 Found 697 hits with Last Name = 'pilatte' and Initial = 'i'
Found 697 hits with Last Name = 'pilatte' and Initial = 'i' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50127246

(8-[Amino-(4-chloro-phenyl)-(3-methyl-3H-imidazol-4...)Show SMILES Cn1cncc1C(N)(c1ccc(Cl)cc1)c1cc2CCn3c2c(c1)c(cc3=O)-c1cccc(Cl)c1 Show InChI InChI=1S/C28H22Cl2N4O/c1-33-16-32-15-25(33)28(31,19-5-7-21(29)8-6-19)20-11-18-9-10-34-26(35)14-23(24(13-20)27(18)34)17-3-2-4-22(30)12-17/h2-8,11-16H,9-10,31H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 1543-7 (2003)
BindingDB Entry DOI: 10.7270/Q2M61JNS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50127246

(8-[Amino-(4-chloro-phenyl)-(3-methyl-3H-imidazol-4...)Show SMILES Cn1cncc1C(N)(c1ccc(Cl)cc1)c1cc2CCn3c2c(c1)c(cc3=O)-c1cccc(Cl)c1 Show InChI InChI=1S/C28H22Cl2N4O/c1-33-16-32-15-25(33)28(31,19-5-7-21(29)8-6-19)20-11-18-9-10-34-26(35)14-23(24(13-20)27(18)34)17-3-2-4-22(30)12-17/h2-8,11-16H,9-10,31H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Geranylgeranylprotein transferase-I at 10 uM |
Bioorg Med Chem Lett 13: 1543-7 (2003)
BindingDB Entry DOI: 10.7270/Q2M61JNS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136383
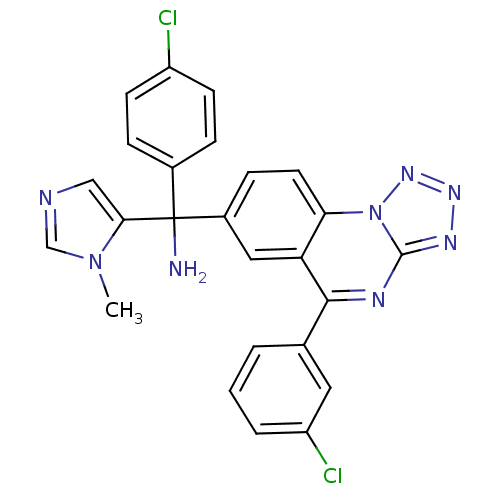
((4-chlorophenyl)(5-(3-chlorophenyl)tetrazolo[1,5-a...)Show SMILES Cn1cncc1C(N)(c1ccc(Cl)cc1)c1ccc2c(c1)c(nc1nnnn21)-c1cccc(Cl)c1 Show InChI InChI=1S/C25H18Cl2N8/c1-34-14-29-13-22(34)25(28,16-5-8-18(26)9-6-16)17-7-10-21-20(12-17)23(15-3-2-4-19(27)11-15)30-24-31-32-33-35(21)24/h2-14H,28H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of Geranylgeranylprotein transferase-I catalyzed incorporation of [3H]-GGPP into biotinYRASNRSCAIL peptide |
Bioorg Med Chem Lett 13: 4365-9 (2003)
BindingDB Entry DOI: 10.7270/Q21R6PX5 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136383

((4-chlorophenyl)(5-(3-chlorophenyl)tetrazolo[1,5-a...)Show SMILES Cn1cncc1C(N)(c1ccc(Cl)cc1)c1ccc2c(c1)c(nc1nnnn21)-c1cccc(Cl)c1 Show InChI InChI=1S/C25H18Cl2N8/c1-34-14-29-13-22(34)25(28,16-5-8-18(26)9-6-16)17-7-10-21-20(12-17)23(15-3-2-4-19(27)11-15)30-24-31-32-33-35(21)24/h2-14H,28H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4365-9 (2003)
BindingDB Entry DOI: 10.7270/Q21R6PX5 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50126335
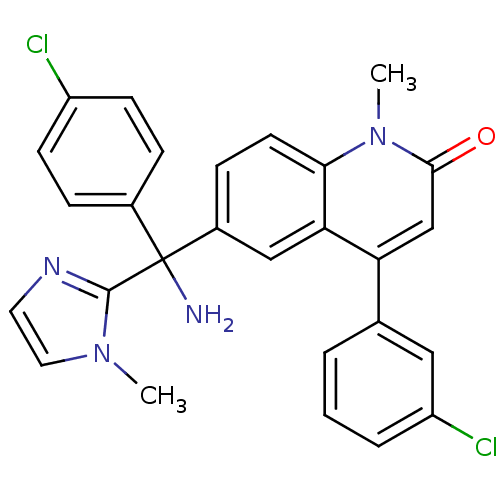
(6-[(R)-Amino-(4-chloro-phenyl)-(3-methyl-3H-imidaz...)Show SMILES Cn1ccnc1C(N)(c1ccc(Cl)cc1)c1ccc2n(C)c(=O)cc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C27H22Cl2N4O/c1-32-13-12-31-26(32)27(30,18-6-9-20(28)10-7-18)19-8-11-24-23(15-19)22(16-25(34)33(24)2)17-4-3-5-21(29)14-17/h3-16H,30H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 1543-7 (2003)
BindingDB Entry DOI: 10.7270/Q2M61JNS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50126335

