 Found 465 hits with Last Name = 'pilla' and Initial = 'm'
Found 465 hits with Last Name = 'pilla' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM142234

(US8933098, 1 | US8933099, 1)Show SMILES Cc1ccc(nc1C(=O)Nc1c(C)cc(cc1C)C(O)=O)N1CCC(O)CC1 Show InChI InChI=1S/C21H25N3O4/c1-12-4-5-17(24-8-6-16(25)7-9-24)22-19(12)20(26)23-18-13(2)10-15(21(27)28)11-14(18)3/h4-5,10-11,16,25H,6-9H2,1-3H3,(H,23,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Eli Lilly and Company
US Patent
| Assay Description
hEP1 and hEP4 membranes are prepared from recombinant HEK293 cells stably expressing human EP1 (Genbank accession number AY275470) or EP4 (Genbank ac... |
US Patent US8933098 (2015)
BindingDB Entry DOI: 10.7270/Q2DJ5DB6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM142234

(US8933098, 1 | US8933099, 1)Show SMILES Cc1ccc(nc1C(=O)Nc1c(C)cc(cc1C)C(O)=O)N1CCC(O)CC1 Show InChI InChI=1S/C21H25N3O4/c1-12-4-5-17(24-8-6-16(25)7-9-24)22-19(12)20(26)23-18-13(2)10-15(21(27)28)11-14(18)3/h4-5,10-11,16,25H,6-9H2,1-3H3,(H,23,26)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| >1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Eli Lilly and Company
US Patent
| Assay Description
hEP1 and hEP4 membranes are prepared from recombinant HEK293 cells stably expressing human EP1 (Genbank accession number AY275470) or EP4 (Genbank ac... |
US Patent US8933098 (2015)
BindingDB Entry DOI: 10.7270/Q2DJ5DB6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM142234

(US8933098, 1 | US8933099, 1)Show SMILES Cc1ccc(nc1C(=O)Nc1c(C)cc(cc1C)C(O)=O)N1CCC(O)CC1 Show InChI InChI=1S/C21H25N3O4/c1-12-4-5-17(24-8-6-16(25)7-9-24)22-19(12)20(26)23-18-13(2)10-15(21(27)28)11-14(18)3/h4-5,10-11,16,25H,6-9H2,1-3H3,(H,23,26)(H,27,28) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| >1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Eli Lilly and Company
US Patent
| Assay Description
hEP1 and hEP4 membranes are prepared from recombinant HEK293 cells stably expressing human EP1 (Genbank accession number AY275470) or EP4 (Genbank ac... |
US Patent US8933098 (2015)
BindingDB Entry DOI: 10.7270/Q2DJ5DB6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM142234

(US8933098, 1 | US8933099, 1)Show SMILES Cc1ccc(nc1C(=O)Nc1c(C)cc(cc1C)C(O)=O)N1CCC(O)CC1 Show InChI InChI=1S/C21H25N3O4/c1-12-4-5-17(24-8-6-16(25)7-9-24)22-19(12)20(26)23-18-13(2)10-15(21(27)28)11-14(18)3/h4-5,10-11,16,25H,6-9H2,1-3H3,(H,23,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| >1.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Eli Lilly and Company
US Patent
| Assay Description
