

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11542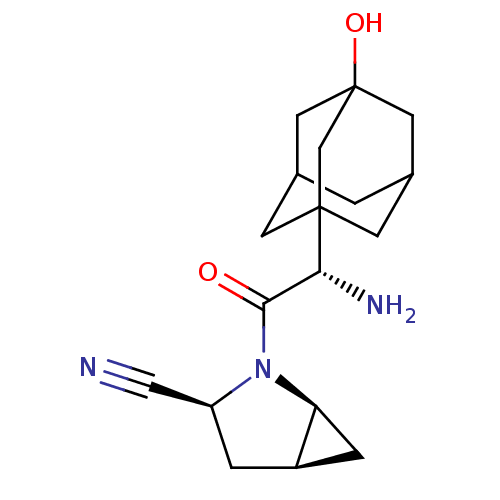 ((1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxyadamantan-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.600 | -52.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11541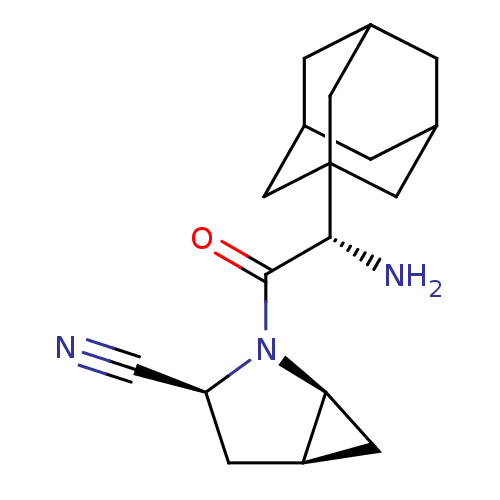 ((1S,3S,5S)-2-[(2S)-2-(adamantan-1-yl)-2-aminoacety...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.900 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11530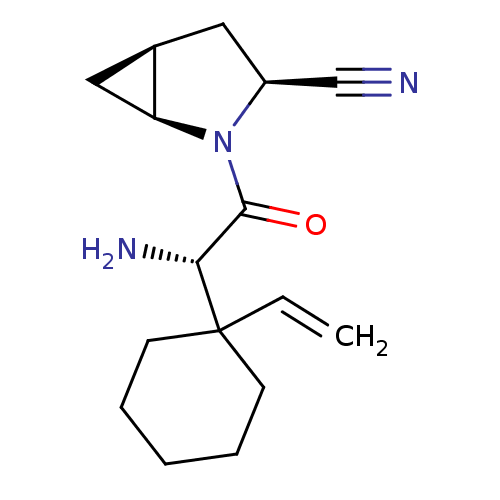 ((1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcyclohexyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | -50.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11544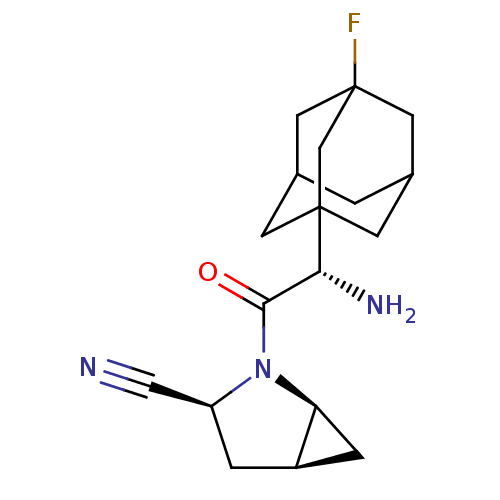 ((1S,3S,5S)-2-[(2S)-2-amino-2-(3-fluoroadamantan-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11543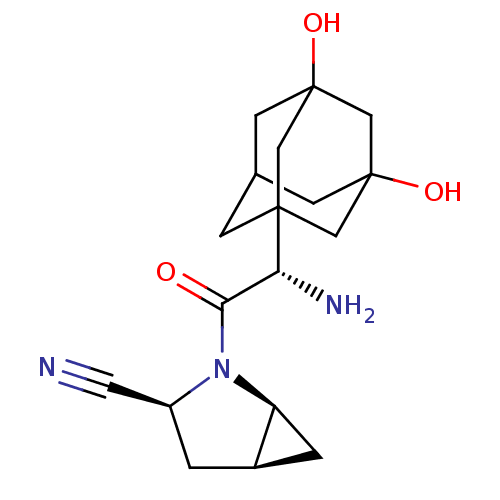 ((1S,3S,5S)-2-[(2S)-2-amino-2-(3,5-dihydroxyadamant...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 2.10 | -49.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11529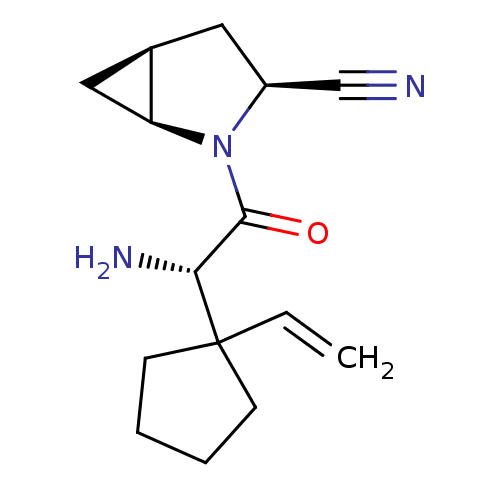 ((1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcyclopentyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.90 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11535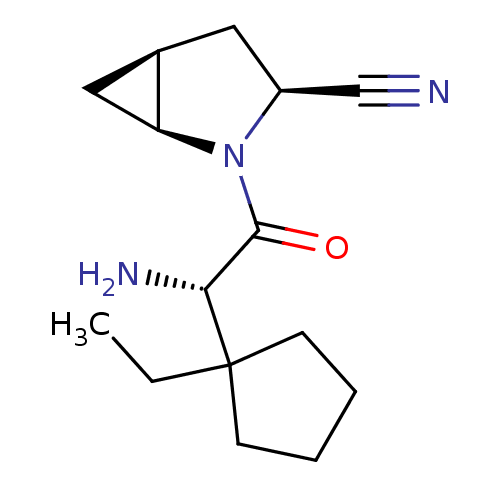 ((1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethylcyclopentyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.5 | -46.