(6-[(R)-Amino-(4-chloro-phenyl)-(3-methyl-3H-imidaz...)Show SMILES Cn1ccnc1C(N)(c1ccc(Cl)cc1)c1ccc2n(C)c(=O)cc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C27H22Cl2N4O/c1-32-13-12-31-26(32)27(30,18-6-9-20(28)10-7-18)19-8-11-24-23(15-19)22(16-25(34)33(24)2)17-4-3-5-21(29)14-17/h3-16H,30H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4361-4 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FPB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50126335

(6-[(R)-Amino-(4-chloro-phenyl)-(3-methyl-3H-imidaz...)Show SMILES Cn1ccnc1C(N)(c1ccc(Cl)cc1)c1ccc2n(C)c(=O)cc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C27H22Cl2N4O/c1-32-13-12-31-26(32)27(30,18-6-9-20(28)10-7-18)19-8-11-24-23(15-19)22(16-25(34)33(24)2)17-4-3-5-21(29)14-17/h3-16H,30H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4365-9 (2003)
BindingDB Entry DOI: 10.7270/Q21R6PX5 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50127248
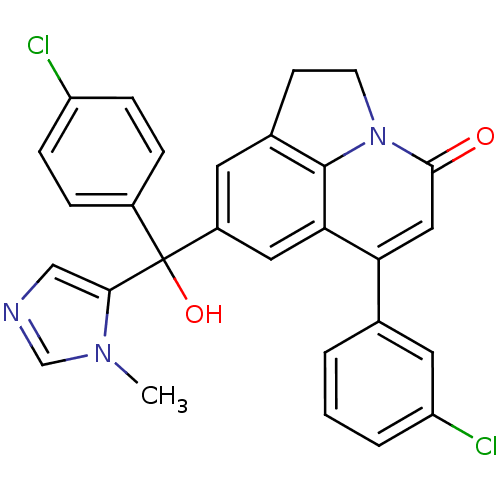
(6-(3-Chloro-phenyl)-8-[(4-chloro-phenyl)-hydroxy-(...)Show SMILES Cn1cncc1C(O)(c1ccc(Cl)cc1)c1cc2CCn3c2c(c1)c(cc3=O)-c1cccc(Cl)c1 Show InChI InChI=1S/C28H21Cl2N3O2/c1-32-16-31-15-25(32)28(35,19-5-7-21(29)8-6-19)20-11-18-9-10-33-26(34)14-23(24(13-20)27(18)33)17-3-2-4-22(30)12-17/h2-8,11-16,35H,9-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 1543-7 (2003)
BindingDB Entry DOI: 10.7270/Q2M61JNS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136377

(4-(3-Chloro-phenyl)-6-[(4-chloro-phenyl)-(4-methyl...)Show SMILES Cn1cnnc1C(c1ccc(Cl)cc1)c1ccc2n(C)c(=O)cc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C26H20Cl2N4O/c1-31-15-29-30-26(31)25(16-6-9-19(27)10-7-16)18-8-11-23-22(13-18)21(14-24(33)32(23)2)17-4-3-5-20(28)12-17/h3-15,25H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4361-4 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FPB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136375

(6-[Amino-(4-chloro-phenyl)-(4-methyl-4H-[1,2,4]tri...)Show SMILES Cn1cnnc1C(N)(c1ccc(Cl)cc1)c1ccc2n(C)c(=O)cc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C26H21Cl2N5O/c1-32-15-30-31-25(32)26(29,17-6-9-19(27)10-7-17)18-8-11-23-22(13-18)21(14-24(34)33(23)2)16-4-3-5-20(28)12-16/h3-15H,29H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4361-4 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FPB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136385
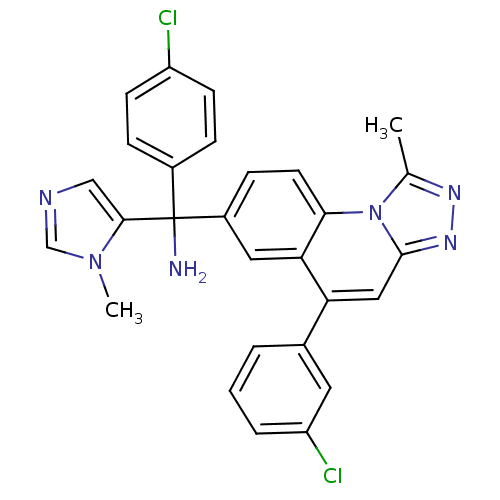
(C-(4-Chloro-phenyl)-C-[5-(3-chloro-phenyl)-1-methy...)Show SMILES Cc1nnc2cc(-c3cccc(Cl)c3)c3cc(ccc3n12)C(N)(c1cncn1C)c1ccc(Cl)cc1 Show InChI InChI=1S/C28H22Cl2N6/c1-17-33-34-27-14-23(18-4-3-5-22(30)12-18)24-13-20(8-11-25(24)36(17)27)28(31,26-15-32-16-35(26)2)19-6-9-21(29)10-7-19/h3-16H,31H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4365-9 (2003)
BindingDB Entry DOI: 10.7270/Q21R6PX5 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50127252
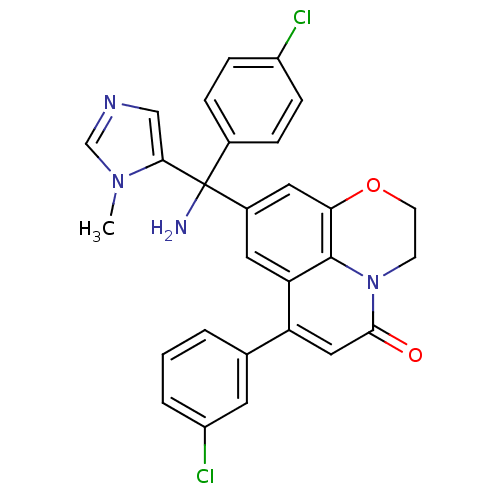
(8-[Amino-(4-chloro-phenyl)-(3-methyl-3H-imidazol-4...)Show SMILES Cn1cncc1C(N)(c1ccc(Cl)cc1)c1cc2OCCn3c2c(c1)c(cc3=O)-c1cccc(Cl)c1 Show InChI InChI=1S/C28H22Cl2N4O2/c1-33-16-32-15-25(33)28(31,18-5-7-20(29)8-6-18)19-12-23-22(17-3-2-4-21(30)11-17)14-26(35)34-9-10-36-24(13-19)27(23)34/h2-8,11-16H,9-10,31H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 1543-7 (2003)
BindingDB Entry DOI: 10.7270/Q2M61JNS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136384