hEP1 and hEP4 membranes are prepared from recombinant HEK293 cells stably expressing human EP1 (Genbank accession number AY275470) or EP4 (Genbank ac... |
US Patent US8933098 (2015)
BindingDB Entry DOI: 10.7270/Q2DJ5DB6 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50483163

(CHEMBL1630067)Show InChI InChI=1S/C18H16N4O3S/c1-11-15(12(2)24-18(23)19-13-7-4-3-5-8-13)25-17-20-16(21-22(11)17)14-9-6-10-26-14/h3-10,12H,1-2H3,(H,19,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilization |
Bioorg Med Chem Lett 20: 7521-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.120
BindingDB Entry DOI: 10.7270/Q2KH0R5D |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50483151
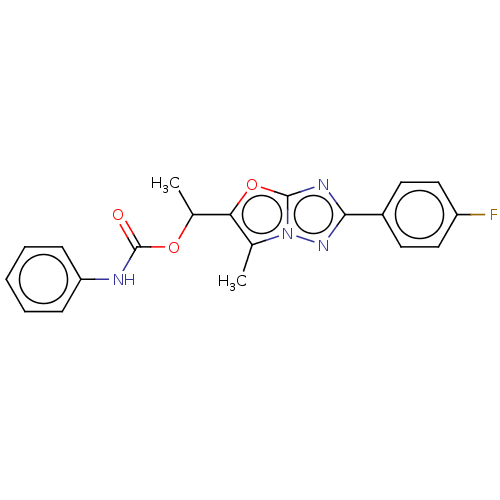
(CHEMBL1630068)Show SMILES CC(OC(=O)Nc1ccccc1)c1oc2nc(nn2c1C)-c1ccc(F)cc1 Show InChI InChI=1S/C20H17FN4O3/c1-12-17(13(2)27-20(26)22-16-6-4-3-5-7-16)28-19-23-18(24-25(12)19)14-8-10-15(21)11-9-14/h3-11,13H,1-2H3,(H,22,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilization |
Bioorg Med Chem Lett 20: 7521-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.120
BindingDB Entry DOI: 10.7270/Q2KH0R5D |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50483149
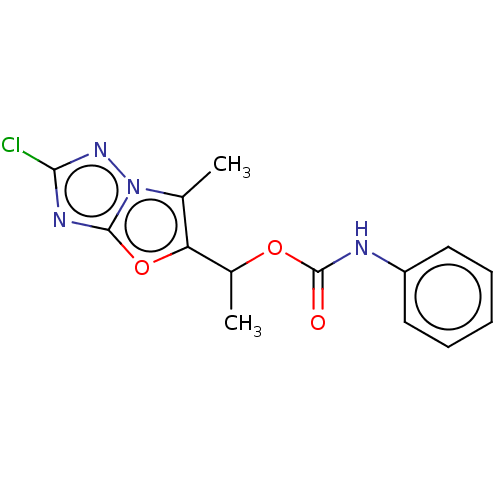
(CHEMBL1630065)Show InChI InChI=1S/C14H13ClN4O3/c1-8-11(22-13-17-12(15)18-19(8)13)9(2)21-14(20)16-10-6-4-3-5-7-10/h3-7,9H,1-2H3,(H,16,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilization |
Bioorg Med Chem Lett 20: 7521-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.120
BindingDB Entry DOI: 10.7270/Q2KH0R5D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50411390
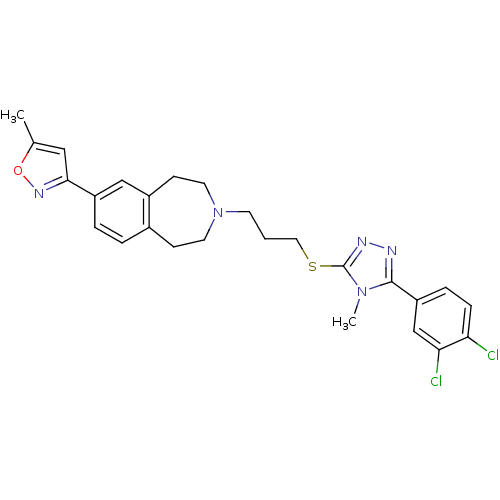
(CHEMBL397222)Show SMILES Cc1cc(no1)-c1ccc2CCN(CCCSc3nnc(-c4ccc(Cl)c(Cl)c4)n3C)CCc2c1 Show InChI InChI=1S/C26H27Cl2N5OS/c1-17-14-24(31-34-17)20-5-4-18-8-11-33(12-9-19(18)15-20)10-3-13-35-26-30-29-25(32(26)2)21-6-7-22(27)23(28)16-21/h4-7,14-16H,3,8-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in CHO cells |
J Med Chem 50: 5076-89 (2007)
Article DOI: 10.1021/jm0705612
BindingDB Entry DOI: 10.7270/Q2DF6QZB |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50483155
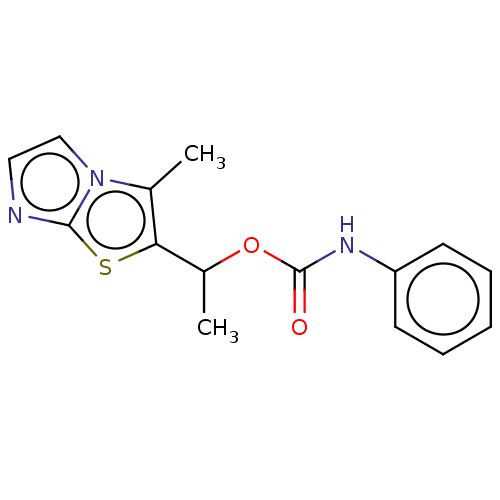
(CHEMBL1630076)Show InChI InChI=1S/C15H15N3O2S/c1-10-13(21-14-16-8-9-18(10)14)11(2)20-15(19)17-12-6-4-3-5-7-12/h3-9,11H,1-2H3,(H,17,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilization |
Bioorg Med Chem Lett 20: 7521-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.120
BindingDB Entry DOI: 10.7270/Q2KH0R5D |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50483150
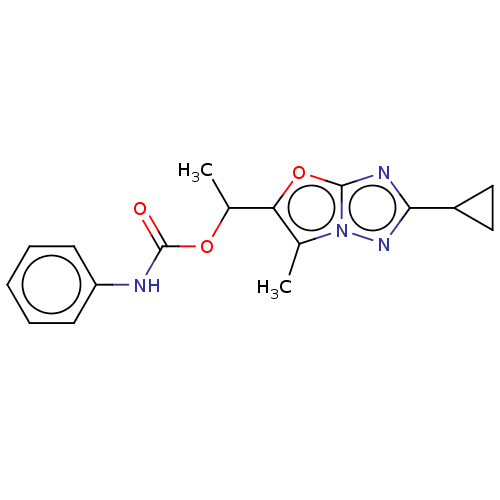