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11533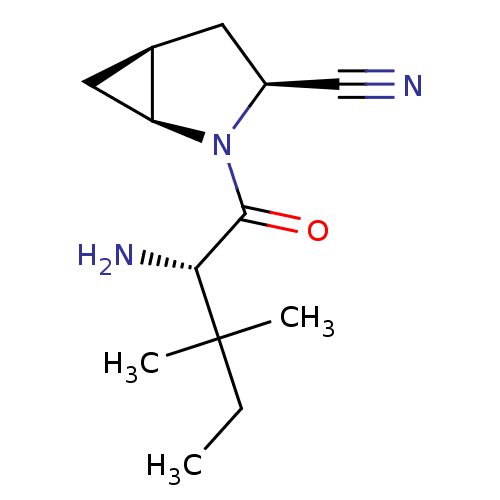 ((1S,3S,5S)-2-[(2S)-2-amino-3,3-dimethylpentanoyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.10 | -46.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11538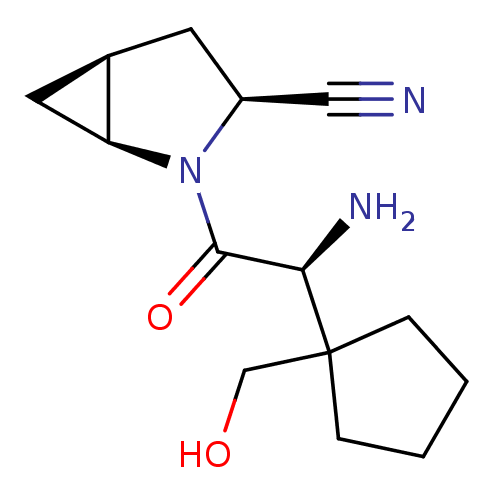 ((1S,3S,5S)-2-[(2S)-2-amino-2-[1-(hydroxymethyl)cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.40 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11539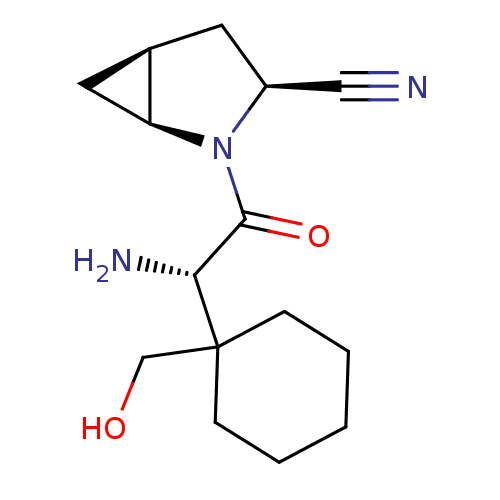 ((1S,3S,5S)-2-[(2S)-2-amino-2-[1-(hydroxymethyl)cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11531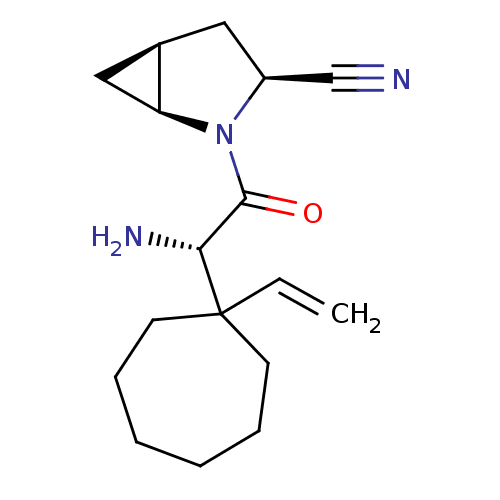 ((1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcycloheptyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11532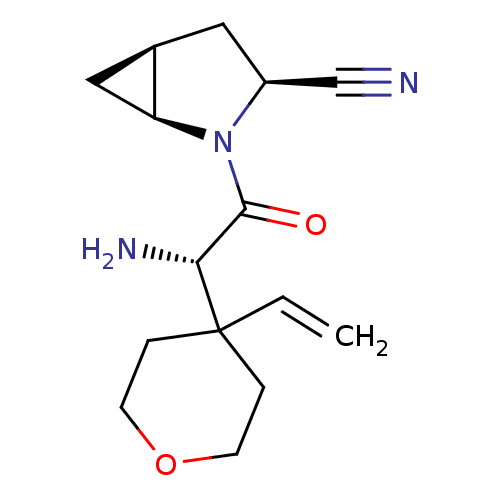 ((1S,3S,5S)-2-[(2S)-2-amino-2-(4-ethenyloxan-4-yl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11528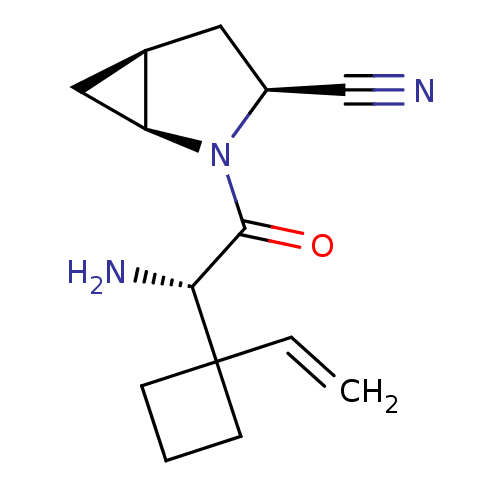 ((1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcyclobutyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | -44.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11536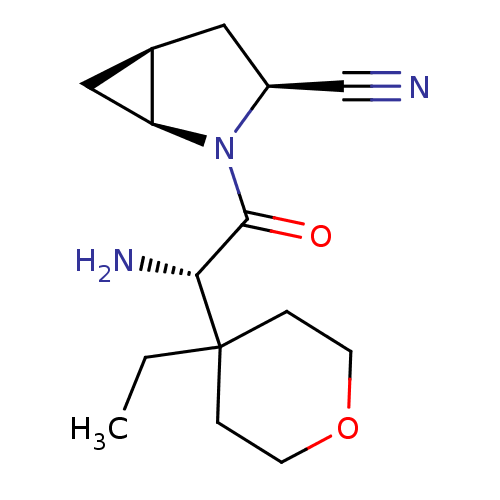 ((1S,3S,5S)-2-[(2S)-2-amino-2-(4-ethyloxan-4-yl)ace...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | -43.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11527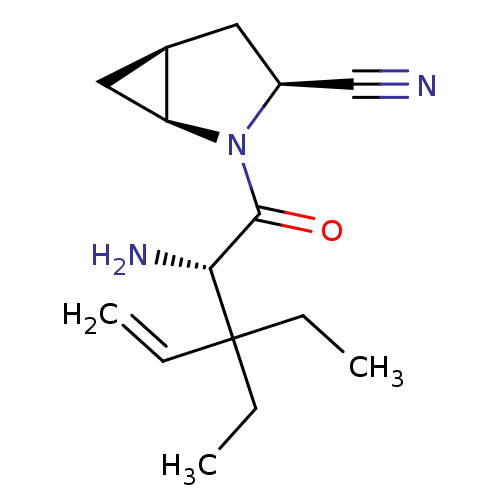 ((1S,3S,5S)-2-[(2S)-2-amino-3,3-diethylpent-4-enoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | -43.