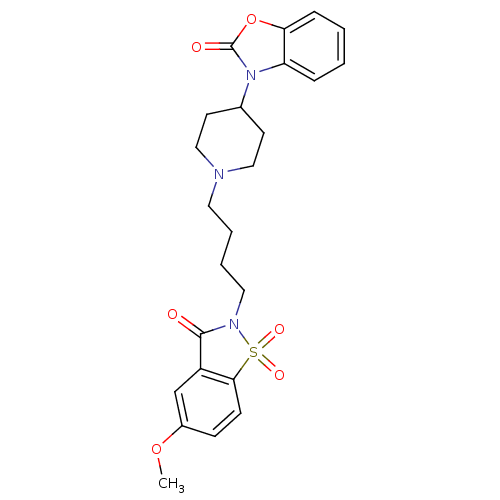 (3-{1-[4-(5-Methoxy-1,1,3-trioxo-1,3-dihydro-1lambd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Ability to displace beta ([125I]-iodo-4-hydroxyphenyl)-ethylaminomethyl tetralone from human cloned Alpha-1a adrenergic receptor stably expressed in ... | Bioorg Med Chem Lett 8: 2467-72 (1999) BindingDB Entry DOI: 10.7270/Q2NP23K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50195684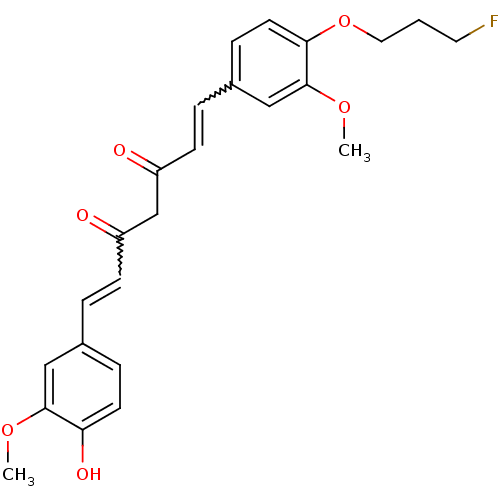 (1-[4-(3-fluoropropoxy)-3-methoxyphenyl]-5-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University School of Medicine Curated by ChEMBL | Assay Description Displacement of [125I]IMSB from beta amyloid protein 40 | J Med Chem 49: 6111-9 (2006) Article DOI: 10.1021/jm0607193 BindingDB Entry DOI: 10.7270/Q2J38TB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50071647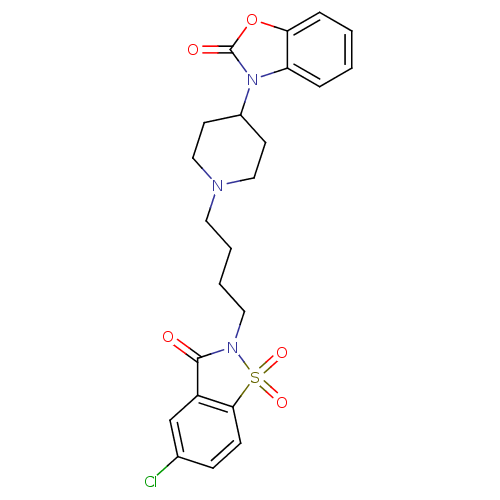 (3-{1-[4-(5-Chloro-1,1,3-trioxo-1,3-dihydro-1lambda...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Compound was evaluated for its affinity for Alpha-1a adrenergic receptor in human aorta preparations | Bioorg Med Chem Lett 8: 2467-72 (1999) BindingDB Entry DOI: 10.7270/Q2NP23K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50071652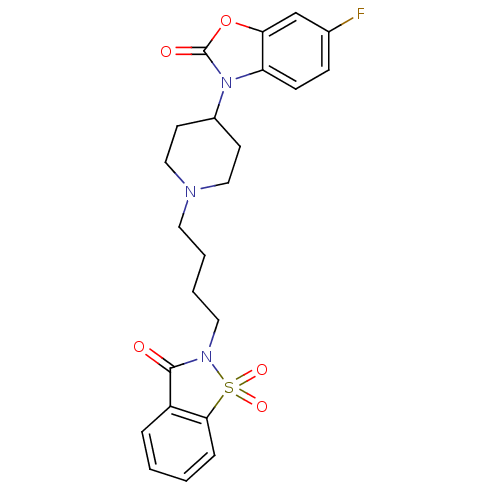 (6-Fluoro-3-{1-[4-(1,1,3-trioxo-1,3-dihydro-1lambda...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Ability to displace beta ([125I]-iodo-4-hydroxyphenyl)-ethylamino methyl tetralone from human cloned Alpha-1a adrenergic receptor stably expressed in... | Bioorg Med Chem Lett 8: 2467-72 (1999) BindingDB Entry DOI: 10.7270/Q2NP23K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50484593 (CHEMBL1939311) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0955 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from nAChR alpha4beta2 in rat brain membrane | J Med Chem 54: 7678-92 (2011) Article DOI: 10.1021/jm201045m BindingDB Entry DOI: 10.7270/Q20Z7646 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50071651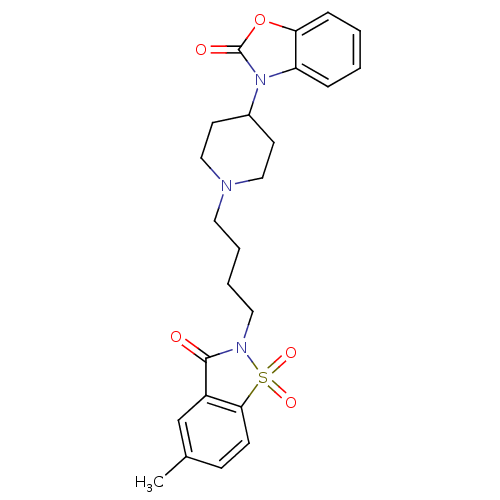 (3-{1-[4-(5-Methyl-1,1,3-trioxo-1,3-dihydro-1lambda...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Ability to displace beta ([125I]-iodo-4-hydroxyphenyl)-ethylaminomethyl tetralone from human cloned Alpha-1a adrenergic receptor stably expressed in ... | Bioorg Med Chem Lett 8: 2467-72 (1999) BindingDB Entry DOI: 10.7270/Q2NP23K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50080879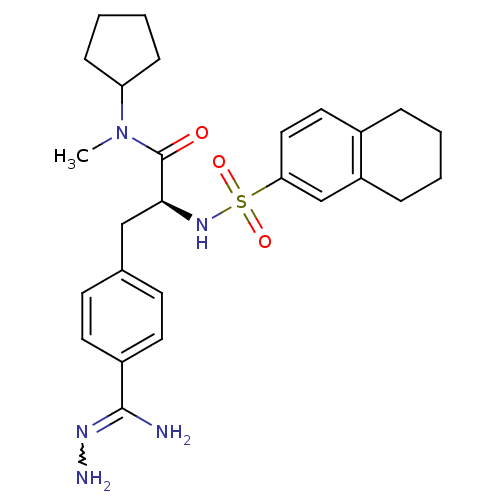 (Benzamidrazone analogue | CHEMBL82057) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human thrombin was determined in vitro. | Bioorg Med Chem Lett 9: 2483-6 (1999) BindingDB Entry DOI: 10.7270/Q2CC0ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131787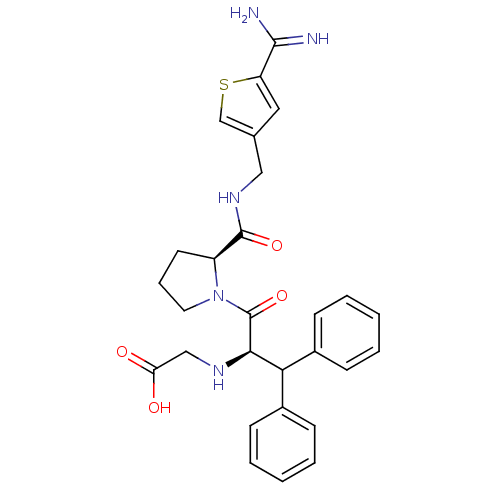 ((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50256547 (CHEMBL4104073) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Research Institute of Pharmaceutical Sciences, College of Pharmacy, Seoul National University, Seoul 08826, Republic of Korea. Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin induced calcium influx pretreated for 6 mins followed... | Bioorg Med Chem 25: 2451-2462 (2017) Article DOI: 10.1016/j.bmc.2017.03.004 BindingDB Entry DOI: 10.7270/Q2BZ68HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111118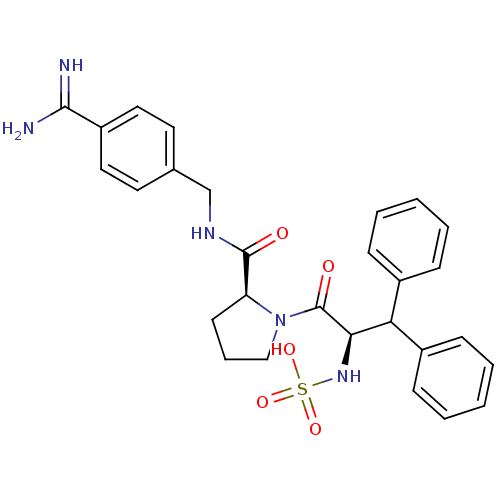 (2N-(4-Benzamidinemethyl)-1-[2-Hydroxysulfonamido-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Life Science R&D, LGCI Curated by ChEMBL | Assay Description Binding affinity against human thrombin | Bioorg Med Chem Lett 12: 1017-22 (2002) BindingDB Entry DOI: 10.7270/Q2CV4J9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50484593 (CHEMBL1939311) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from nAChR alpha4beta2 in rat brain membrane | J Med Chem 54: 7678-92 (2011) Article DOI: 10.1021/jm201045m BindingDB Entry DOI: 10.7270/Q20Z7646 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50007518 ((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation cou... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01353 BindingDB Entry DOI: 10.7270/Q2BV7MF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50007518 ((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]N-methylspiperone from human D2L receptor expressed in HEK293 cell membranes measured after 60 mins by MicroBeta scintillation co... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01353 BindingDB Entry DOI: 10.7270/Q2BV7MF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50071646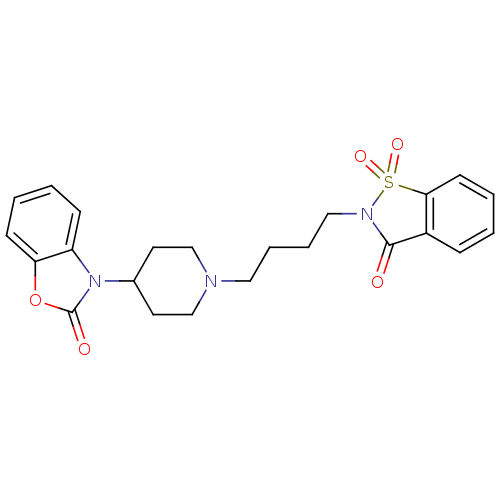 (3-{1-[4-(1,1,3-Trioxo-1,3-dihydro-1lambda*6*-benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Ability to displace beta ([125I]-iodo-4-hydroxyphenyl)-ethylamino methyl tetralone from human cloned Alpha-1a adrenergic receptor stably expressed in... | Bioorg Med Chem Lett 8: 2467-72 (1999) BindingDB Entry DOI: 10.7270/Q2NP23K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 31161 total ) | Next | Last >> |