

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50105264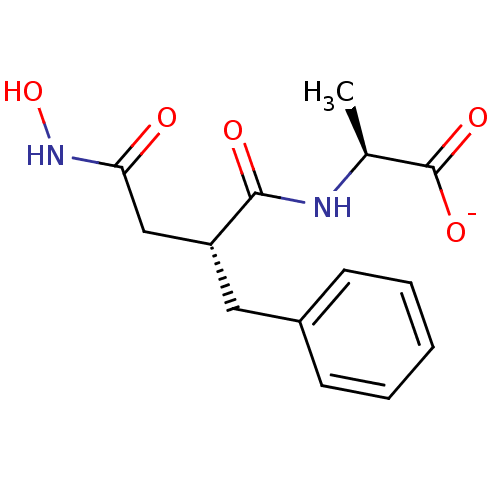 (2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085261 (CHEMBL163478 | [2-(4-Benzyl-phenoxy)-ethyl]-dimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085288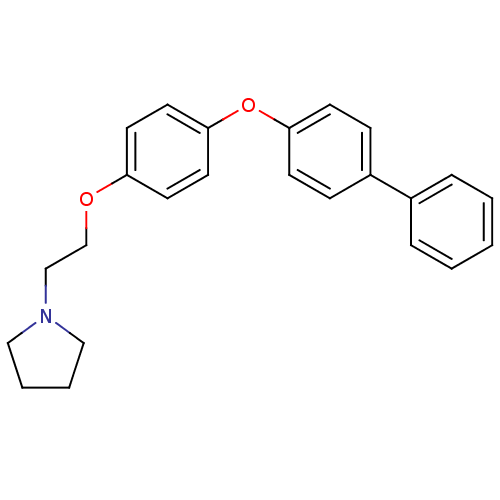 (1-{2-[4-(Biphenyl-4-yloxy)-phenoxy]-ethyl}-pyrroli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085282 (5-Benzyl-2-(2-pyrrolidin-1-yl-ethoxy)-pyridine | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085290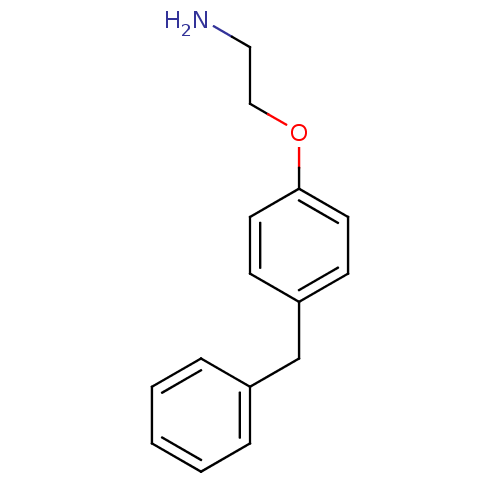 (2-(4-Benzyl-phenoxy)-ethylamine | 2-(4-benzylpheno...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085277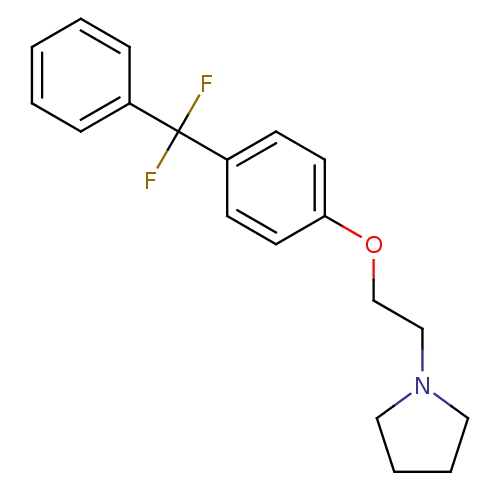 (1-{2-[4-(Difluoro-phenyl-methyl)-phenoxy]-ethyl}-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085270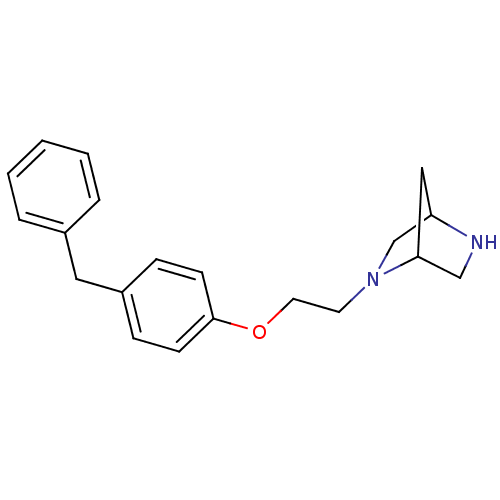 (2-[2-(4-Benzyl-phenoxy)-ethyl]-2,5-diaza-bicyclo[2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085297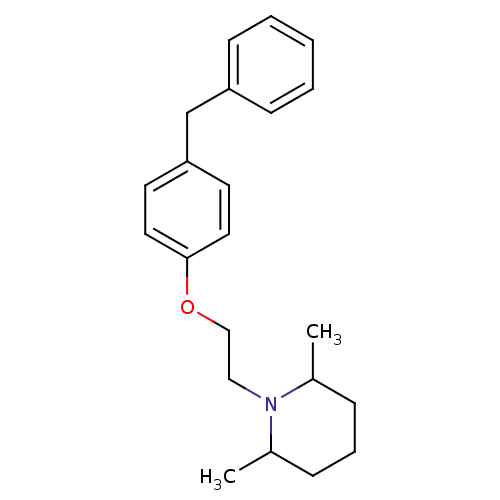 (1-[2-(4-Benzyl-phenoxy)-ethyl]-2,6-dimethyl-piperi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50094519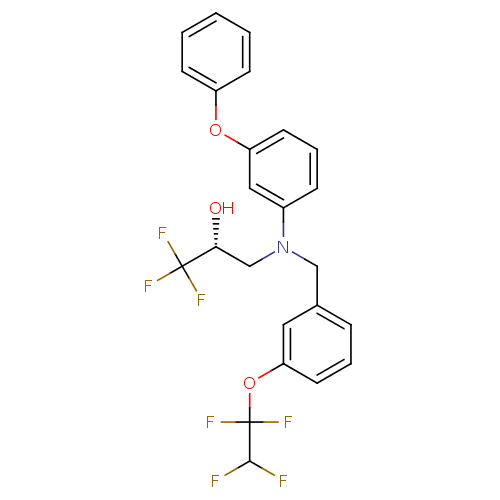 ((R)-1,1,1-Trifluoro-3-{(3-phenoxy-phenyl)-[3-(1,1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Discovery Research Curated by ChEMBL | Assay Description Inhibition of recombinant human CETP-mediated