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11534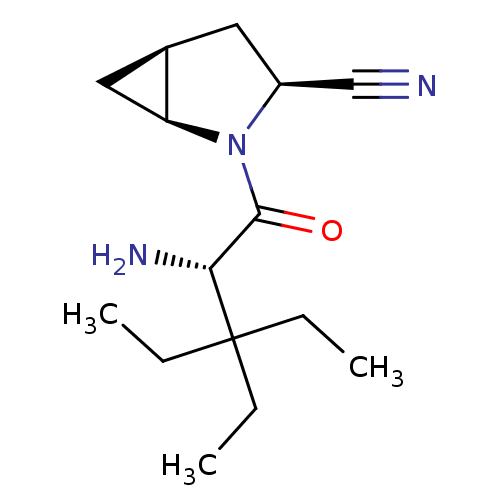 ((1S,3S,5S)-2-[(2S)-2-amino-3,3-diethylpentanoyl]-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 31 | -42.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11537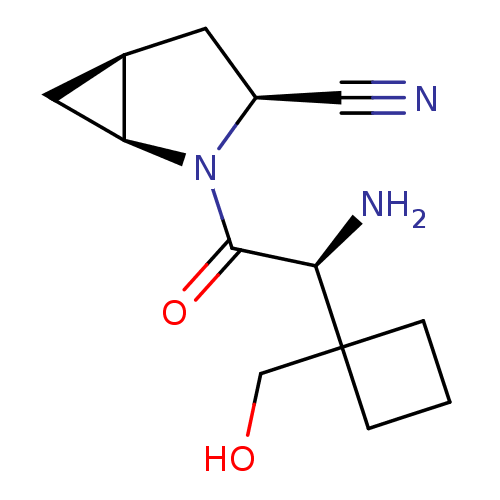 ((1S,3S,5S)-2-[(2S)-2-amino-2-[1-(hydroxymethyl)cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 42 | -41.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11525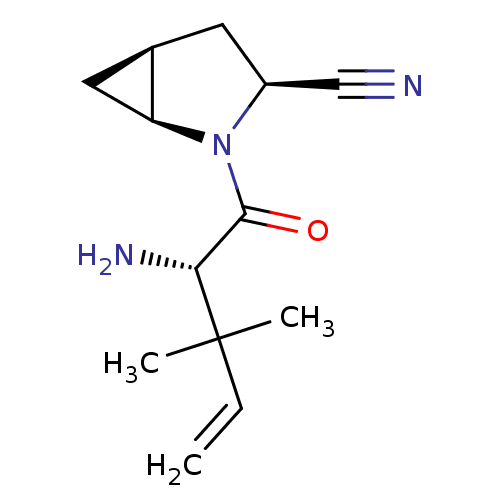 ((1S,3S,5S)-2-[(2S)-2-amino-3,3-dimethylpent-4-enoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 57 | -40.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50438750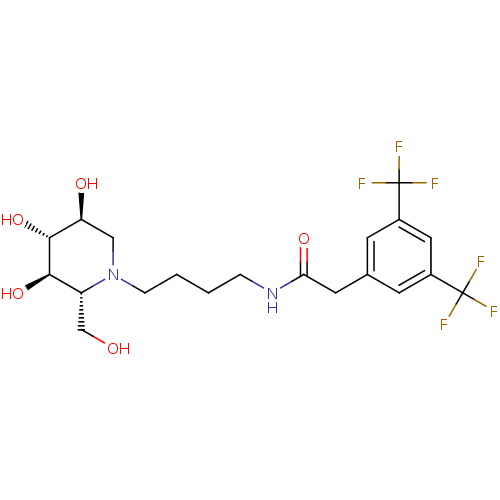 (CHEMBL2414884) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11540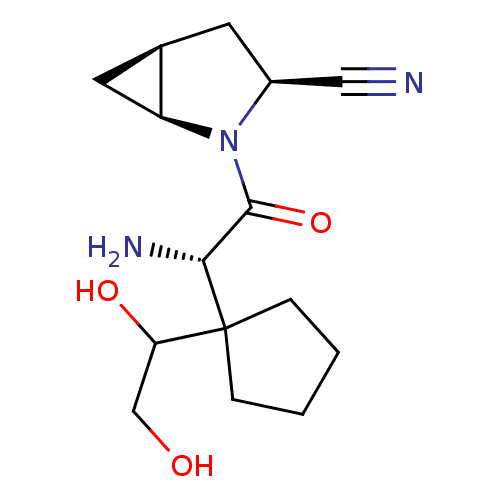 ((1S,3S,5S)-2-[(2S)-2-amino-2-[1-(1,2-dihydroxyethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 143 | -38.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50438754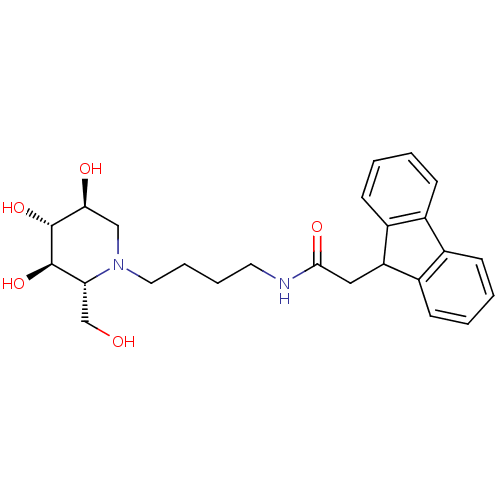 (CHEMBL2414880) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50438752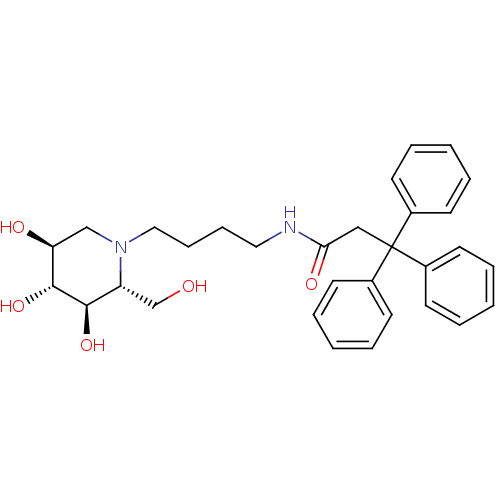 (CHEMBL2414882) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50438753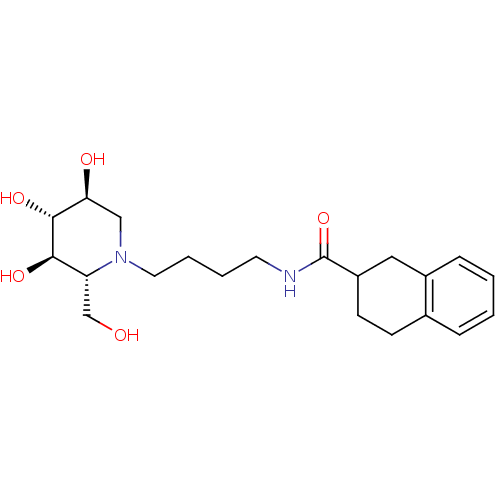 (CHEMBL2414881) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50438748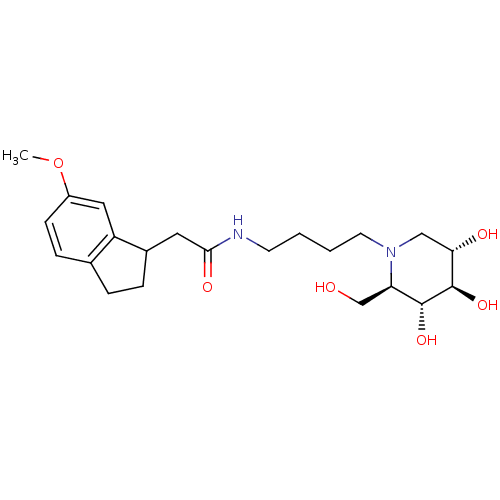 (CHEMBL2414879) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Non-competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18358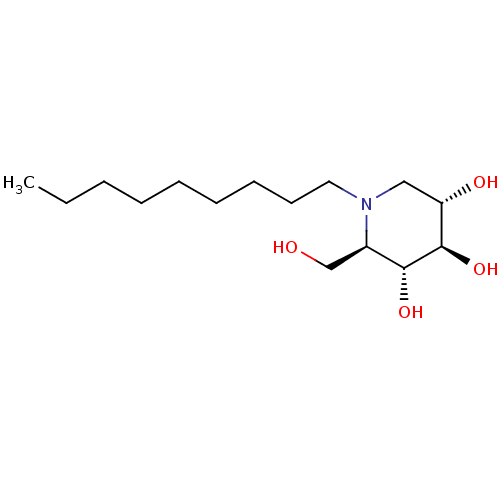 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-nonylpiperidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50438751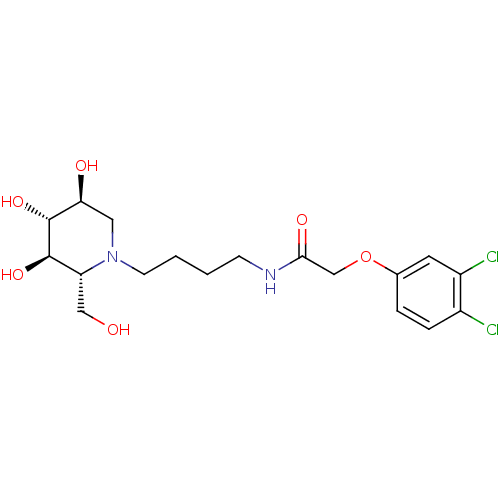 (CHEMBL2414883) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50438749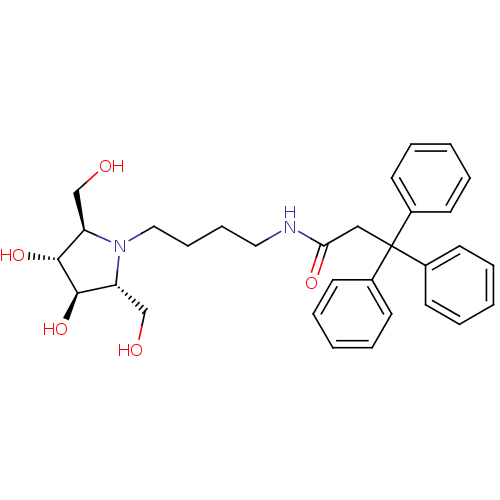 (CHEMBL2414888) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Research Center Curated by ChEMBL | Assay Description Competitive inhibition of human beta-glucocerebrosidase by Lineweaver-Burk double reciprocal plot method | Bioorg Med Chem 21: 5021-8 (2013) Article DOI: 10.1016/j.bmc.2013.06.054 BindingDB Entry DOI: 10.7270/Q2XG9SJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent RNA helicase RhlE (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM13307 (1-Methyl-1H-imidazole-4-sulfonic Acid Benzyl-{2-[(...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Yale University | Assay Description Assays for PFT activity were performed with a PFT-specific scintillation assay (SPA) kit (Amersham Biosciences, Piscataway, NJ). IC50 values were cal... | J Med Chem 49: 5710-27 (2006) Article DOI: 10.1021/jm060081v BindingDB Entry DOI: 10.7270/Q2G44NJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent RNA helicase RhlE (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM13311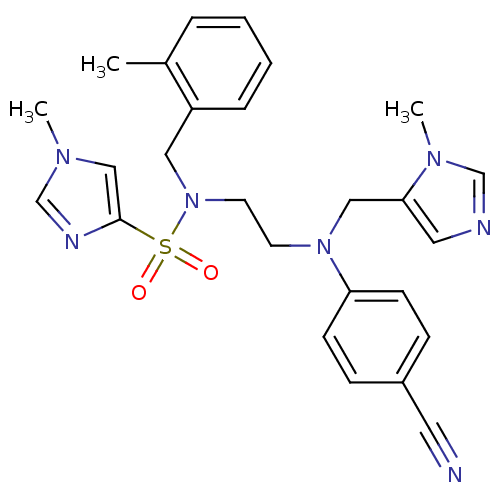 (1-Methyl-1H-imidazole-4-sulfonic Acid {2-[(4-Cyano...