((4-chlorophenyl)(5-(3-chlorophenyl)tetrazolo[1,5-a...)Show SMILES Cn1cncc1C(N)(c1ccc(Cl)cc1)c1ccc2c(c1)c(cc1nnnn21)-c1cccc(Cl)c1 Show InChI InChI=1S/C26H19Cl2N7/c1-34-15-30-14-24(34)26(29,17-5-8-19(27)9-6-17)18-7-10-23-22(12-18)21(13-25-31-32-33-35(23)25)16-3-2-4-20(28)11-16/h2-15H,29H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4365-9 (2003)
BindingDB Entry DOI: 10.7270/Q21R6PX5 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136384

((4-chlorophenyl)(5-(3-chlorophenyl)tetrazolo[1,5-a...)Show SMILES Cn1cncc1C(N)(c1ccc(Cl)cc1)c1ccc2c(c1)c(cc1nnnn21)-c1cccc(Cl)c1 Show InChI InChI=1S/C26H19Cl2N7/c1-34-15-30-14-24(34)26(29,17-5-8-19(27)9-6-17)18-7-10-23-22(12-18)21(13-25-31-32-33-35(23)25)16-3-2-4-20(28)11-16/h2-15H,29H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of Geranylgeranylprotein transferase-I catalyzed incorporation of [3H]-GGPP into biotinYRASNRSCAIL peptide |
Bioorg Med Chem Lett 13: 4365-9 (2003)
BindingDB Entry DOI: 10.7270/Q21R6PX5 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50127254
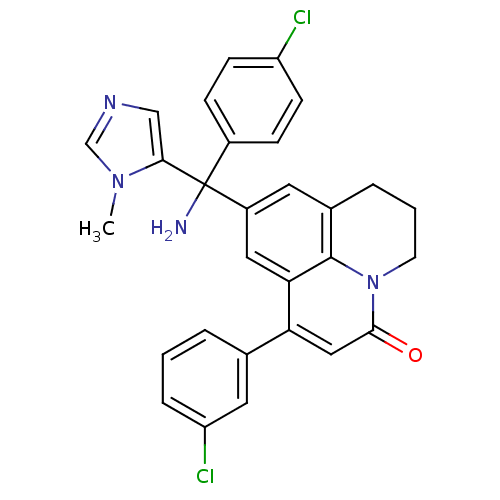
(9-[Amino-(4-chloro-phenyl)-(3-methyl-3H-imidazol-4...)Show SMILES Cn1cncc1C(N)(c1ccc(Cl)cc1)c1cc2CCCn3c2c(c1)c(cc3=O)-c1cccc(Cl)c1 Show InChI InChI=1S/C29H24Cl2N4O/c1-34-17-33-16-26(34)29(32,20-7-9-22(30)10-8-20)21-12-19-5-3-11-35-27(36)15-24(25(14-21)28(19)35)18-4-2-6-23(31)13-18/h2,4,6-10,12-17H,3,5,11,32H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 1543-7 (2003)
BindingDB Entry DOI: 10.7270/Q2M61JNS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136379
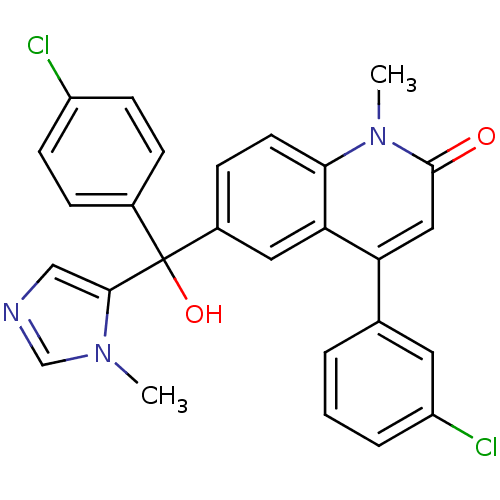
(4-(3-Chloro-phenyl)-6-[(4-chloro-phenyl)-hydroxy-(...)Show SMILES Cn1cncc1C(O)(c1ccc(Cl)cc1)c1ccc2n(C)c(=O)cc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C27H21Cl2N3O2/c1-31-16-30-15-25(31)27(34,18-6-9-20(28)10-7-18)19-8-11-24-23(13-19)22(14-26(33)32(24)2)17-4-3-5-21(29)12-17/h3-16,34H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4361-4 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FPB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50304782

(CHEMBL609583 | N-hydroxy-2-(4-(naphthalen-2-ylsulf...)Show SMILES ONC(=O)c1cnc(nc1)N1CCN(CC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N5O4S/c25-18(22-26)16-12-20-19(21-13-16)23-7-9-24(10-8-23)29(27,28)17-6-5-14-3-1-2-4-15(14)11-17/h1-6,11-13,26H,7-10H2,(H,22,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC11 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50304782