(CHEMBL1630066)Show InChI InChI=1S/C17H18N4O3/c1-10-14(24-16-19-15(12-8-9-12)20-21(10)16)11(2)23-17(22)18-13-6-4-3-5-7-13/h3-7,11-12H,8-9H2,1-2H3,(H,18,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilization |
Bioorg Med Chem Lett 20: 7521-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.120
BindingDB Entry DOI: 10.7270/Q2KH0R5D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50411385
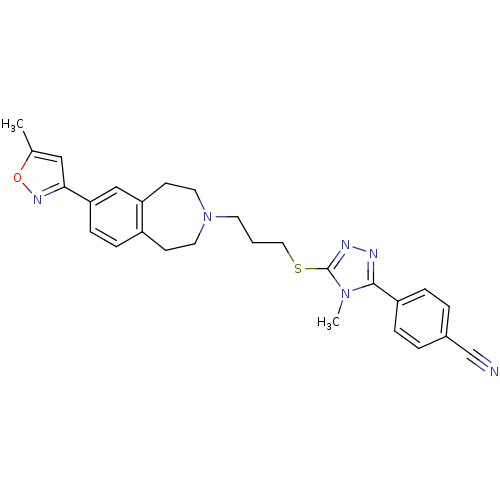
(CHEMBL243896)Show SMILES Cc1cc(no1)-c1ccc2CCN(CCCSc3nnc(-c4ccc(cc4)C#N)n3C)CCc2c1 Show InChI InChI=1S/C27H28N6OS/c1-19-16-25(31-34-19)24-9-8-21-10-13-33(14-11-23(21)17-24)12-3-15-35-27-30-29-26(32(27)2)22-6-4-20(18-28)5-7-22/h4-9,16-17H,3,10-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in CHO cells |
J Med Chem 50: 5076-89 (2007)
Article DOI: 10.1021/jm0705612
BindingDB Entry DOI: 10.7270/Q2DF6QZB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50411382
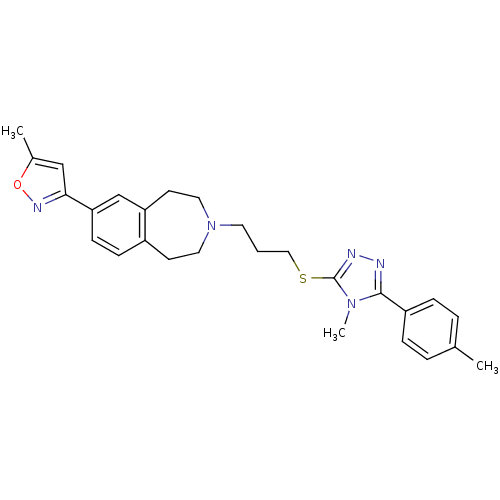
(CHEMBL395742)Show SMILES Cc1cc(no1)-c1ccc2CCN(CCCSc3nnc(-c4ccc(C)cc4)n3C)CCc2c1 Show InChI InChI=1S/C27H31N5OS/c1-19-5-7-22(8-6-19)26-28-29-27(31(26)3)34-16-4-13-32-14-11-21-9-10-24(18-23(21)12-15-32)25-17-20(2)33-30-25/h5-10,17-18H,4,11-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in CHO cells |
J Med Chem 50: 5076-89 (2007)
Article DOI: 10.1021/jm0705612
BindingDB Entry DOI: 10.7270/Q2DF6QZB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50411387
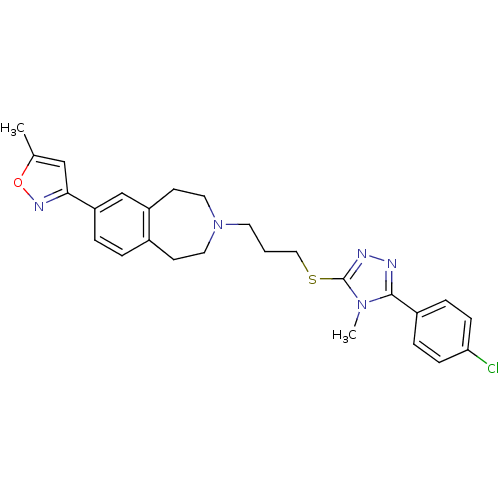
(CHEMBL244279)Show SMILES Cc1cc(no1)-c1ccc2CCN(CCCSc3nnc(-c4ccc(Cl)cc4)n3C)CCc2c1 Show InChI InChI=1S/C26H28ClN5OS/c1-18-16-24(30-33-18)22-5-4-19-10-13-32(14-11-21(19)17-22)12-3-15-34-26-29-28-25(31(26)2)20-6-8-23(27)9-7-20/h4-9,16-17H,3,10-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in CHO cells |
J Med Chem 50: 5076-89 (2007)
Article DOI: 10.1021/jm0705612
BindingDB Entry DOI: 10.7270/Q2DF6QZB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50411392
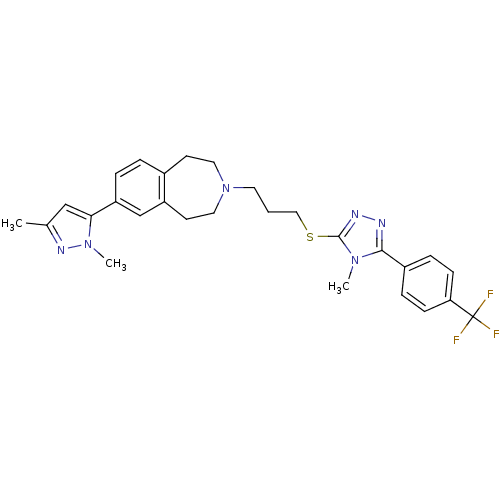
(CHEMBL390649)Show SMILES Cc1cc(-c2ccc3CCN(CCCSc4nnc(-c5ccc(cc5)C(F)(F)F)n4C)CCc3c2)n(C)n1 Show InChI InChI=1S/C28H31F3N6S/c1-19-17-25(36(3)34-19)23-6-5-20-11-14-37(15-12-22(20)18-23)13-4-16-38-27-33-32-26(35(27)2)21-7-9-24(10-8-21)28(29,30)31/h5-10,17-18H,4,11-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in CHO cells |
J Med Chem 50: 5076-89 (2007)
Article DOI: 10.1021/jm0705612
BindingDB Entry DOI: 10.7270/Q2DF6QZB |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Mus musculus) | BDBM50483145
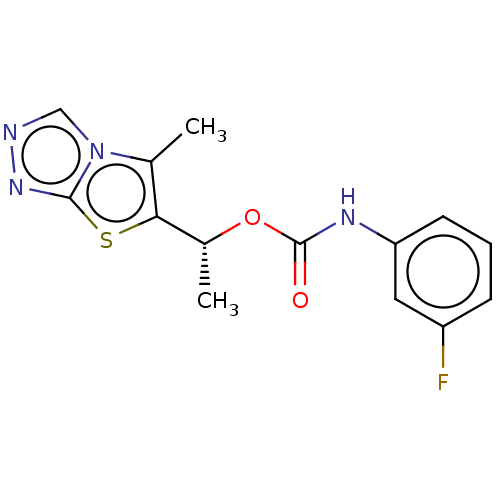
(CHEMBL1630080)Show SMILES C[C@@H](OC(=O)Nc1cccc(F)c1)c1sc2nncn2c1C |r| Show InChI InChI=1S/C14H13FN4O2S/c1-8-12(22-13-18-16-7-19(8)13)9(2)21-14(20)17-11-5-3-4-10(15)6-11/h3-7,9H,1-2H3,(H,17,20)/t9-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at mGluR5 in mouse astrocytes assessed as inhibition of L-quisqualate induced calcium release by FLIPR assay |
