

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM190405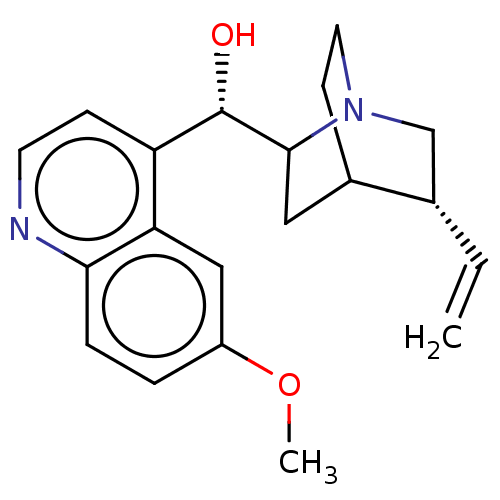 (US9180183, Quinidine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9173935 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM190405 (US9180183, Quinidine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9180183 (2015) BindingDB Entry DOI: 10.7270/Q2B27T2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM151585 (US11739089, Compound Ketoconazole | US8987315, Ket...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Similars | DrugBank PDB US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9173935 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P8S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM151585 (US11739089, Compound Ketoconazole | US8987315, Ket...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Similars | DrugBank PDB US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9180183 (2015) BindingDB Entry DOI: 10.7270/Q2B27T2W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50461837 (CHEMBL4226608) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Antagonist activity at TRPV1 (unknown origin) expressed in human SH-SY5Y cells assessed as inhibition of capsaicin-induced calcium increase after 25 ... | J Med Chem 61: 4436-4455 (2018) Article DOI: 10.1021/acs.jmedchem.8b00109 BindingDB Entry DOI: 10.7270/Q2PK0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM151585 (US11739089, Compound Ketoconazole | US8987315, Ket...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9173935 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM151585 (US11739089, Compound Ketoconazole | US8987315, Ket...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9180183 (2015) BindingDB Entry DOI: 10.7270/Q2B27T2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50090677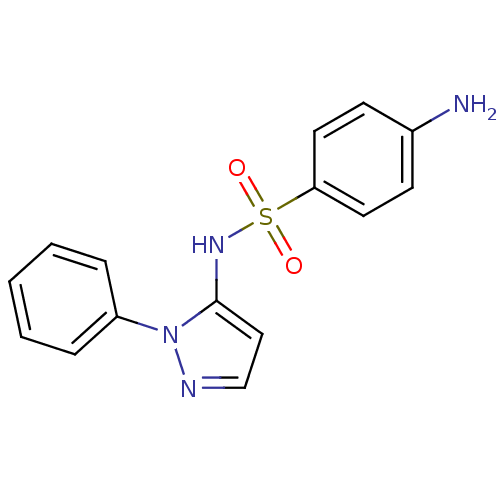 (4-Amino-N-(2-phenyl-2H-pyrazol-3-yl)-benzenesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank US Patent | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9180183 (2015) BindingDB Entry DOI: 10.7270/Q2B27T2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50090677 (4-Amino-N-(2-phenyl-2H-pyrazol-3-yl)-benzenesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank US Patent | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9173935 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50010231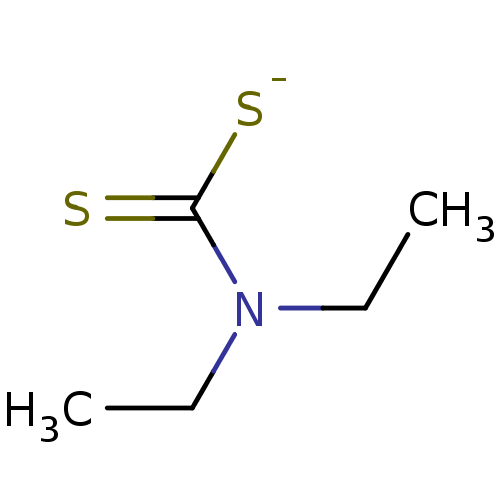 (CHEMBL107217 | Ditiocarb sodium | Sodium salt of D...