(CHEMBL609583 | N-hydroxy-2-(4-(naphthalen-2-ylsulf...)Show SMILES ONC(=O)c1cnc(nc1)N1CCN(CC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N5O4S/c25-18(22-26)16-12-20-19(21-13-16)23-7-9-24(10-8-23)29(27,28)17-6-5-14-3-1-2-4-15(14)11-17/h1-6,11-13,26H,7-10H2,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136389

(C-(4-Chloro-phenyl)-C-[5-(3-chloro-phenyl)-[1,2,4]...)Show SMILES Cn1cncc1C(N)(c1ccc(Cl)cc1)c1ccc2c(c1)c(nc1nncn21)-c1cccc(Cl)c1 Show InChI InChI=1S/C26H19Cl2N7/c1-34-14-30-13-23(34)26(29,17-5-8-19(27)9-6-17)18-7-10-22-21(12-18)24(16-3-2-4-20(28)11-16)32-25-33-31-15-35(22)25/h2-15H,29H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4365-9 (2003)
BindingDB Entry DOI: 10.7270/Q21R6PX5 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136376
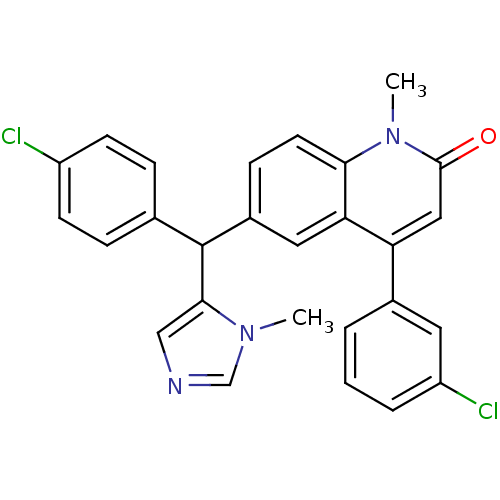
(4-(3-Chloro-phenyl)-6-[(4-chloro-phenyl)-(3-methyl...)Show SMILES Cn1cncc1C(c1ccc(Cl)cc1)c1ccc2n(C)c(=O)cc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C27H21Cl2N3O/c1-31-16-30-15-25(31)27(17-6-9-20(28)10-7-17)19-8-11-24-23(13-19)22(14-26(33)32(24)2)18-4-3-5-21(29)12-18/h3-16,27H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4361-4 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FPB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50127255
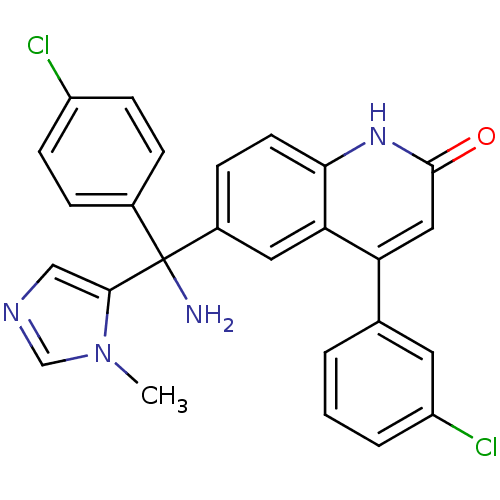
(6-[Amino-(4-chloro-phenyl)-(3-methyl-3H-imidazol-4...)Show SMILES Cn1cncc1C(N)(c1ccc(Cl)cc1)c1ccc2[nH]c(=O)cc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C26H20Cl2N4O/c1-32-15-30-14-24(32)26(29,17-5-8-19(27)9-6-17)18-7-10-23-22(12-18)21(13-25(33)31-23)16-3-2-4-20(28)11-16/h2-15H,29H2,1H3,(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 1543-7 (2003)
BindingDB Entry DOI: 10.7270/Q2M61JNS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136384

((4-chlorophenyl)(5-(3-chlorophenyl)tetrazolo[1,5-a...)Show SMILES Cn1cncc1C(N)(c1ccc(Cl)cc1)c1ccc2c(c1)c(cc1nnnn21)-c1cccc(Cl)c1 Show InChI InChI=1S/C26H19Cl2N7/c1-34-15-30-14-24(34)26(29,17-5-8-19(27)9-6-17)18-7-10-23-22(12-18)21(13-25-31-32-33-35(23)25)16-3-2-4-20(28)11-16/h2-15H,29H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of Geranylgeranylprotein transferase-I catalyzed incorporation of [3H]-GGPP into biotinYRASNRSCAIL peptide |
Bioorg Med Chem Lett 13: 4365-9 (2003)
BindingDB Entry DOI: 10.7270/Q21R6PX5 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136380