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Yale University | Assay Description Assays for PFT activity were performed with a PFT-specific scintillation assay (SPA) kit (Amersham Biosciences, Piscataway, NJ). IC50 values were cal... | J Med Chem 49: 5710-27 (2006) Article DOI: 10.1021/jm060081v BindingDB Entry DOI: 10.7270/Q2G44NJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM571674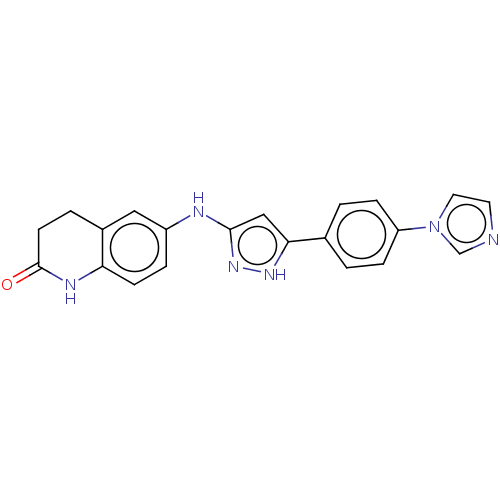 (6-((5-(4-(1H-imidazol-1- yl)phenyl)-1H-pyrazol-3- ...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11447469-20220920-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24X5C2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM571620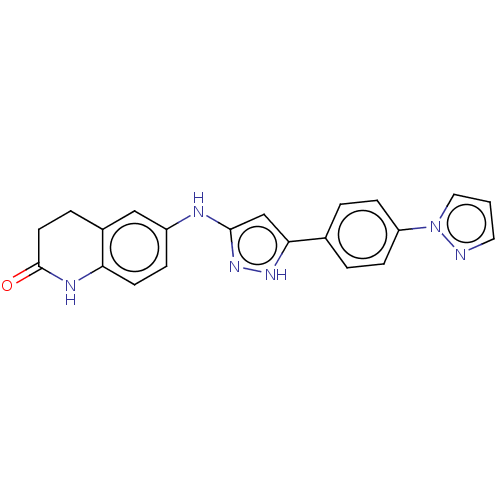 (6-((5-(4-(1H-pyrazol-1- yl)phenyl)-1H-pyrazol-3- y...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11447469-20220920-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24X5C2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM571666 (5-((5-(3-fluoro-4- hydroxyphenyl)-1H- pyrazol-3- y...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11447469-20220920-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24X5C2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579738 (N-(4-((5-(4-(1H-pyrazol-1- yl)phenyl)-1H-pyrazol-3...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579704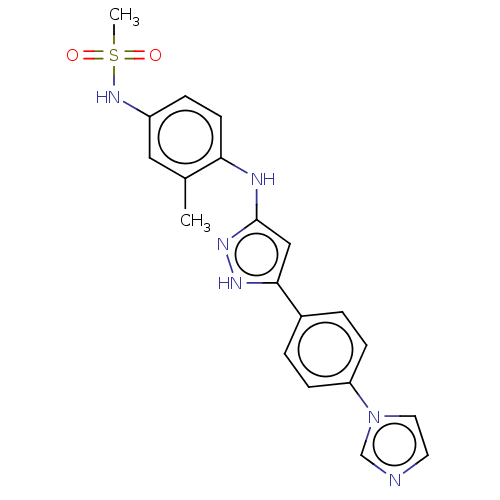 (N-(4-((5-(4-(1H-imidazol-1- yl)phenyl)-1H-pyrazol-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579709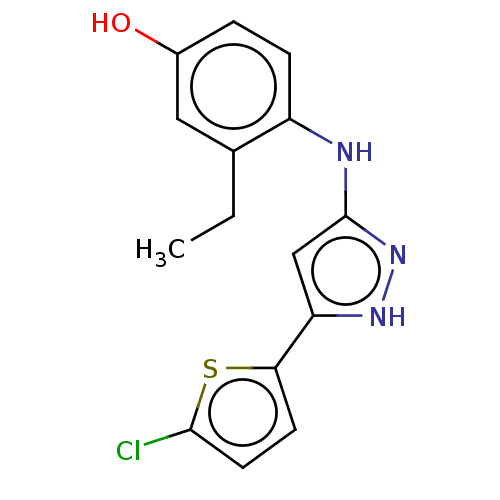 (4-((5-(5-chlorothiophen-2- yl)-1H-pyrazol-3-yl)ami...