Bioorg Med Chem Lett 20: 7521-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.120
BindingDB Entry DOI: 10.7270/Q2KH0R5D |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Mus musculus) | BDBM50483144

(CHEMBL1630057 | GSK2210875)Show InChI InChI=1S/C14H14N4O2S/c1-9-12(21-13-15-8-16-18(9)13)10(2)20-14(19)17-11-6-4-3-5-7-11/h3-8,10H,1-2H3,(H,17,19)/t10-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at mGluR5 in mouse astrocytes assessed as inhibition of L-quisqualate induced calcium release by FLIPR assay |
Bioorg Med Chem Lett 20: 7521-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.120
BindingDB Entry DOI: 10.7270/Q2KH0R5D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50411367
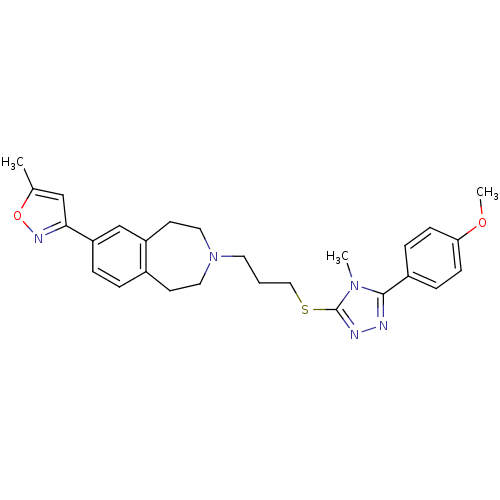
(CHEMBL242600)Show SMILES COc1ccc(cc1)-c1nnc(SCCCN2CCc3ccc(cc3CC2)-c2cc(C)on2)n1C Show InChI InChI=1S/C27H31N5O2S/c1-19-17-25(30-34-19)23-6-5-20-11-14-32(15-12-22(20)18-23)13-4-16-35-27-29-28-26(31(27)2)21-7-9-24(33-3)10-8-21/h5-10,17-18H,4,11-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in CHO cells |
J Med Chem 50: 5076-89 (2007)
Article DOI: 10.1021/jm0705612
BindingDB Entry DOI: 10.7270/Q2DF6QZB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50411376
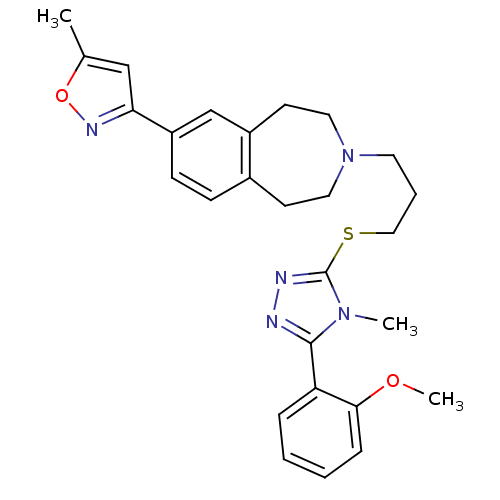
(CHEMBL390429)Show SMILES COc1ccccc1-c1nnc(SCCCN2CCc3ccc(cc3CC2)-c2cc(C)on2)n1C Show InChI InChI=1S/C27H31N5O2S/c1-19-17-24(30-34-19)22-10-9-20-11-14-32(15-12-21(20)18-22)13-6-16-35-27-29-28-26(31(27)2)23-7-4-5-8-25(23)33-3/h4-5,7-10,17-18H,6,11-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in CHO cells |
J Med Chem 50: 5076-89 (2007)
Article DOI: 10.1021/jm0705612
BindingDB Entry DOI: 10.7270/Q2DF6QZB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50411394
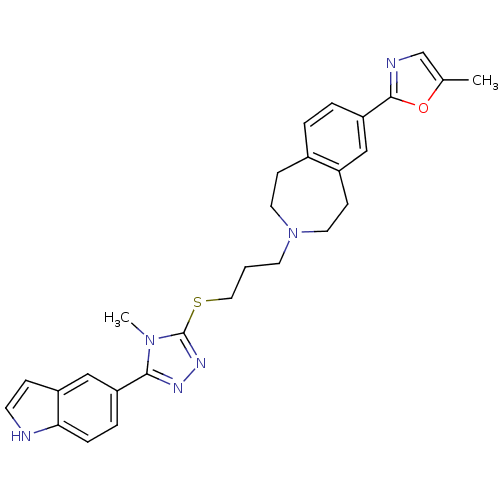
(CHEMBL244276)Show SMILES Cc1cnc(o1)-c1ccc2CCN(CCCSc3nnc(-c4ccc5[nH]ccc5c4)n3C)CCc2c1 Show InChI InChI=1S/C28H30N6OS/c1-19-18-30-27(35-19)24-5-4-20-9-13-34(14-10-21(20)16-24)12-3-15-36-28-32-31-26(33(28)2)23-6-7-25-22(17-23)8-11-29-25/h4-8,11,16-18,29H,3,9-10,12-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in CHO cells |
J Med Chem 50: 5076-89 (2007)
Article DOI: 10.1021/jm0705612
BindingDB Entry DOI: 10.7270/Q2DF6QZB |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50483144

(CHEMBL1630057 | GSK2210875)Show InChI InChI=1S/C14H14N4O2S/c1-9-12(21-13-15-8-16-18(9)13)10(2)20-14(19)17-11-6-4-3-5-7-11/h3-8,10H,1-2H3,(H,17,19)/t10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilization |
Bioorg Med Chem Lett 20: 7521-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.120
BindingDB Entry DOI: 10.7270/Q2KH0R5D |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50483145

(CHEMBL1630080)Show SMILES C[C@@H](OC(=O)Nc1cccc(F)c1)c1sc2nncn2c1C |r| Show InChI InChI=1S/C14H13FN4O2S/c1-8-12(22-13-18-16-7-19(8)13)9(2)21-14(20)17-11-5-3-4-10(15)6-11/h3-7,9H,1-2H3,(H,17,20)/t9-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilization |
Bioorg Med Chem Lett 20: 7521-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.120
BindingDB Entry DOI: 10.7270/Q2KH0R5D |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50483152
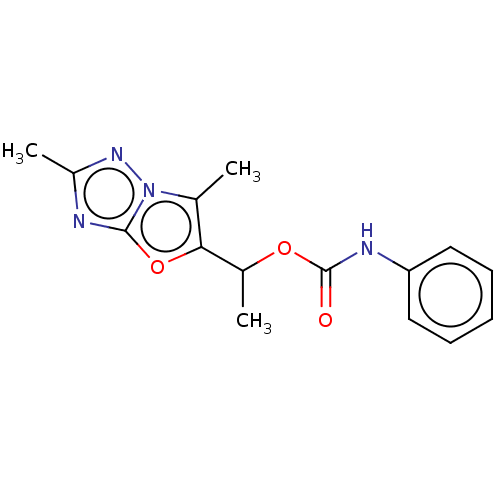
(CHEMBL1630069)Show InChI InChI=1S/C15H16N4O3/c1-9-13(22-14-16-11(3)18-19(9)14)10(2)21-15(20)17-12-7-5-4-6-8-12/h4-8,10H,1-3H3,(H,17,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilization |
Bioorg Med Chem Lett 20: 7521-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.120
BindingDB Entry DOI: 10.7270/Q2KH0R5D |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50483153
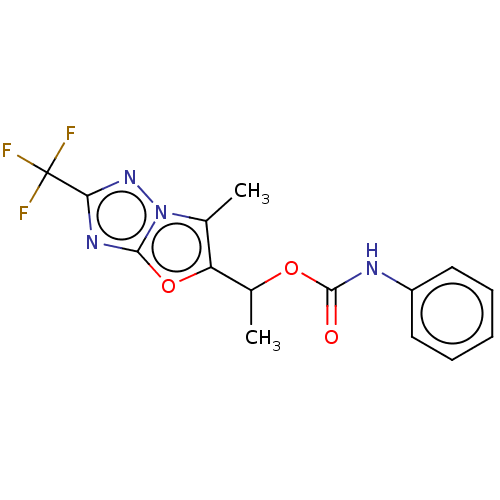
(CHEMBL1630070)Show InChI InChI=1S/C15H13F3N4O3/c1-8-11(25-13-20-12(15(16,17)18)21-22(8)13)9(2)24-14(23)19-10-6-4-3-5-7-10/h3-7,9H,1-2H3,(H,19,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilization |
Bioorg Med Chem Lett 20: 7521-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.120
BindingDB Entry DOI: 10.7270/Q2KH0R5D |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50483147
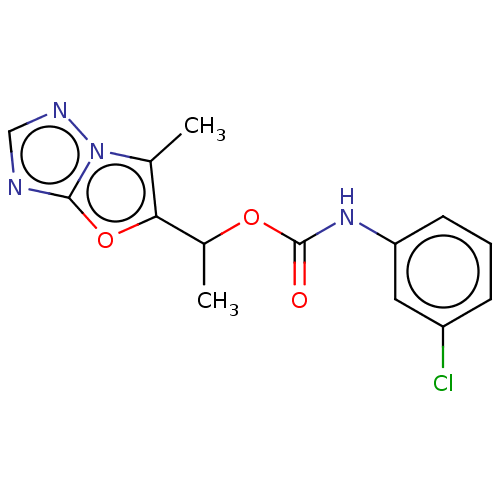
(CHEMBL1630061)Show InChI InChI=1S/C14H13ClN4O3/c1-8-12(22-13-16-7-17-19(8)13)9(2)21-14(20)18-11-5-3-4-10(15)6-11/h3-7,9H,1-2H3,(H,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilization |
Bioorg Med Chem Lett 20: 7521-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.120
BindingDB Entry DOI: 10.7270/Q2KH0R5D |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50483160
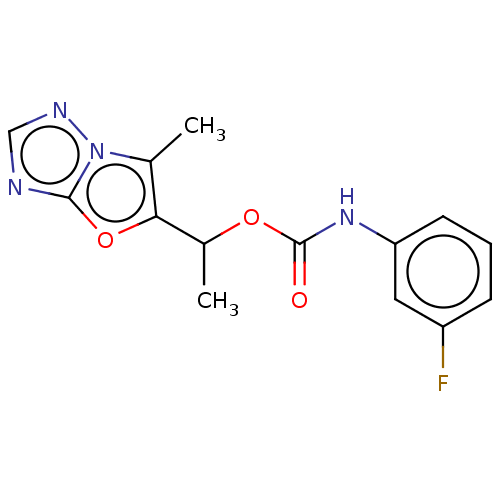
(CHEMBL1630064)Show InChI InChI=1S/C14H13FN4O3/c1-8-12(22-13-16-7-17-19(8)13)9(2)21-14(20)18-11-5-3-4-10(15)6-11/h3-7,9H,1-2H3,(H,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilization |
Bioorg Med Chem Lett 20: 7521-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.120
BindingDB Entry DOI: 10.7270/Q2KH0R5D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50411388
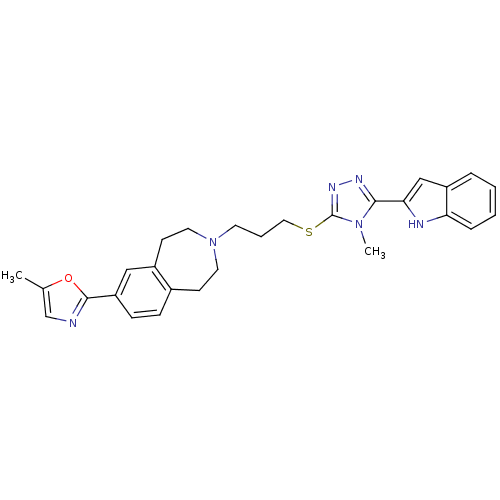
(CHEMBL397429)Show SMILES Cc1cnc(o1)-c1ccc2CCN(CCCSc3nnc(-c4cc5ccccc5[nH]4)n3C)CCc2c1 Show InChI InChI=1S/C28H30N6OS/c1-19-18-29-27(35-19)23-9-8-20-10-13-34(14-11-21(20)16-23)12-5-15-36-28-32-31-26(33(28)2)25-17-22-6-3-4-7-24(22)30-25/h3-4,6-9,16-18,30H,5,10-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in CHO cells |