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9180183 (2015) BindingDB Entry DOI: 10.7270/Q2B27T2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50110231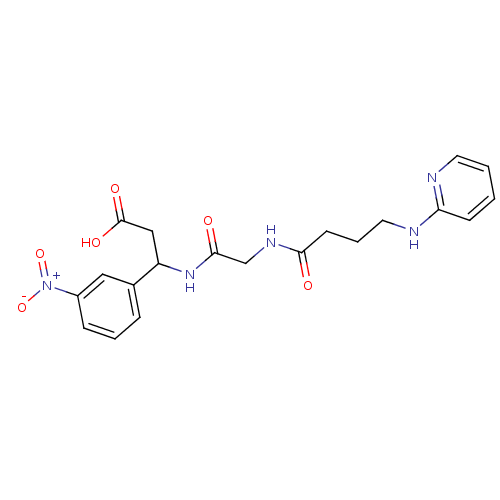 (3-(3-Nitro-phenyl)-3-{2-[4-(pyridin-2-ylamino)-but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | US Patent | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9173935 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50461844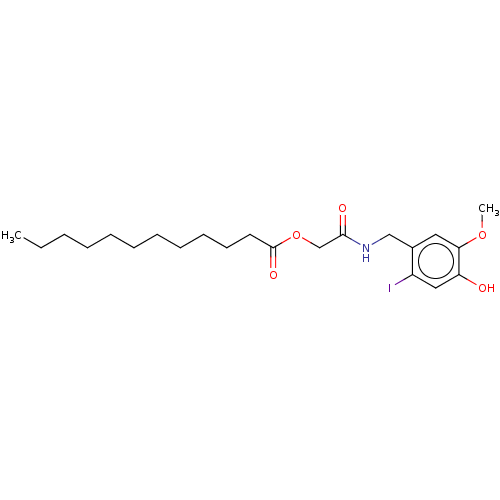 (CHEMBL4227926) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Antagonist activity at TRPV1 (unknown origin) expressed in human SH-SY5Y cells assessed as inhibition of capsaicin-induced calcium increase after 25 ... | J Med Chem 61: 4436-4455 (2018) Article DOI: 10.1021/acs.jmedchem.8b00109 BindingDB Entry DOI: 10.7270/Q2PK0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50461847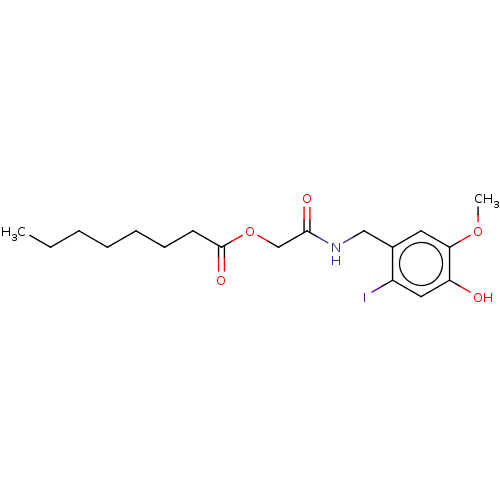 (CHEMBL4226335) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Antagonist activity at TRPV1 (unknown origin) expressed in human SH-SY5Y cells assessed as inhibition of capsaicin-induced calcium increase after 25 ... | J Med Chem 61: 4436-4455 (2018) Article DOI: 10.1021/acs.jmedchem.8b00109 BindingDB Entry DOI: 10.7270/Q2PK0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50236897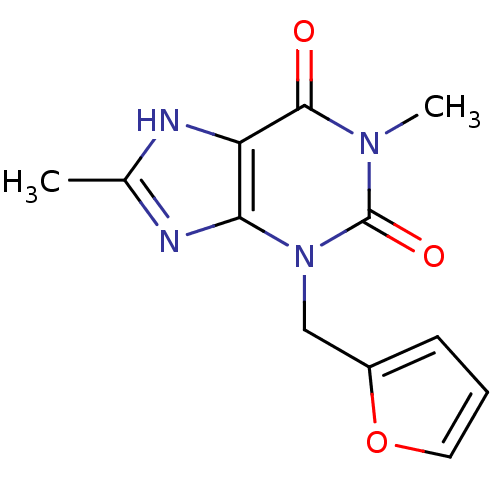 (3-(furan-2-ylmethyl)-1,8-dimethyl-1H-purine-2,6(3H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9173935 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50236897 (3-(furan-2-ylmethyl)-1,8-dimethyl-1H-purine-2,6(3H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9180183 (2015) BindingDB Entry DOI: 10.7270/Q2B27T2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9180183 (2015) BindingDB Entry DOI: 10.7270/Q2B27T2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9173935 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50113851 ((+/-)-Tranylcypromine | 2-PCPA | 