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579718 (3-ethyl-4-((5-(4-iodophenyl)- 1H-pyrazol-3-yl)amin...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent RNA helicase RhlE (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM13309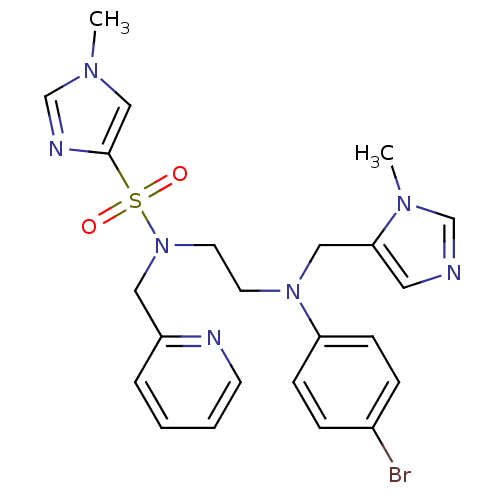 (N-(2-{(4-bromophenyl)[(1-methyl-1H-imidazol-5-yl)m...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Yale University | Assay Description Assays for PFT activity were performed with a PFT-specific scintillation assay (SPA) kit (Amersham Biosciences, Piscataway, NJ). IC50 values were cal... | J Med Chem 49: 5710-27 (2006) Article DOI: 10.1021/jm060081v BindingDB Entry DOI: 10.7270/Q2G44NJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579739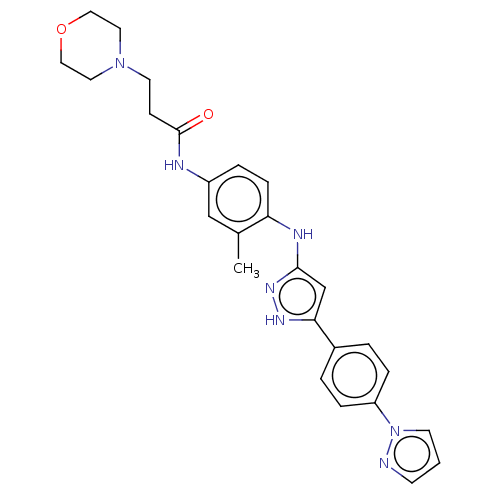 (N-(4-((5-(4-(1H-pyrazol-1- yl)phenyl)-1H-pyrazol-3...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent RNA helicase RhlE (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM13308 (N-benzyl-N-(2-{(4-bromophenyl)[(1-methyl-1H-imidaz...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Yale University | Assay Description Assays for PFT activity were performed with a PFT-specific scintillation assay (SPA) kit (Amersham Biosciences, Piscataway, NJ). IC50 values were cal... | J Med Chem 49: 5710-27 (2006) Article DOI: 10.1021/jm060081v BindingDB