J Med Chem 50: 5076-89 (2007)
Article DOI: 10.1021/jm0705612
BindingDB Entry DOI: 10.7270/Q2DF6QZB |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50483157
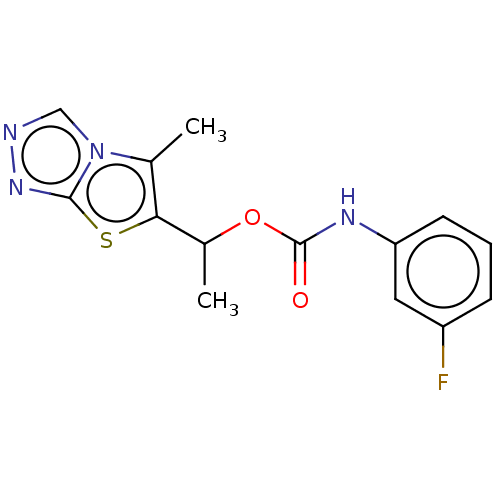
(CHEMBL1630079)Show InChI InChI=1S/C14H13FN4O2S/c1-8-12(22-13-18-16-7-19(8)13)9(2)21-14(20)17-11-5-3-4-10(15)6-11/h3-7,9H,1-2H3,(H,17,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilization |
Bioorg Med Chem Lett 20: 7521-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.120
BindingDB Entry DOI: 10.7270/Q2KH0R5D |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50483146
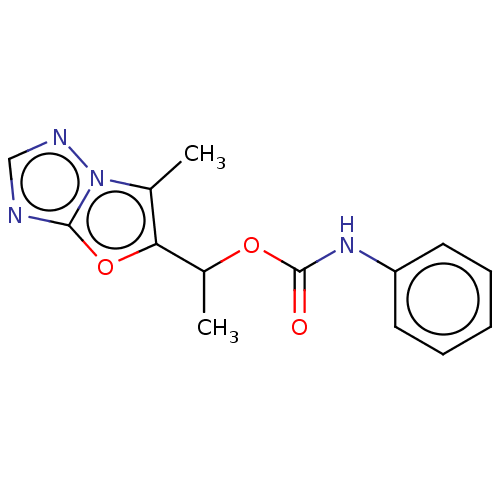
(CHEMBL1630059)Show InChI InChI=1S/C14H14N4O3/c1-9-12(21-13-15-8-16-18(9)13)10(2)20-14(19)17-11-6-4-3-5-7-11/h3-8,10H,1-2H3,(H,17,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human mGluR5 expressed in CHO cells assessed as doxycycline induced calcium mobilization |
Bioorg Med Chem Lett 20: 7521-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.120
BindingDB Entry DOI: 10.7270/Q2KH0R5D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50411383
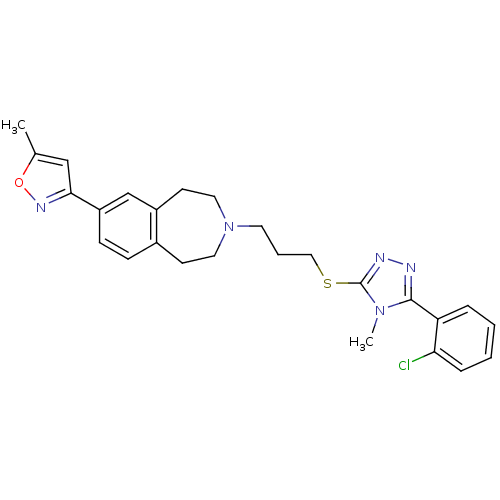
(CHEMBL244087)Show SMILES Cc1cc(no1)-c1ccc2CCN(CCCSc3nnc(-c4ccccc4Cl)n3C)CCc2c1 Show InChI InChI=1S/C26H28ClN5OS/c1-18-16-24(30-33-18)21-9-8-19-10-13-32(14-11-20(19)17-21)12-5-15-34-26-29-28-25(31(26)2)22-6-3-4-7-23(22)27/h3-4,6-9,16-17H,5,10-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in CHO cells |
J Med Chem 50: 5076-89 (2007)
Article DOI: 10.1021/jm0705612
BindingDB Entry DOI: 10.7270/Q2DF6QZB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50411369
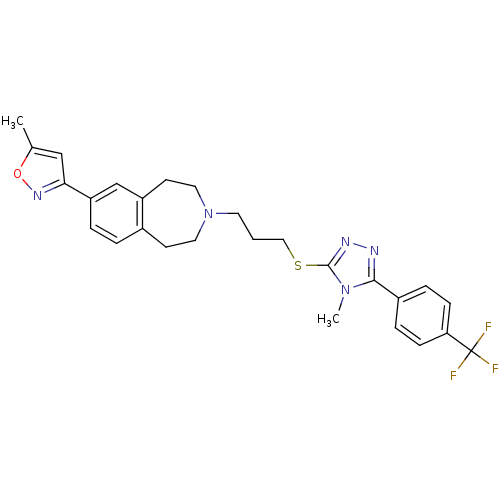
(CHEMBL243897)Show SMILES Cc1cc(no1)-c1ccc2CCN(CCCSc3nnc(-c4ccc(cc4)C(F)(F)F)n3C)CCc2c1 Show InChI InChI=1S/C27H28F3N5OS/c1-18-16-24(33-36-18)22-5-4-19-10-13-35(14-11-21(19)17-22)12-3-15-37-26-32-31-25(34(26)2)20-6-8-23(9-7-20)27(28,29)30/h4-9,16-17H,3,10-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in CHO cells |
J Med Chem 50: 5076-89 (2007)
Article DOI: 10.1021/jm0705612
BindingDB Entry DOI: 10.7270/Q2DF6QZB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50411396
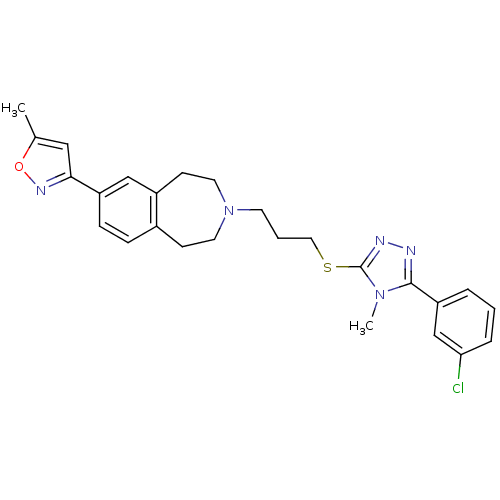
(CHEMBL244278)Show SMILES Cc1cc(no1)-c1ccc2CCN(CCCSc3nnc(-c4cccc(Cl)c4)n3C)CCc2c1 Show InChI InChI=1S/C26H28ClN5OS/c1-18-15-24(30-33-18)21-8-7-19-9-12-32(13-10-20(19)16-21)11-4-14-34-26-29-28-25(31(26)2)22-5-3-6-23(27)17-22/h3,5-8,15-17H,4,9-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79.4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in CHO cells |