2-Phenyl-cyclopro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9173935 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50240772 ((1R,2S)-(-)-2-phenylcyclopropylamine | (1R,2S)-2-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9180183 (2015) BindingDB Entry DOI: 10.7270/Q2B27T2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50240772 ((1R,2S)-(-)-2-phenylcyclopropylamine | (1R,2S)-2-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9180183 (2015) BindingDB Entry DOI: 10.7270/Q2B27T2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50113851 ((+/-)-Tranylcypromine | 2-PCPA | 2-Phenyl-cyclopro...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9173935 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM190404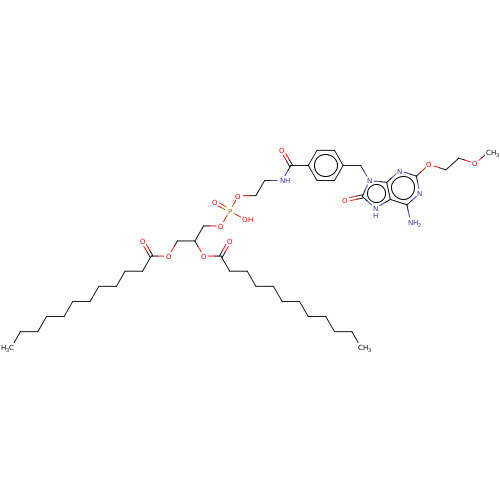 (US9180183, SC12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9180183 (2015) BindingDB Entry DOI: 10.7270/Q2B27T2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM189322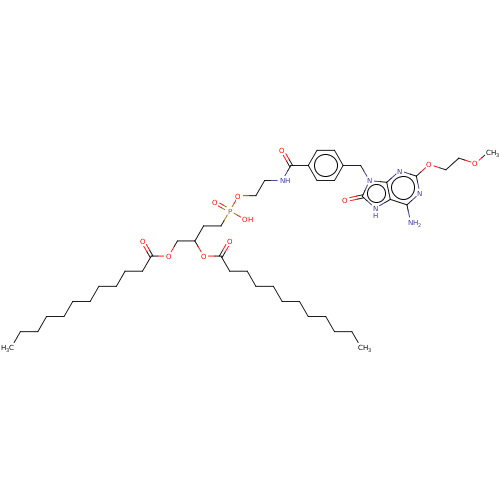 (US9173935, SC12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9173935 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM190404 (US9180183, SC12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9180183 (2015) BindingDB Entry DOI: 10.7270/Q2B27T2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM189322 (US9173935, SC12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9173935 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM189322 (US9173935, SC12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9173935 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM190404 (US9180183, SC12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9180183 (2015) BindingDB Entry DOI: 10.7270/Q2B27T2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM190404 (US9180183, SC12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 3.34E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9180183 (2015) BindingDB Entry DOI: 10.7270/Q2B27T2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM189322 (US9173935, SC12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9173935 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM189322 (US9173935, SC12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9173935 