Entry DOI: 10.7270/Q2G44NJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent RNA helicase RhlE (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM13310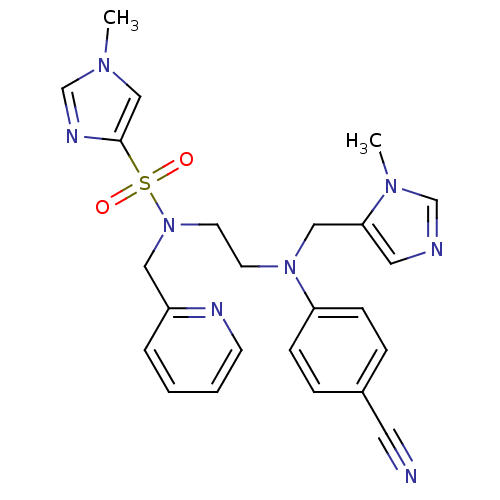 (N-(2-{(4-cyanophenyl)[(1-methyl-1H-imidazol-5-yl)m...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Yale University | Assay Description Assays for PFT activity were performed with a PFT-specific scintillation assay (SPA) kit (Amersham Biosciences, Piscataway, NJ). IC50 values were cal... | J Med Chem 49: 5710-27 (2006) Article DOI: 10.1021/jm060081v BindingDB Entry DOI: 10.7270/Q2G44NJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579743 (N-(4-((5-(4-(1H-pyrazol-1- yl)phenyl)-1H-pyrazol-3...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579689 (N-(4-((5-(4-hydroxyphenyl)- 1H-pyrazol-3-yl)amino)...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579794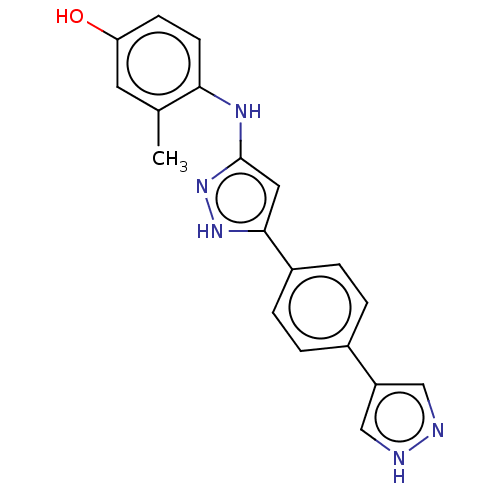 (4-((5-(4-(1H-pyrazol-4- yl)phenyl)-1H-pyrazol-3- y...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent RNA helicase RhlE (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM13315 (1-Methyl-1H-imidazole-4-sulfonic Acid {2-[(4-Cyano...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Yale University | Assay Description Assays for PFT activity were performed with a PFT-specific scintillation assay (SPA) kit (Amersham Biosciences, Piscataway, NJ). IC50 values were cal... | J Med Chem 49: 5710-27 (2006) Article DOI: 10.1021/jm060081v BindingDB Entry DOI: 10.7270/Q2G44NJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579761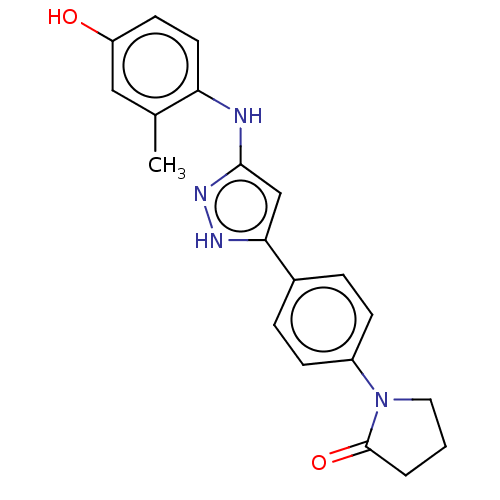 (1-(4-(3-((4-hydroxy-2- methylphenyl)amino)-1H- pyr...