J Med Chem 50: 5076-89 (2007)
Article DOI: 10.1021/jm0705612
BindingDB Entry DOI: 10.7270/Q2DF6QZB |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit beta-1
(Rat) | BDBM625827
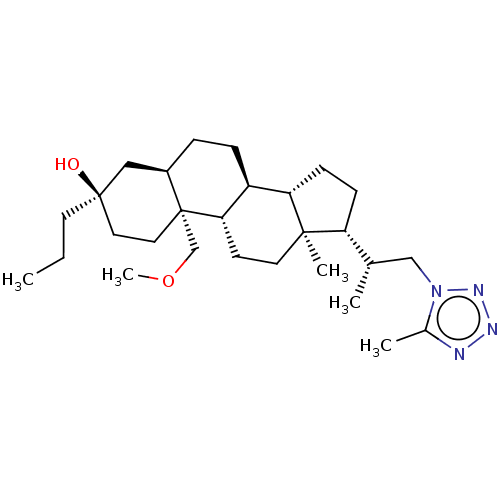
(US20230322846, Example 321)Show SMILES CCC[C@@]1(O)CC[C@@]2(COC)[C@H](CC[C@H]3[C@@H]4CC[C@H]([C@@H](C)Cn5nnnc5C)[C@@]4(C)CC[C@H]23)C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit beta-1
(Rat) | BDBM625828
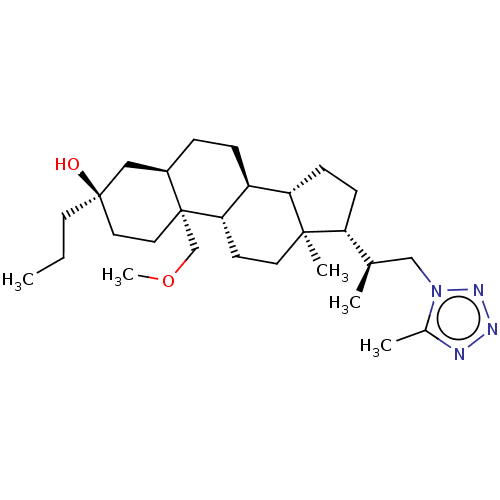
(US20230322846, Example 322)Show SMILES CCC[C@@]1(O)CC[C@@]2(COC)[C@H](CC[C@H]3[C@@H]4CC[C@H]([C@H](C)Cn5nnnc5C)[C@@]4(C)CC[C@H]23)C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit beta-1
(Rat) | BDBM625829
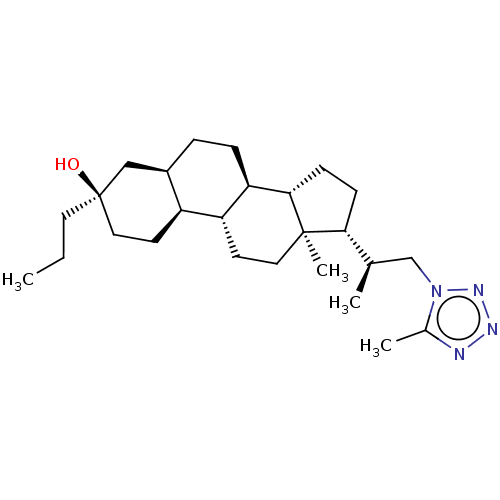
((3R,5R,8R,9R,10S,13S,14S,17R)-13-methyl-17-(1-(5-m...)Show SMILES CCC[C@@]1(O)CC[C@H]2[C@H](CC[C@H]3[C@@H]4CC[C@H]([C@H](C)Cn5nnnc5C)[C@@]4(C)CC[C@H]23)C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit beta-1
(Rat) | BDBM625830
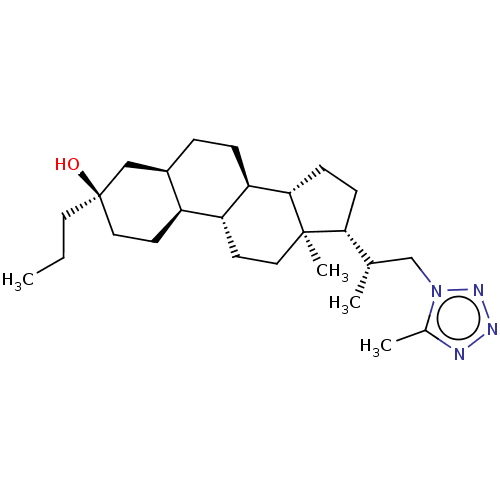
(US20230322846, Example 324)Show SMILES CCC[C@@]1(O)CC[C@H]2[C@H](CC[C@H]3[C@@H]4CC[C@H]([C@@H](C)Cn5nnnc5C)[C@@]4(C)CC[C@H]23)C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit beta-1
(Rat) | BDBM625831
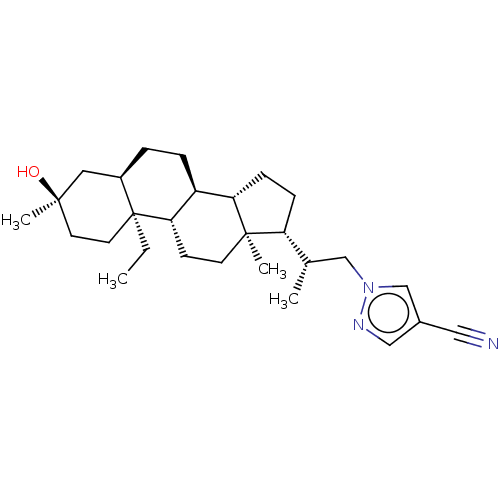
(1-((S)-2-((3R,5R,8S,9S,10S,13S,14S,17R)-10-ethyl-3...)Show SMILES CC[C@]12CC[C@@](C)(O)C[C@H]1CC[C@H]1[C@@H]3CC[C@H]([C@@H](C)Cn4cc(cn4)C#N)[C@@]3(C)CC[C@H]21 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit beta-1
(Rat) | BDBM625832
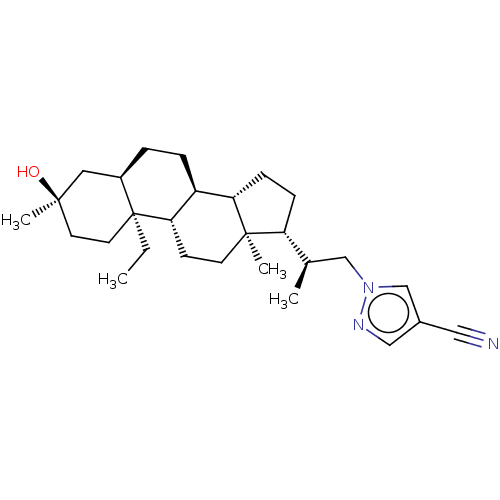
(US20230322846, Example 326)Show SMILES CC[C@]12CC[C@@](C)(O)C[C@H]1CC[C@H]1[C@@H]3CC[C@H]([C@H](C)Cn4cc(cn4)C#N)[C@@]3(C)CC[C@H]21 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit beta-1
(Rat) | BDBM625845
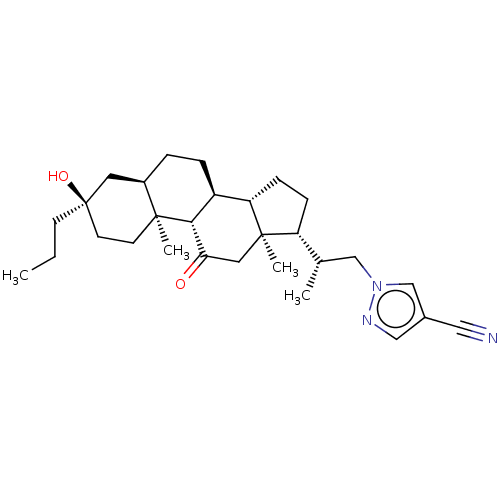
(US20230322846, Example 339)Show SMILES CCC[C@@]1(O)CC[C@@]2(C)[C@H](CC[C@H]3[C@@H]4CC[C@H]([C@@H](C)Cn5cc(cn5)C#N)[C@@]4(C)CC(=O)[C@H]23)C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit beta-1
(Rat) | BDBM625846
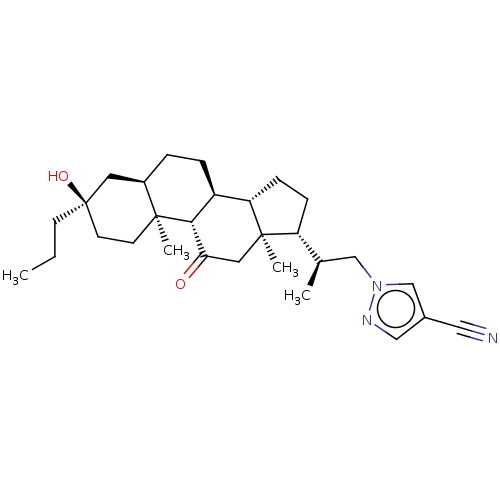
(US20230322846, Example 340)Show SMILES CCC[C@@]1(O)CC[C@@]2(C)[C@H](CC[C@H]3[C@@H]4CC[C@H]([C@H](C)Cn5cc(cn5)C#N)[C@@]4(C)CC(=O)[C@H]23)C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit beta-1
(Rat) | BDBM625847
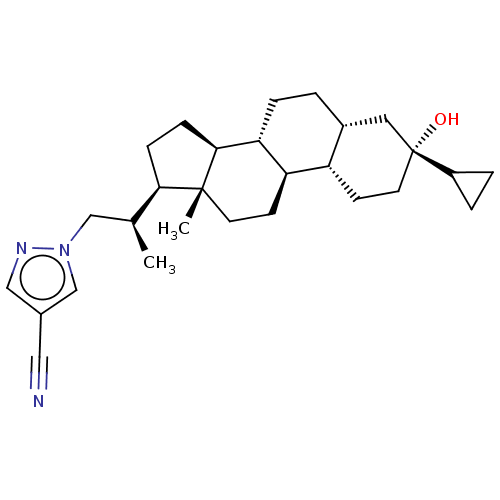
(Synthesis of 1-((R)-2-((3R,5R,8R,9R,10S,13S,14S,17...)Show SMILES C[C@@H](Cn1cc(cn1)C#N)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@](O)(CC[C@@H]4[C@H]3CC[C@]12C)C1CC1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit beta-1
(Rat) | BDBM625861
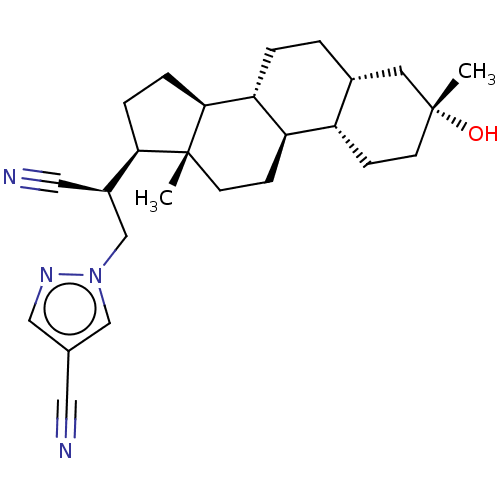