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM190404 (US9180183, SC12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9180183 (2015) BindingDB Entry DOI: 10.7270/Q2B27T2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM190404 (US9180183, SC12) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9180183 (2015) BindingDB Entry DOI: 10.7270/Q2B27T2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM190404 (US9180183, SC12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9180183 (2015) BindingDB Entry DOI: 10.7270/Q2B27T2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM190404 (US9180183, SC12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9180183 (2015) BindingDB Entry DOI: 10.7270/Q2B27T2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM189322 (US9173935, SC12) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9173935 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM189322 (US9173935, SC12) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9173935 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM189322 (US9173935, SC12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9173935 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM189322 (US9173935, SC12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9173935 (2015) BindingDB Entry DOI: 10.7270/Q2JS9P8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM190404 (US9180183, SC12) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | 37 |
Telormedix SA US Patent | Assay Description The interaction of SC12 with cytochrome P450 enzymes was tested using Fluorescent High Throughput P450 assays (Gentest); The IC50s of the compounds w... | US Patent US9180183 (2015) BindingDB Entry DOI: 10.7270/Q2B27T2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50461840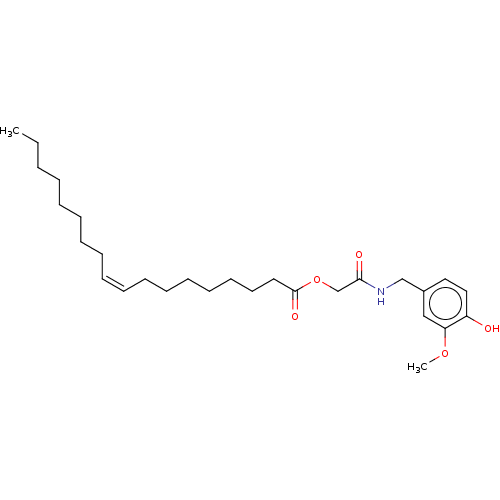 (CHEMBL4228832) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Agonist activity at TRPV1 (unknown origin) expressed in human SH-SY5Y cells assessed as increase in calcium influx after 25 mins by Fluo-4 NW dye-bas... | J Med Chem 61: 4436-4455 (2018) Article DOI: 10.1021/acs.jmedchem.8b00109 BindingDB Entry DOI: 10.7270/Q2PK0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50461841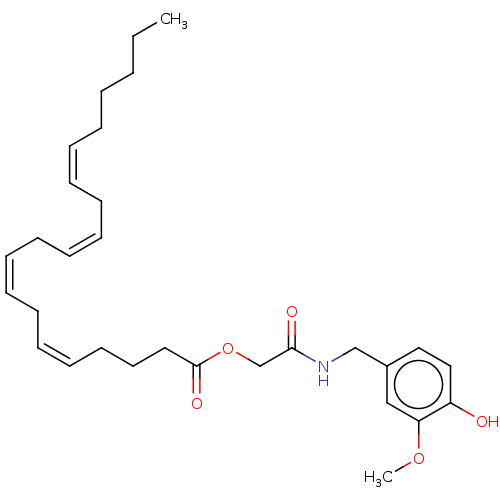 (CHEMBL4224748) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Agonist activity at TRPV1 (unknown origin) expressed in human SH-SY5Y cells assessed as increase in calcium influx after 25 mins by Fluo-4 NW dye-bas... | J Med Chem 61: 4436-4455 (2018) Article DOI: 10.1021/acs.jmedchem.8b00109 BindingDB Entry DOI: 10.7270/Q2PK0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50461842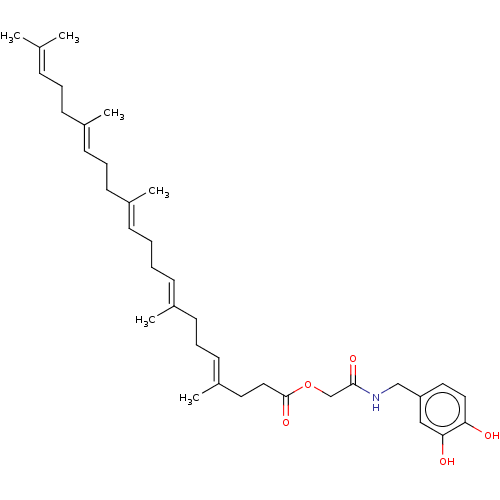 (CHEMBL4226128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Agonist activity at TRPV1 (unknown origin) expressed in human SH-SY5Y cells assessed as increase in calcium influx after 25 mins by Fluo-4 NW dye-bas... | J Med Chem 61: 4436-4455 (2018) Article DOI: 10.1021/acs.jmedchem.8b00109 BindingDB Entry DOI: 10.7270/Q2PK0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM20461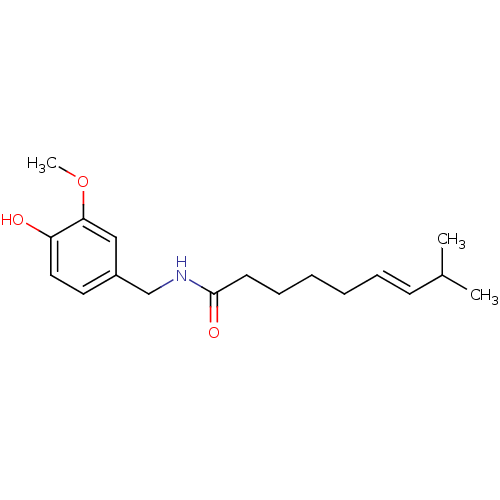 ((6E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Agonist activity at TRPV1 (unknown origin) expressed in human SH-SY5Y cells assessed as increase in calcium influx after 25 mins by Fluo-4 NW dye-bas... | J Med Chem 61: 4436-4455 (2018) Article DOI: 10.1021/acs.jmedchem.8b00109 BindingDB Entry DOI: 10.7270/Q2PK0JSM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50461843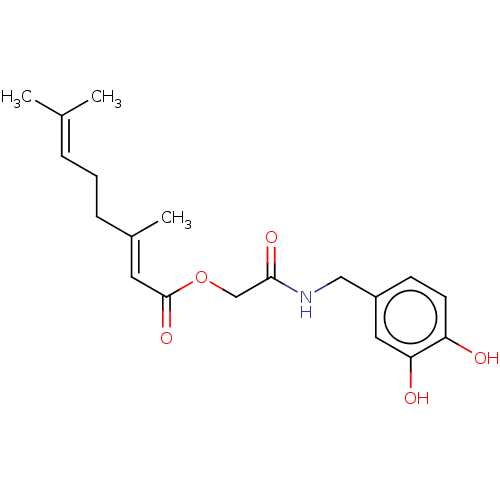 (CHEMBL4225006) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 410 | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Agonist activity at TRPV1 (unknown origin) expressed in human SH-SY5Y cells assessed as increase in calcium influx after 25 mins by Fluo-4 NW dye-bas... | J Med Chem 61: 4436-4455 (2018) Article DOI: 10.1021/acs.jmedchem.8b00109 BindingDB Entry DOI: 10.7270/Q2PK0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50461839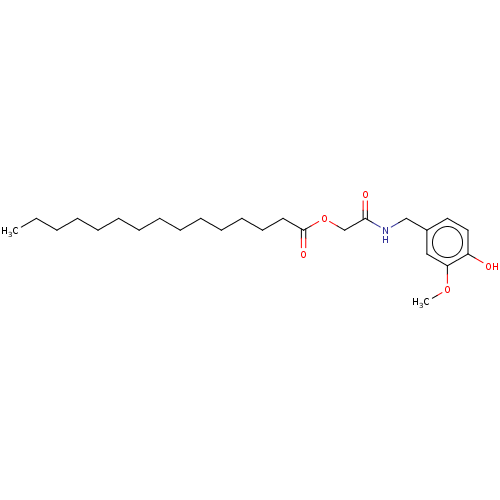 (CHEMBL4227432) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Agonist activity at TRPV1 (unknown origin) expressed in human SH-SY5Y cells assessed as increase in calcium influx after 25 mins by Fluo-4 NW dye-bas... | J Med Chem 61: 4436-4455 (2018) Article DOI: 10.1021/acs.jmedchem.8b00109 BindingDB Entry DOI: 10.7270/Q2PK0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50461845 (CHEMBL4226647) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Agonist activity at TRPV1 (unknown origin) expressed in human SH-SY5Y cells assessed as increase in calcium influx after 25 mins by Fluo-4 NW dye-bas... | J Med Chem 61: 4436-4455 (2018) Article DOI: 10.1021/acs.jmedchem.8b00109 BindingDB Entry DOI: 10.7270/Q2PK0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50461846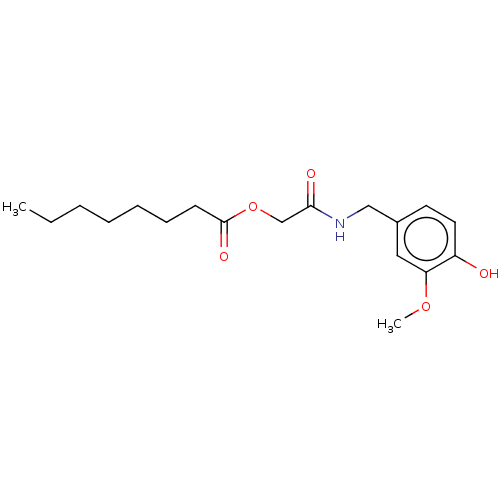 (CHEMBL4226064) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Agonist activity at TRPV1 (unknown origin) expressed in human SH-SY5Y cells assessed as increase in calcium influx after 25 mins by Fluo-4 NW dye-bas... | J Med Chem 61: 4436-4455 (2018) Article DOI: 10.1021/acs.jmedchem.8b00109 BindingDB Entry DOI: 10.7270/Q2PK0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50461850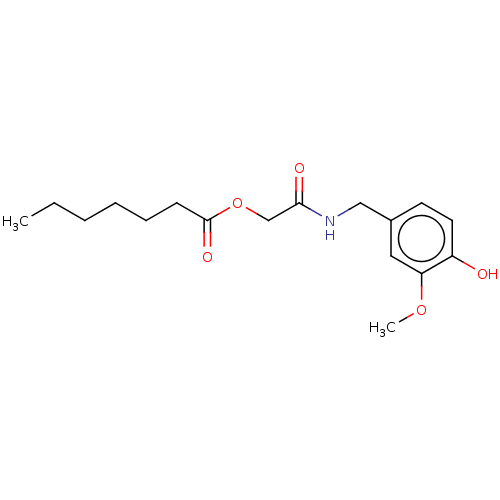 (CHEMBL4226124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Agonist activity at TRPV1 (unknown origin) expressed in human SH-SY5Y cells assessed as increase in calcium influx after 25 mins by Fluo-4 NW dye-bas... | J Med Chem 61: 4436-4455 (2018) Article DOI: 10.1021/acs.jmedchem.8b00109 BindingDB Entry DOI: 10.7270/Q2PK0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50461848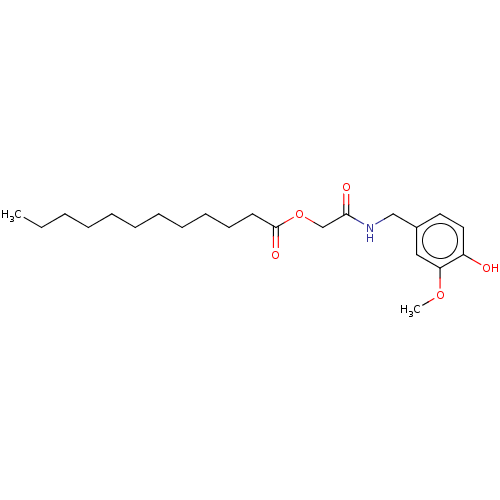 (CHEMBL4226582) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 370 | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Agonist activity at TRPV1 (unknown origin) expressed in human SH-SY5Y cells assessed as increase in calcium influx after 25 mins by Fluo-4 NW dye-bas... | J Med Chem 61: 4436-4455 (2018) Article DOI: 10.1021/acs.jmedchem.8b00109 BindingDB Entry DOI: 10.7270/Q2PK0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50461849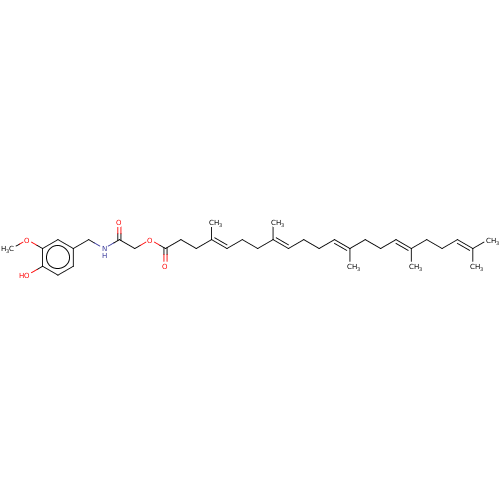 (CHEMBL4225866) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale Curated by ChEMBL | Assay Description Agonist activity at TRPV1 (unknown origin) expressed in human SH-SY5Y cells assessed as increase in calcium influx after 25 mins by Fluo-4 NW dye-bas... | J Med Chem 61: 4436-4455 (2018) Article DOI: 10.1021/acs.jmedchem.8b00109 BindingDB Entry DOI: 10.7270/Q2PK0JSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 51 total ) | Next | Last >> |