(4-(3-Chloro-phenyl)-6-[1-(4-chloro-phenyl)-1-(3-me...)Show SMILES Cn1cncc1C(C)(c1ccc(Cl)cc1)c1ccc2n(C)c(=O)cc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C28H23Cl2N3O/c1-28(26-16-31-17-32(26)2,19-7-10-21(29)11-8-19)20-9-12-25-24(14-20)23(15-27(34)33(25)3)18-5-4-6-22(30)13-18/h4-17H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4361-4 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FPB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136378
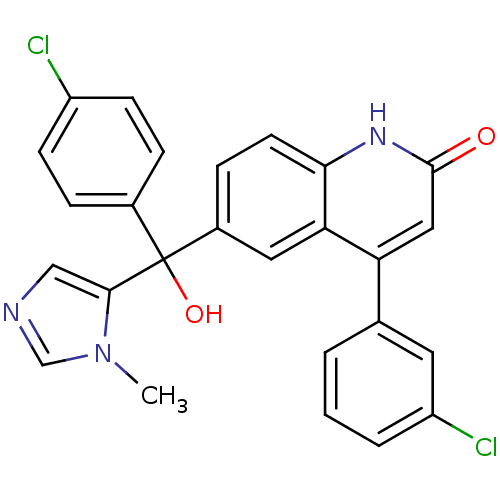
(4-(3-Chloro-phenyl)-6-[(4-chloro-phenyl)-hydroxy-(...)Show SMILES Cn1cncc1C(O)(c1ccc(Cl)cc1)c1ccc2[nH]c(=O)cc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C26H19Cl2N3O2/c1-31-15-29-14-24(31)26(33,17-5-8-19(27)9-6-17)18-7-10-23-22(12-18)21(13-25(32)30-23)16-3-2-4-20(28)11-16/h2-15,33H,1H3,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4361-4 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FPB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50127247
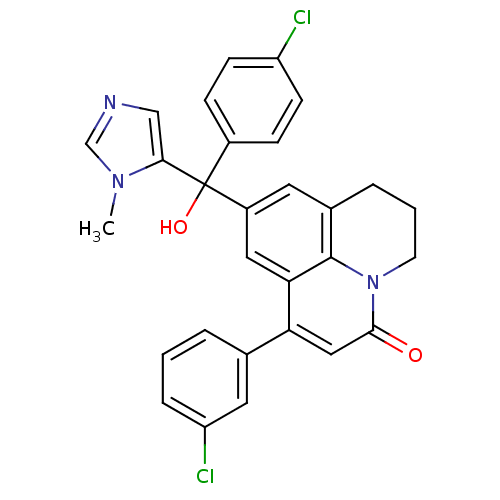
(1-(3-Chloro-phenyl)-9-[(4-chloro-phenyl)-hydroxy-(...)Show SMILES Cn1cncc1C(O)(c1ccc(Cl)cc1)c1cc2CCCn3c2c(c1)c(cc3=O)-c1cccc(Cl)c1 Show InChI InChI=1S/C29H23Cl2N3O2/c1-33-17-32-16-26(33)29(36,20-7-9-22(30)10-8-20)21-12-19-5-3-11-34-27(35)15-24(25(14-21)28(19)34)18-4-2-6-23(31)13-18/h2,4,6-10,12-17,36H,3,5,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 1543-7 (2003)
BindingDB Entry DOI: 10.7270/Q2M61JNS |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50304782

(CHEMBL609583 | N-hydroxy-2-(4-(naphthalen-2-ylsulf...)Show SMILES ONC(=O)c1cnc(nc1)N1CCN(CC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N5O4S/c25-18(22-26)16-12-20-19(21-13-16)23-7-9-24(10-8-23)29(27,28)17-6-5-14-3-1-2-4-15(14)11-17/h1-6,11-13,26H,7-10H2,(H,22,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC10 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50304782

(CHEMBL609583 | N-hydroxy-2-(4-(naphthalen-2-ylsulf...)Show SMILES ONC(=O)c1cnc(nc1)N1CCN(CC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N5O4S/c25-18(22-26)16-12-20-19(21-13-16)23-7-9-24(10-8-23)29(27,28)17-6-5-14-3-1-2-4-15(14)11-17/h1-6,11-13,26H,7-10H2,(H,22,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC4 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136386

(C-(4-Chloro-phenyl)-C-[5-(3-chloro-phenyl)-imidazo...)Show SMILES Cn1cncc1C(N)(c1ccc(Cl)cc1)c1ccc2c(c1)c(nc1nccn21)-c1cccc(Cl)c1 Show InChI InChI=1S/C27H20Cl2N6/c1-34-16-31-15-24(34)27(30,18-5-8-20(28)9-6-18)19-7-10-23-22(14-19)25(17-3-2-4-21(29)13-17)33-26-32-11-12-35(23)26/h2-16H,30H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4365-9 (2003)
BindingDB Entry DOI: 10.7270/Q21R6PX5 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136382

(4-(3-Chloro-phenyl)-6-[1-(4-chloro-phenyl)-1-(4-me...)Show SMILES Cn1cnnc1C(C)(c1ccc(Cl)cc1)c1ccc2n(C)c(=O)cc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C27H22Cl2N4O/c1-27(26-31-30-16-32(26)2,18-7-10-20(28)11-8-18)19-9-12-24-23(14-19)22(15-25(34)33(24)3)17-5-4-6-21(29)13-17/h4-16H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4361-4 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FPB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136381