(Synthesis of 1-((S)-2-cyano-2-((3R,5R,8R,9R,10S,13...)Show SMILES C[C@]12CC[C@H]3[C@@H](CC[C@@H]4C[C@](C)(O)CC[C@H]34)[C@@H]1CC[C@@H]2[C@@H](Cn1cc(cn1)C#N)C#N |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit beta-1
(Rat) | BDBM625862
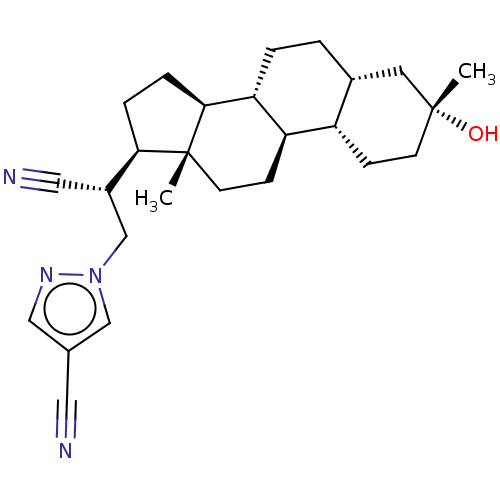
(US20230322846, Example 356)Show SMILES C[C@]12CC[C@H]3[C@@H](CC[C@@H]4C[C@](C)(O)CC[C@H]34)[C@@H]1CC[C@@H]2[C@H](Cn1cc(cn1)C#N)C#N |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit beta-1
(Rat) | BDBM625870
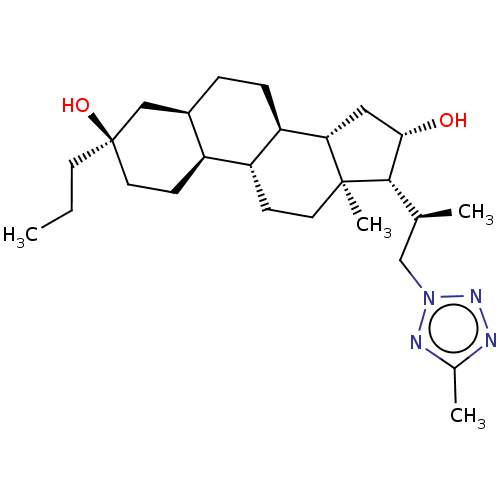
(US20230322846, Example 365)Show SMILES CCC[C@@]1(O)CC[C@H]2[C@H](CC[C@H]3[C@@H]4C[C@H](O)[C@H]([C@@H](C)Cn5nnc(C)n5)[C@@]4(C)CC[C@H]23)C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit beta-1
(Rat) | BDBM625871
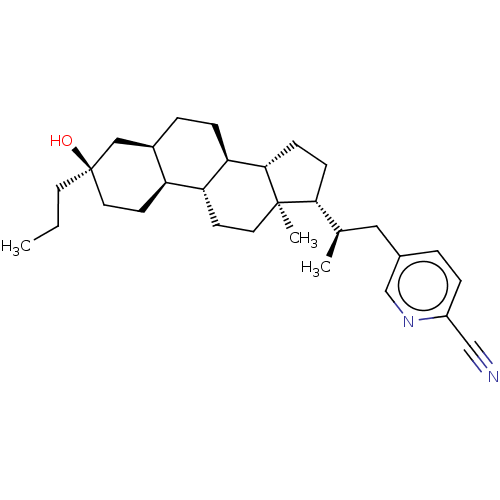
(Synthesis of 5-((S)-2-((3R,5R,8R,9R,10S,13R,14S,17...)Show SMILES CCC[C@@]1(O)CC[C@H]2[C@H](CC[C@H]3[C@@H]4CC[C@H]([C@H](C)Cc5ccc(nc5)C#N)[C@@]4(C)CC[C@H]23)C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit beta-1
(Rat) | BDBM625872
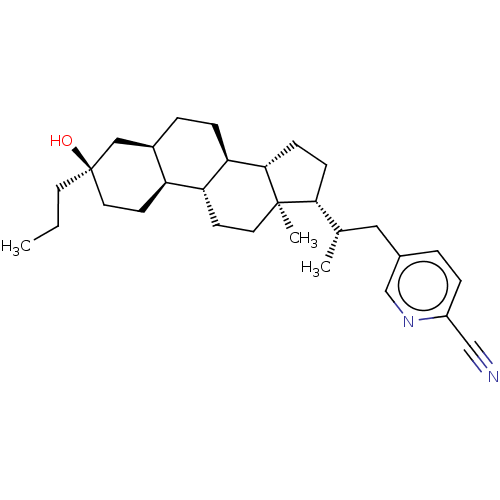
(US20230322846, Example 401)Show SMILES CCC[C@@]1(O)CC[C@H]2[C@H](CC[C@H]3[C@@H]4CC[C@H]([C@@H](C)Cc5ccc(nc5)C#N)[C@@]4(C)CC[C@H]23)C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit beta-1
(Rat) | BDBM625873
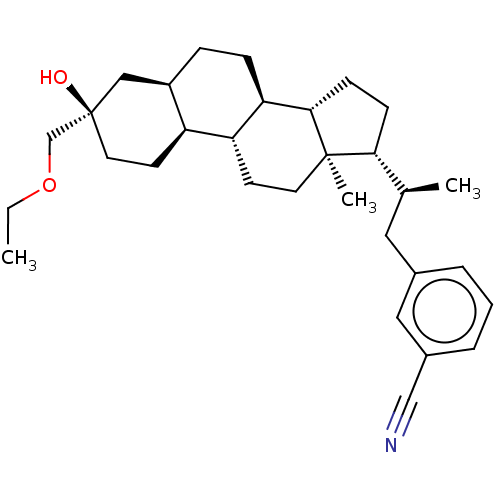
(Synthesis of 3-((S)-2-((3R,5R,8R,9R,10S,13R,14S,17...)Show SMILES CCOC[C@@]1(O)CC[C@H]2[C@H](CC[C@H]3[C@@H]4CC[C@H]([C@@H](C)Cc5cccc(c5)C#N)[C@@]4(C)CC[C@H]23)C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit beta-1
(Rat) | BDBM625875
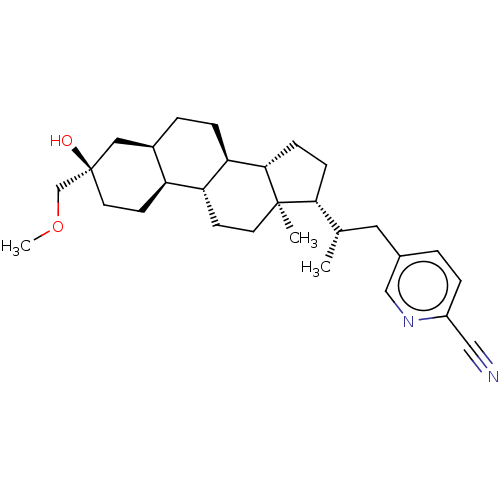
(Synthesis of 5-((S)-2-((3R,5R,8R,9R,10S,13R,14S,17...)Show SMILES COC[C@@]1(O)CC[C@H]2[C@H](CC[C@H]3[C@@H]4CC[C@H]([C@@H](C)Cc5ccc(nc5)C#N)[C@@]4(C)CC[C@H]23)C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit beta-1
(Rat) | BDBM625876
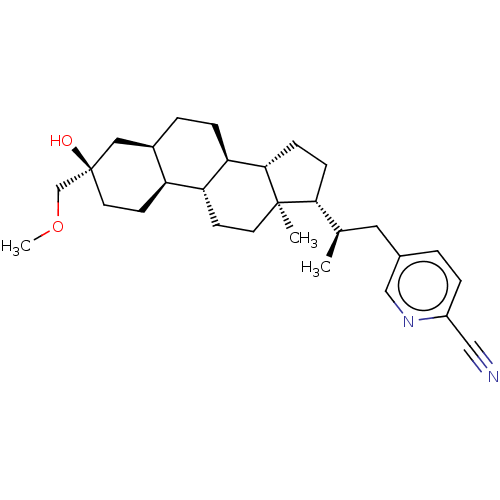
(US20230322846, Example 405)Show SMILES COC[C@@]1(O)CC[C@H]2[C@H](CC[C@H]3[C@@H]4CC[C@H]([C@H](C)Cc5ccc(nc5)C#N)[C@@]4(C)CC[C@H]23)C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit beta-1
(Rat) | BDBM625877
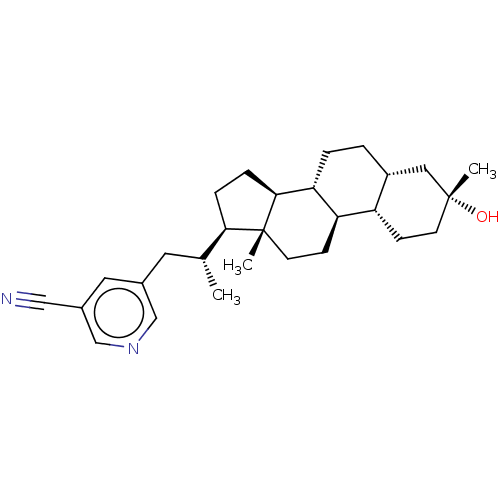
(Synthesis of 5-((R)-2-((3R,5R,8R,9R,10S,13R,14S,17...)Show SMILES C[C@H](Cc1cncc(c1)C#N)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@](C)(O)CC[C@@H]4[C@H]3CC[C@]12C |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit beta-1
(Rat) | BDBM625878
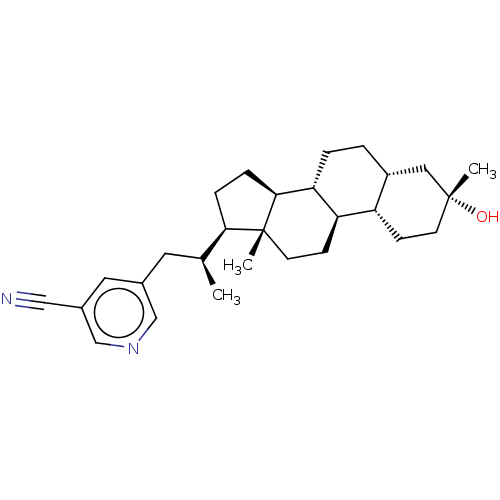
(US20230322846, Example 407)Show SMILES C[C@@H](Cc1cncc(c1)C#N)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@](C)(O)CC[C@@H]4[C@H]3CC[C@]12C |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data