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579714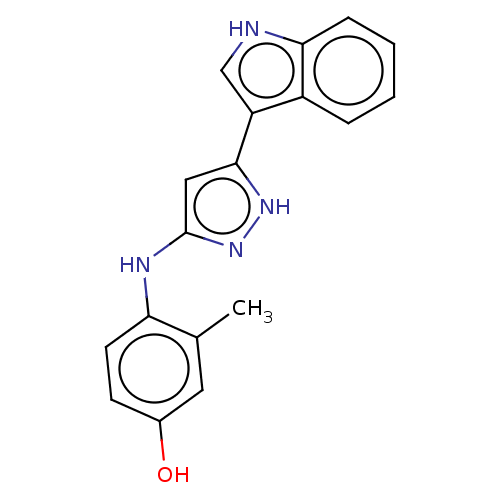 (4-((5-(1H-indol-3-yl)-1H- pyrazol-3-yl)amino)-3- m...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM571724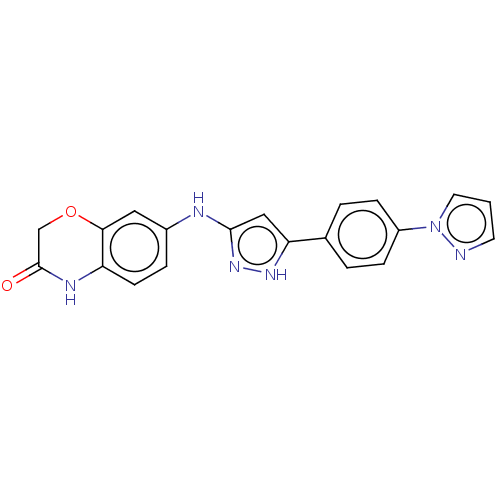 (7-((5-(4-(1H-pyrazol-1- yl)phenyl)-1H-pyrazol-3- y...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11447469-20220920-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24X5C2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579764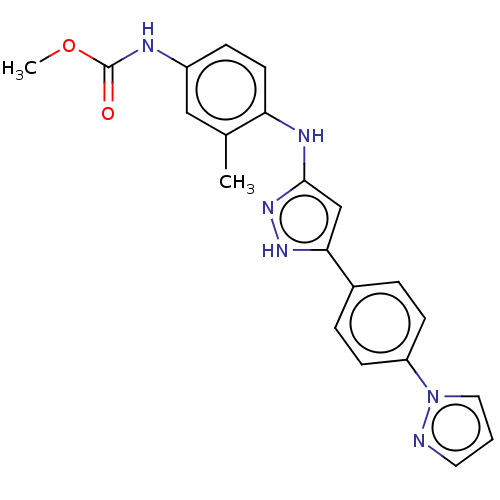 (US11485711, Compound 208 | methyl (4-((5-(4-(1H-py...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579740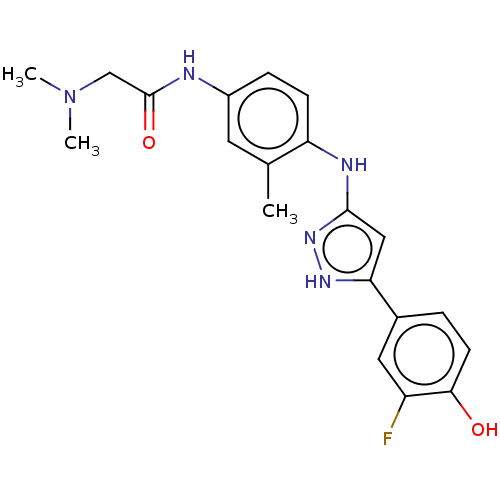 (2-(dimethylamino)-N-(4-((5- (3-fluoro-4-hydroxyphe...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus (rat)) | BDBM13315 (1-Methyl-1H-imidazole-4-sulfonic Acid {2-[(4-Cyano...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Yale University | Assay Description Assays for PFT activity were performed with a PFT-specific scintillation assay (SPA) kit (Amersham Biosciences, Piscataway, NJ). IC50 values were cal... | J Med Chem 49: 5710-27 (2006) Article DOI: 10.1021/jm060081v BindingDB Entry DOI: 10.7270/Q2G44NJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 414 total ) | Next | Last >> |