(4-(3-Chloro-phenyl)-6-[(4-chloro-phenyl)-hydroxy-(...)Show SMILES Cn1cnnc1C(O)(c1ccc(Cl)cc1)c1ccc2n(C)c(=O)cc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C26H20Cl2N4O2/c1-31-15-29-30-25(31)26(34,17-6-9-19(27)10-7-17)18-8-11-23-22(13-18)21(14-24(33)32(23)2)16-4-3-5-20(28)12-16/h3-15,34H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4361-4 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FPB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50127257
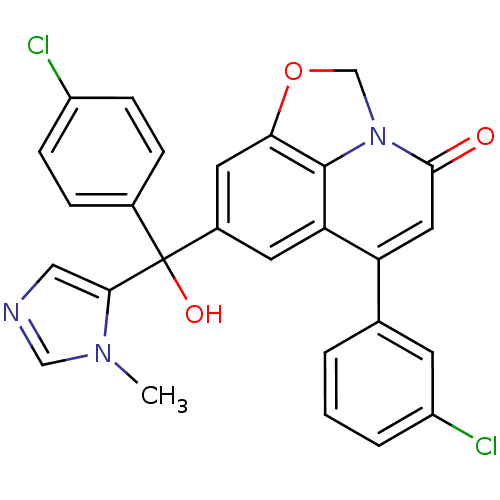
(6-(3-Chloro-phenyl)-8-[(4-chloro-phenyl)-hydroxy-(...)Show SMILES Cn1cncc1C(O)(c1ccc(Cl)cc1)c1cc2OCn3c2c(c1)c(cc3=O)-c1cccc(Cl)c1 Show InChI InChI=1S/C27H19Cl2N3O3/c1-31-14-30-13-24(31)27(34,17-5-7-19(28)8-6-17)18-10-22-21(16-3-2-4-20(29)9-16)12-25(33)32-15-35-23(11-18)26(22)32/h2-14,34H,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 1543-7 (2003)
BindingDB Entry DOI: 10.7270/Q2M61JNS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50127249
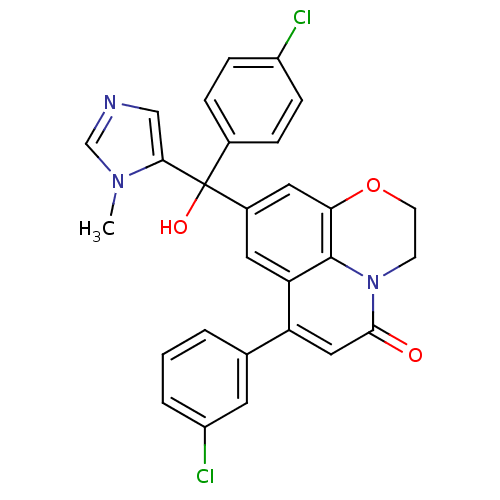
(6-(3-Chloro-phenyl)-8-[(4-chloro-phenyl)-hydroxy-(...)Show SMILES Cn1cncc1C(O)(c1ccc(Cl)cc1)c1cc2OCCn3c2c(c1)c(cc3=O)-c1cccc(Cl)c1 Show InChI InChI=1S/C28H21Cl2N3O3/c1-32-16-31-15-25(32)28(35,18-5-7-20(29)8-6-18)19-12-23-22(17-3-2-4-21(30)11-17)14-26(34)33-9-10-36-24(13-19)27(23)33/h2-8,11-16,35H,9-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 1543-7 (2003)
BindingDB Entry DOI: 10.7270/Q2M61JNS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50127250
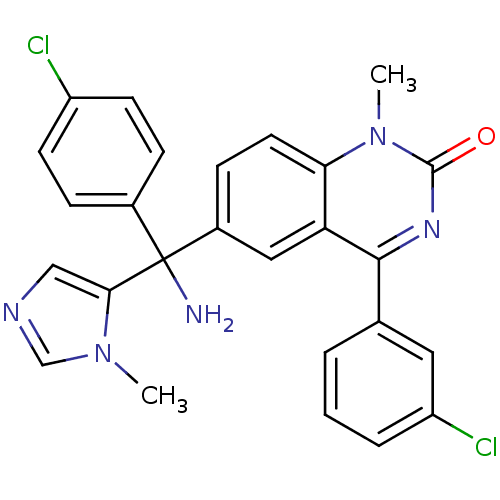
(6-[Amino-(4-chloro-phenyl)-(3-methyl-3H-imidazol-4...)Show SMILES Cn1cncc1C(N)(c1ccc(Cl)cc1)c1ccc2n(C)c(=O)nc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C26H21Cl2N5O/c1-32-15-30-14-23(32)26(29,17-6-9-19(27)10-7-17)18-8-11-22-21(13-18)24(31-25(34)33(22)2)16-4-3-5-20(28)12-16/h3-15H,29H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 1543-7 (2003)
BindingDB Entry DOI: 10.7270/Q2M61JNS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136387

(C-(4-Chloro-phenyl)-C-[5-(3-chloro-phenyl)-imidazo...)Show SMILES Cn1cncc1C(N)(c1ccc(Cl)cc1)c1ccc2c(c1)c(cc1nccn21)-c1cccc(Cl)c1 Show InChI InChI=1S/C28H21Cl2N5/c1-34-17-32-16-26(34)28(31,19-5-8-21(29)9-6-19)20-7-10-25-24(14-20)23(15-27-33-11-12-35(25)27)18-3-2-4-22(30)13-18/h2-17H,31H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4365-9 (2003)
BindingDB Entry DOI: 10.7270/Q21R6PX5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50304782