transfer of [3H]-CE from HDL to LDL | J Med Chem 43: 4575-8 (2001) BindingDB Entry DOI: 10.7270/Q2X0669S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50094519 ((R)-1,1,1-Trifluoro-3-{(3-phenoxy-phenyl)-[3-(1,1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of cholesteryl ester transfer protein in presence of buffer. | J Med Chem 45: 3891-904 (2002) BindingDB Entry DOI: 10.7270/Q2JW8D7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085279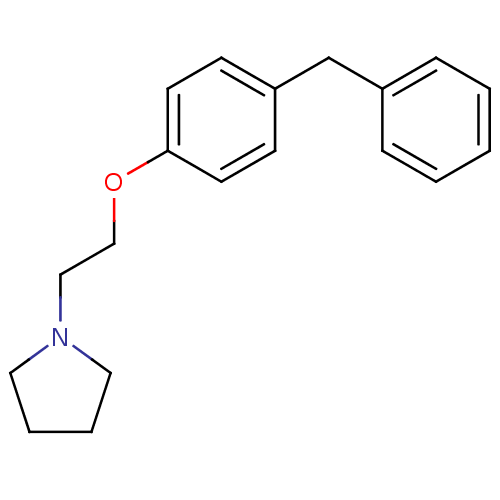 (1-[2-(4-Benzyl-phenoxy)-ethyl]-pyrrolidine | 1-[2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085252 (CHEMBL162180 | Phenyl-[4-(2-pyrrolidin-1-yl-ethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 85/88 kDa calcium-independent phospholipase A2 (Homo sapiens (Human)) | BDBM50046270 ((E)-6-Iodomethylene-3-naphthalen-1-yl-tetrahydro-p...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Compounds were evaluated for the inhibitory activity against canine myocardial cytosolic calcium dependent phospholipase A2 . | J Med Chem 36: 95-100 (1993) BindingDB Entry DOI: 10.7270/Q22J69XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085248 (1-[2-(4-Benzyloxy-phenoxy)-ethyl]-pyrrolidine | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085245 (1-[2-(4-Phenoxy-phenoxy)-ethyl]-pyrrolidine | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085299 (1-[2-(4-Thiophen-3-ylmethyl-phenoxy)-ethyl]-pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085262 (1-[2-(5-Benzyl-thiophen-2-yloxy)-ethyl]-pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085283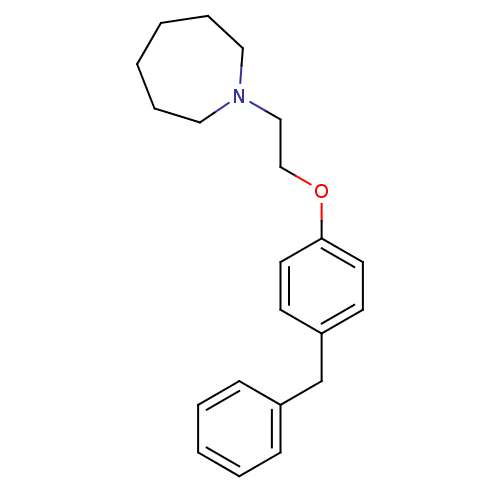 (1-[2-(4-Benzyl-phenoxy)-ethyl]-azepane | CHEMBL159...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085276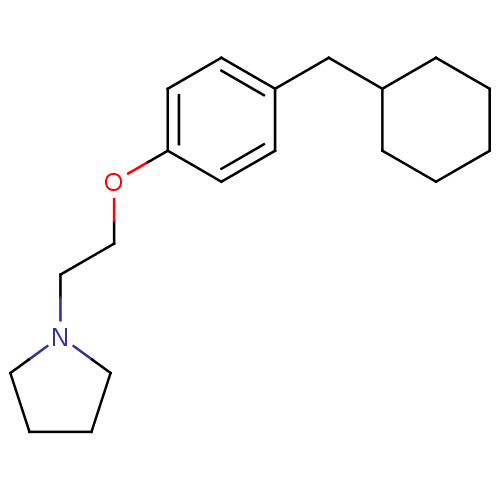 (1-[2-(4-Cyclohexylmethyl-phenoxy)-ethyl]-pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085255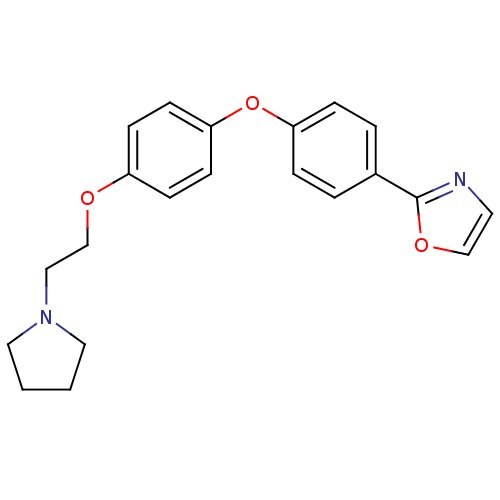 (2-{4-[4-(2-Pyrrolidin-1-yl-ethoxy)-phenoxy]-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085292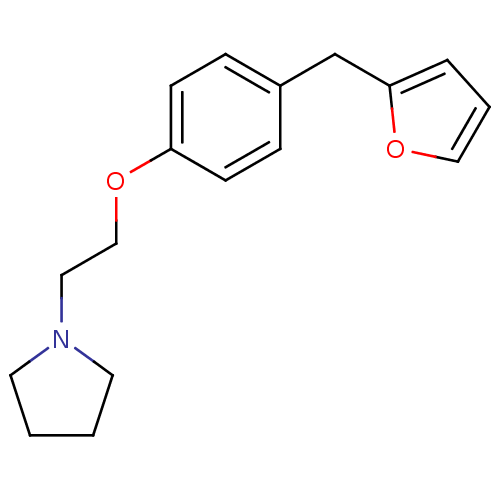 (1-[2-(4-Furan-2-ylmethyl-phenoxy)-ethyl]-pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085268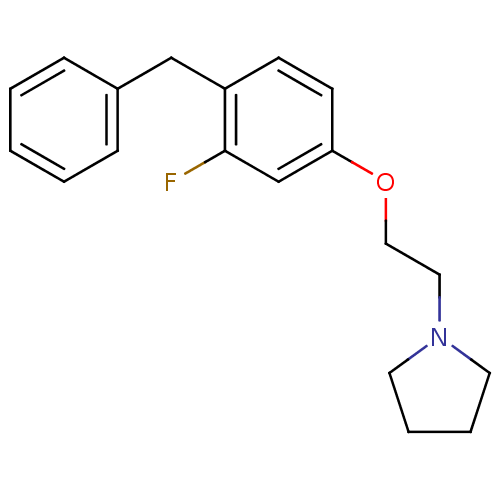 (1-[2-(4-Benzyl-3-fluoro-phenoxy)-ethyl]-pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085285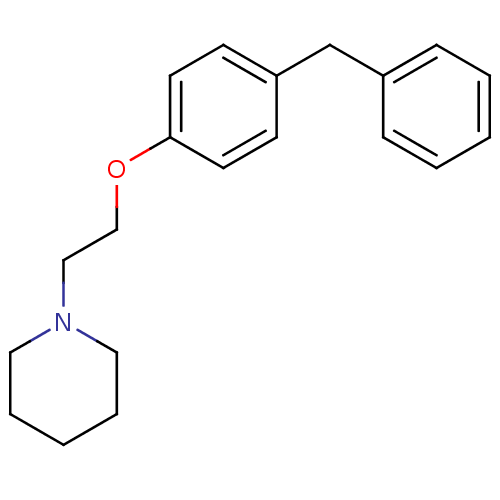 (1-[2-(4-Benzyl-phenoxy)-ethyl]-piperidine | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085255 (2-{4-[4-(2-Pyrrolidin-1-yl-ethoxy)-phenoxy]-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined for LTB4 production in human whole blood. | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085253 (1-[2-(4-Benzyl-2-fluoro-phenoxy)-ethyl]-pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085281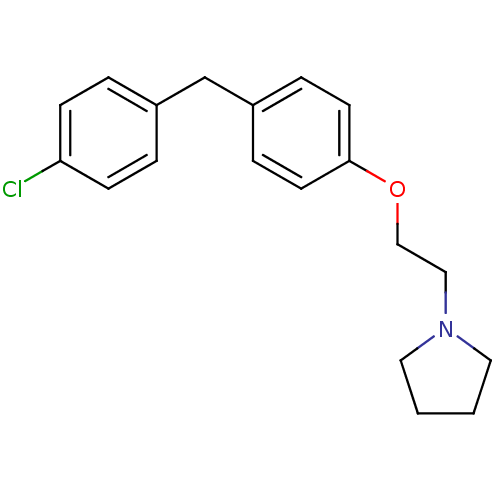 (1-{2-[4-(4-Chloro-benzyl)-phenoxy]-ethyl}-pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085311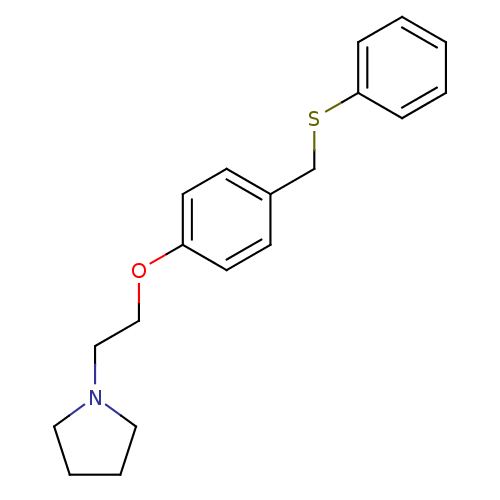 (1-[2-(4-Phenylsulfanylmethyl-phenoxy)-ethyl]-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085291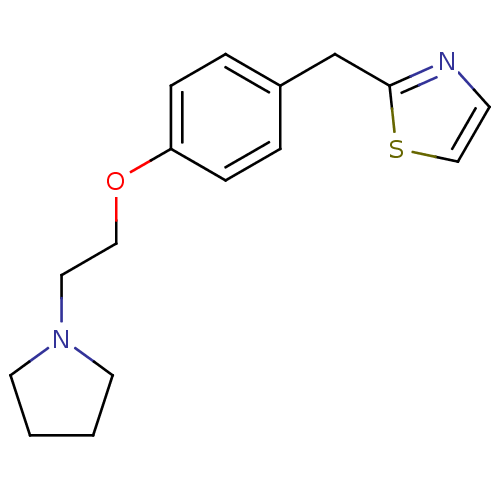 (2-[4-(2-Pyrrolidin-1-yl-ethoxy)-benzyl]-thiazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085308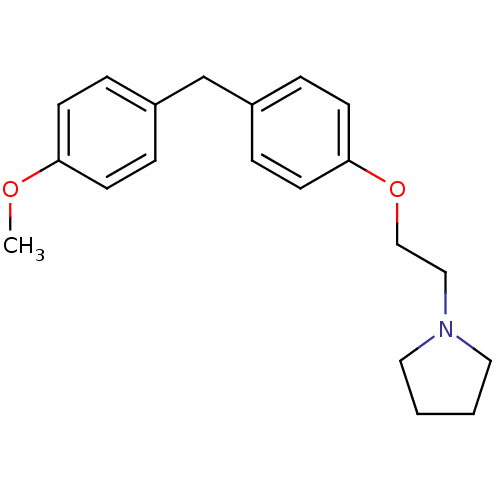 (1-{2-[4-(4-Methoxy-benzyl)-phenoxy]-ethyl}-pyrroli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085261 (CHEMBL163478 | [2-(4-Benzyl-phenoxy)-ethyl]-dimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined for LTB4 production in human whole blood. | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085289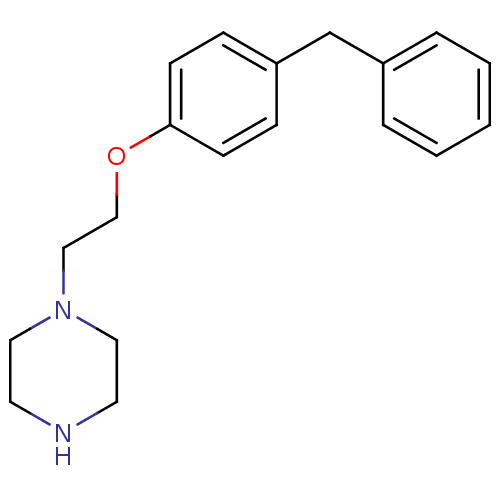 (1-[2-(4-Benzyl-phenoxy)-ethyl]-piperazine | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085292 (1-[2-(4-Furan-2-ylmethyl-phenoxy)-ethyl]-pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined for LTB4 production in human whole blood. | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085298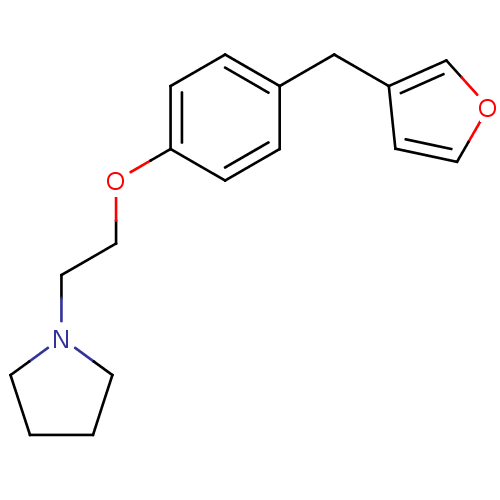 (1-[2-(4-Furan-3-ylmethyl-phenoxy)-ethyl]-pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085301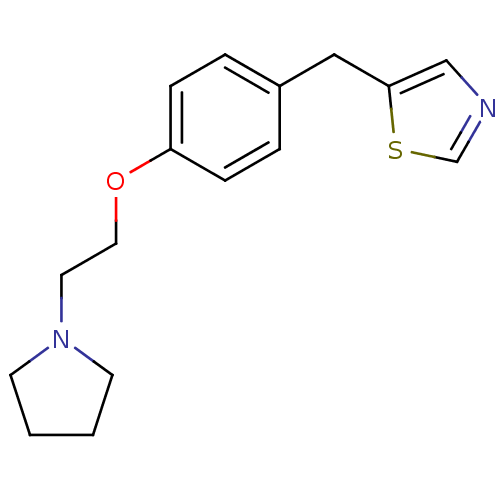 (5-[4-(2-Pyrrolidin-1-yl-ethoxy)-benzyl]-thiazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085245 (1-[2-(4-Phenoxy-phenoxy)-ethyl]-pyrrolidine | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined for LTB4 production in human whole blood. | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085305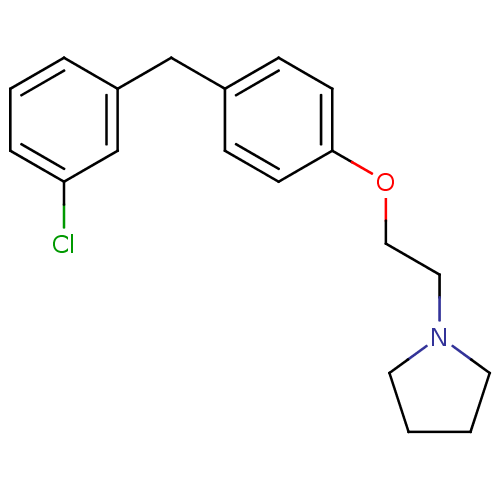 (1-{2-[4-(3-Chloro-benzyl)-phenoxy]-ethyl}-pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085282 (5-Benzyl-2-(2-pyrrolidin-1-yl-ethoxy)-pyridine | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined for LTB4 production in human whole blood. | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085246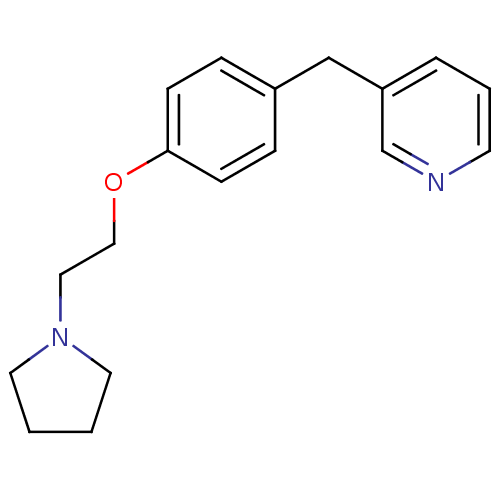 (3-[4-(2-Pyrrolidin-1-yl-ethoxy)-benzyl]-pyridine |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 85/88 kDa calcium-independent phospholipase A2 (Homo sapiens (Human)) | BDBM50046271 ((E)-3-(naphthalen-1-yl)-6-(prop-2-yn-1-ylidene)tet...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Compounds were evaluated for the inhibitory activity against canine myocardial cytosolic calcium dependent phospholipase A2 . | J Med Chem 36: 95-100 (1993) BindingDB Entry DOI: 10.7270/Q22J69XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085299 (1-[2-(4-Thiophen-3-ylmethyl-phenoxy)-ethyl]-pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined for LTB4 production in human whole blood. | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085263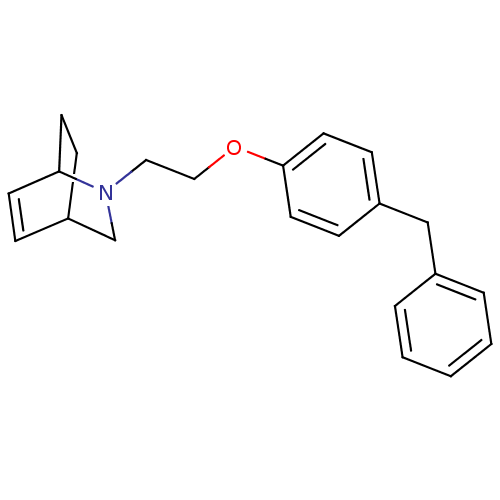 (2-[2-(4-Benzyl-phenoxy)-ethyl]-2-aza-bicyclo[2.2.2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 85/88 kDa calcium-independent phospholipase A2 (Homo sapiens (Human)) | BDBM50046268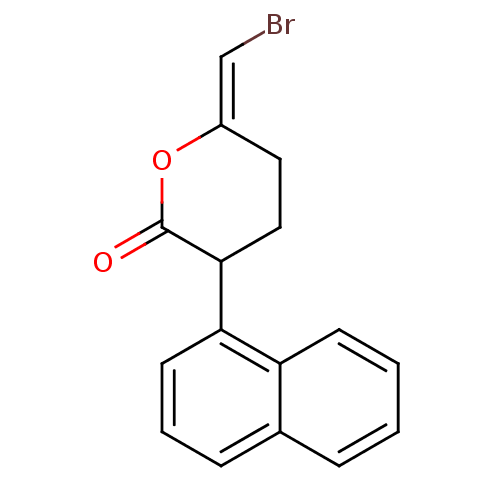 (6-Bromomethylene-3-naphthalen-1-yl-tetrahydro-pyra...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Compounds were evaluated for the inhibitory activity against canine myocardial cytosolic calcium dependent phospholipase A2 . | J Med Chem 36: 95-100 (1993) BindingDB Entry DOI: 10.7270/Q22J69XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085306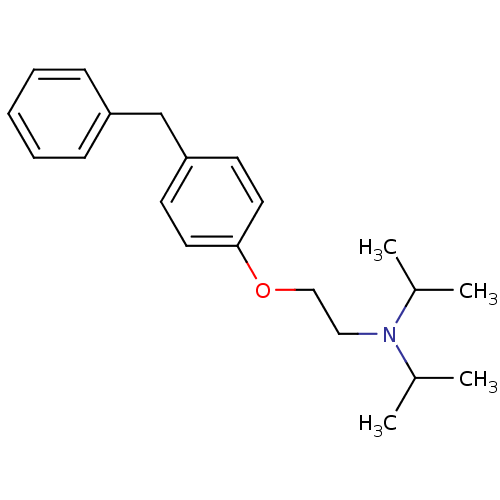 (CHEMBL164168 | [2-(4-Benzyl-phenoxy)-ethyl]-diisop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined against Leukotriene A4 hydrolase | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085283 (1-[2-(4-Benzyl-phenoxy)-ethyl]-azepane | CHEMBL159...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined for LTB4 production in human whole blood. | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085279 (1-[2-(4-Benzyl-phenoxy)-ethyl]-pyrrolidine | 1-[2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined for LTB4 production in human whole blood. | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 85/88 kDa calcium-independent phospholipase A2 (Homo sapiens (Human)) | BDBM50046268 (6-Bromomethylene-3-naphthalen-1-yl-tetrahydro-pyra...