(CHEMBL609583 | N-hydroxy-2-(4-(naphthalen-2-ylsulf...)Show SMILES ONC(=O)c1cnc(nc1)N1CCN(CC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N5O4S/c25-18(22-26)16-12-20-19(21-13-16)23-7-9-24(10-8-23)29(27,28)17-6-5-14-3-1-2-4-15(14)11-17/h1-6,11-13,26H,7-10H2,(H,22,25) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC5 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50304783
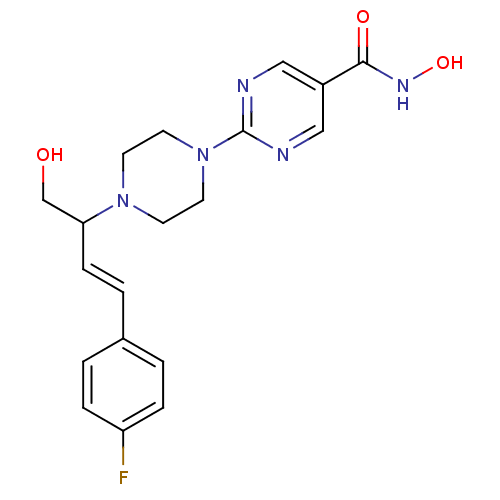
((+)-2-(4-(4-(4-fluorophenyl)-1-hydroxybut-3-en-2-y...)Show SMILES OCC(\C=C\c1ccc(F)cc1)N1CCN(CC1)c1ncc(cn1)C(=O)NO Show InChI InChI=1S/C19H22FN5O3/c20-16-4-1-14(2-5-16)3-6-17(13-26)24-7-9-25(10-8-24)19-21-11-15(12-22-19)18(27)23-28/h1-6,11-12,17,26,28H,7-10,13H2,(H,23,27)/b6-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50304782

(CHEMBL609583 | N-hydroxy-2-(4-(naphthalen-2-ylsulf...)Show SMILES ONC(=O)c1cnc(nc1)N1CCN(CC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N5O4S/c25-18(22-26)16-12-20-19(21-13-16)23-7-9-24(10-8-23)29(27,28)17-6-5-14-3-1-2-4-15(14)11-17/h1-6,11-13,26H,7-10H2,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC2 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50304782

(CHEMBL609583 | N-hydroxy-2-(4-(naphthalen-2-ylsulf...)Show SMILES ONC(=O)c1cnc(nc1)N1CCN(CC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N5O4S/c25-18(22-26)16-12-20-19(21-13-16)23-7-9-24(10-8-23)29(27,28)17-6-5-14-3-1-2-4-15(14)11-17/h1-6,11-13,26H,7-10H2,(H,22,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC3 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM335695
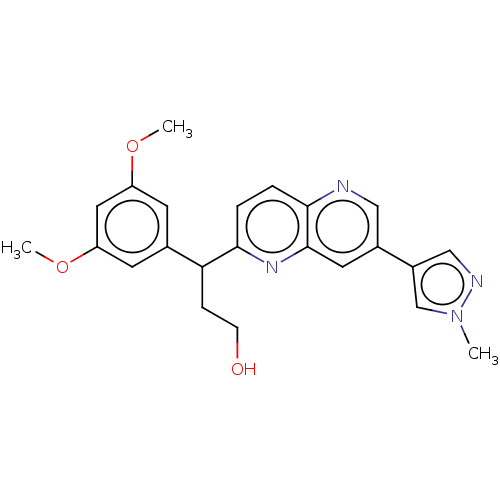
(US9737544, Compound 13)Show SMILES COc1cc(OC)cc(c1)C(CCO)c1ccc2ncc(cc2n1)-c1cnn(C)c1 Show InChI InChI=1S/C23H24N4O3/c1-27-14-17(13-25-27)16-10-23-22(24-12-16)5-4-21(26-23)20(6-7-28)15-8-18(29-2)11-19(9-15)30-3/h4-5,8-14,20,28H,6-7H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED
US Patent
| Assay Description
In a final reaction volume of 30 μL, FGFR3 (h) (40 ng/ml) was incubated with 50 mM HEPES pH 7.5, 6 mM MnCl2, 1 mM DTT, 0.1 mM Na3VO4, 0.01% Trit... |
US Patent US9737544 (2017)
BindingDB Entry DOI: 10.7270/Q2H70HZF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50304783

((+)-2-(4-(4-(4-fluorophenyl)-1-hydroxybut-3-en-2-y...)Show SMILES OCC(\C=C\c1ccc(F)cc1)N1CCN(CC1)c1ncc(cn1)C(=O)NO Show InChI InChI=1S/C19H22FN5O3/c20-16-4-1-14(2-5-16)3-6-17(13-26)24-7-9-25(10-8-24)19-21-11-15(12-22-19)18(27)23-28/h1-6,11-12,17,26,28H,7-10,13H2,(H,23,27)/b6-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC2 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50304783

((+)-2-(4-(4-(4-fluorophenyl)-1-hydroxybut-3-en-2-y...)Show SMILES OCC(\C=C\c1ccc(F)cc1)N1CCN(CC1)c1ncc(cn1)C(=O)NO Show InChI InChI=1S/C19H22FN5O3/c20-16-4-1-14(2-5-16)3-6-17(13-26)24-7-9-25(10-8-24)19-21-11-15(12-22-19)18(27)23-28/h1-6,11-12,17,26,28H,7-10,13H2,(H,23,27)/b6-3+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC11 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50127256

(7-[Amino-(4-chloro-phenyl)-(3-methyl-3H-imidazol-4...)Show SMILES CN1c2ccc(cc2C(=NCC1=O)c1cccc(Cl)c1)C(N)(c1cncn1C)c1ccc(Cl)cc1 |c:9| Show InChI InChI=1S/C27H23Cl2N5O/c1-33-16-31-14-24(33)27(30,18-6-9-20(28)10-7-18)19-8-11-23-22(13-19)26(32-15-25(35)34(23)2)17-4-3-5-21(29)12-17/h3-14,16H,15,30H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of farnesyl transferase at 0.1 uM |
Bioorg Med Chem Lett 13: 1543-7 (2003)
BindingDB Entry DOI: 10.7270/Q2M61JNS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50304783

((+)-2-(4-(4-(4-fluorophenyl)-1-hydroxybut-3-en-2-y...)Show SMILES OCC(\C=C\c1ccc(F)cc1)N1CCN(CC1)c1ncc(cn1)C(=O)NO Show InChI InChI=1S/C19H22FN5O3/c20-16-4-1-14(2-5-16)3-6-17(13-26)24-7-9-25(10-8-24)19-21-11-15(12-22-19)18(27)23-28/h1-6,11-12,17,26,28H,7-10,13H2,(H,23,27)/b6-3+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC5 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136383

((4-chlorophenyl)(5-(3-chlorophenyl)tetrazolo[1,5-a...)Show SMILES Cn1cncc1C(N)(c1ccc(Cl)cc1)c1ccc2c(c1)c(nc1nnnn21)-c1cccc(Cl)c1 Show InChI InChI=1S/C25H18Cl2N8/c1-34-14-29-13-22(34)25(28,16-5-8-18(26)9-6-16)17-7-10-21-20(12-17)23(15-3-2-4-19(27)11-15)30-24-31-32-33-35(21)24/h2-14H,28H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of Geranylgeranylprotein transferase-I catalyzed incorporation of [3H]-GGPP into biotinYRASNRSCAIL peptide at 10 uM |
Bioorg Med Chem Lett 13: 4365-9 (2003)
BindingDB Entry DOI: 10.7270/Q21R6PX5 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50136374

(4-(3-Chloro-phenyl)-6-[(4-chloro-phenyl)-hydroxy-(...)Show SMILES Cn1cnnc1C(O)(c1ccc(Cl)cc1)c1ccc2[nH]c(=O)cc(-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C25H18Cl2N4O2/c1-31-14-28-30-24(31)25(33,16-5-8-18(26)9-6-16)17-7-10-22-21(12-17)20(13-23(32)29-22)15-3-2-4-19(27)11-15/h2-14,33H,1H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Department Johnson & Johnson Pharmaceutical Research & Development (J&JPRD)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated lamin B peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 4361-4 (2003)
BindingDB Entry DOI: 10.7270/Q25H7FPB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50304782

(CHEMBL609583 | N-hydroxy-2-(4-(naphthalen-2-ylsulf...)Show SMILES ONC(=O)c1cnc(nc1)N1CCN(CC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N5O4S/c25-18(22-26)16-12-20-19(21-13-16)23-7-9-24(10-8-23)29(27,28)17-6-5-14-3-1-2-4-15(14)11-17/h1-6,11-13,26H,7-10H2,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC8 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50304782

(CHEMBL609583 | N-hydroxy-2-(4-(naphthalen-2-ylsulf...)Show SMILES ONC(=O)c1cnc(nc1)N1CCN(CC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N5O4S/c25-18(22-26)16-12-20-19(21-13-16)23-7-9-24(10-8-23)29(27,28)17-6-5-14-3-1-2-4-15(14)11-17/h1-6,11-13,26H,7-10H2,(H,22,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC9 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50127258
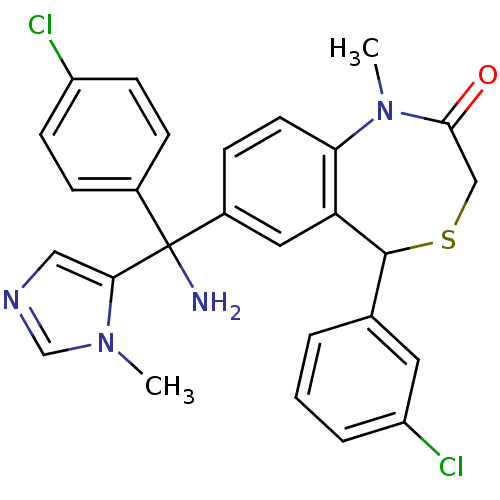
(2-[Amino-(4-chloro-phenyl)-(3-methyl-3H-imidazol-4...)Show SMILES CN1c2ccc(cc2C(SCC1=O)c1cccc(Cl)c1)C(N)(c1cncn1C)c1ccc(Cl)cc1 Show InChI InChI=1S/C27H24Cl2N4OS/c1-32-16-31-14-24(32)27(30,18-6-9-20(28)10-7-18)19-8-11-23-22(13-19)26(35-15-25(34)33(23)2)17-4-3-5-21(29)12-17/h3-14,16,26H,15,30H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotinylated laminB peptide by farnesyl transferase |
Bioorg Med Chem Lett 13: 1543-7 (2003)
BindingDB Entry DOI: 10.7270/Q2M61JNS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50304783

((+)-2-(4-(4-(4-fluorophenyl)-1-hydroxybut-3-en-2-y...)Show SMILES OCC(\C=C\c1ccc(F)cc1)N1CCN(CC1)c1ncc(cn1)C(=O)NO Show InChI InChI=1S/C19H22FN5O3/c20-16-4-1-14(2-5-16)3-6-17(13-26)24-7-9-25(10-8-24)19-21-11-15(12-22-19)18(27)23-28/h1-6,11-12,17,26,28H,7-10,13H2,(H,23,27)/b6-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC8 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50304783

((+)-2-(4-(4-(4-fluorophenyl)-1-hydroxybut-3-en-2-y...)Show SMILES OCC(\C=C\c1ccc(F)cc1)N1CCN(CC1)c1ncc(cn1)C(=O)NO Show InChI InChI=1S/C19H22FN5O3/c20-16-4-1-14(2-5-16)3-6-17(13-26)24-7-9-25(10-8-24)19-21-11-15(12-22-19)18(27)23-28/h1-6,11-12,17,26,28H,7-10,13H2,(H,23,27)/b6-3+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC3 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data