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Compounds were evaluated for the inhibitory activity against canine myocardial cytosolic calcium dependent phospholipase A2 . | J Med Chem 36: 95-100 (1993) BindingDB Entry DOI: 10.7270/Q22J69XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085306 (CHEMBL164168 | [2-(4-Benzyl-phenoxy)-ethyl]-diisop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined for LTB4 production in human whole blood. | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085288 (1-{2-[4-(Biphenyl-4-yloxy)-phenoxy]-ethyl}-pyrroli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined for LTB4 production in human whole blood. | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50094513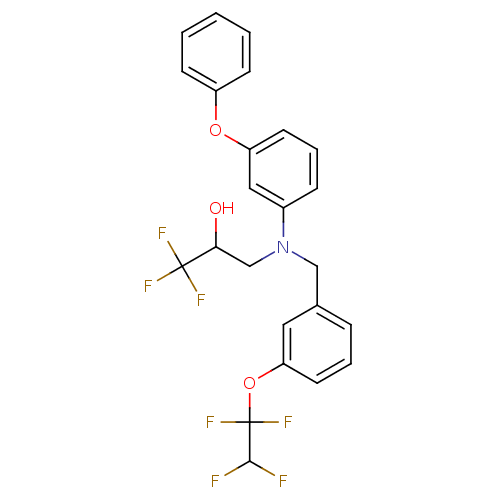 (1,1,1-Trifluoro-3-{(3-phenoxy-phenyl)-[3-(1,1,2,2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of cholesteryl ester transfer protein in presence of buffer. | J Med Chem 45: 3891-904 (2002) BindingDB Entry DOI: 10.7270/Q2JW8D7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085252 (CHEMBL162180 | Phenyl-[4-(2-pyrrolidin-1-yl-ethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity was determined for LTB4 production in human whole blood. | J Med Chem 43: 721-35 (2000) BindingDB Entry DOI: 10.7270/Q2CN74MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 230 total ) | Next | Last >> |