 Found 389 hits with Last Name = 'pinney' and Initial = 'kg'
Found 389 hits with Last Name = 'pinney' and Initial = 'kg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Procathepsin L
(Homo sapiens (Human)) | BDBM50330030

(CHEMBL1269632 | [(3-Bromophenyl)-(3-hydroxyphenyl)...)Show SMILES NC(=S)NN=C(c1cccc(O)c1)c1cccc(Br)c1 |w:4.3| Show InChI InChI=1S/C14H12BrN3OS/c15-11-5-1-3-9(7-11)13(17-18-14(16)20)10-4-2-6-12(19)8-10/h1-8,19H,(H3,16,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Covalent/reversible inhibition of human liver cathepsin-L using Z-FR-AMC as substrate preincubated for 5 mins followed by substrate addition measured... |
Bioorg Med Chem Lett 27: 1304-1310 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.039
BindingDB Entry DOI: 10.7270/Q23B62C1 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50232473

(CHEMBL4087627)Show InChI InChI=1S/C14H11Br2N3OS/c15-10-4-9(5-11(16)7-10)13(18-19-14(17)21)8-2-1-3-12(20)6-8/h1-7,20H,(H3,17,19,21)/b18-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Covalent/reversible inhibition of human liver cathepsin-L using Z-FR-AMC as substrate preincubated for 5 mins followed by substrate addition measured... |
Bioorg Med Chem Lett 27: 1304-1310 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.039
BindingDB Entry DOI: 10.7270/Q23B62C1 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50128821

(CHEMBL3629203)Show SMILES NC(=S)N\N=C(/c1cc(Br)cc(c1)C(=O)c1ccccc1F)c1ccccc1F Show InChI InChI=1S/C21H14BrF2N3OS/c22-14-10-12(9-13(11-14)20(28)16-6-2-4-8-18(16)24)19(26-27-21(25)29)15-5-1-3-7-17(15)23/h1-11H,(H3,25,27,29)/b26-19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L using Z-FR-AMC as substrate measured every 15 sec for 5 mins by fluorescence assay |
Bioorg Med Chem 23: 6974-92 (2015)
Article DOI: 10.1016/j.bmc.2015.09.036
BindingDB Entry DOI: 10.7270/Q2T43VX1 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM138145
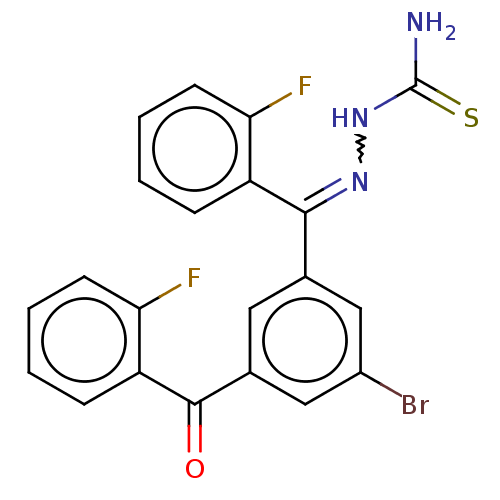
(US8877967, 11)Show SMILES NC(=S)NN=C(c1cc(Br)cc(c1)C(=O)c1ccccc1F)c1ccccc1F |w:4.3| Show InChI InChI=1S/C21H14BrF2N3OS/c22-14-10-12(9-13(11-14)20(28)16-6-2-4-8-18(16)24)19(26-27-21(25)29)15-5-1-3-7-17(15)23/h1-11H,(H3,25,27,29)/b26-19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.12 | n/a | n/a | n/a | n/a | 5.5 | n/a |
OXiGENE, Inc.; Baylor University
US Patent
| Assay Description
Human liver Cathepsin L (Sigma) was preincubated with test compounds at various concentrations for 5 minutes at 25C. The assay was initiated by addit... |
US Patent US8877967 (2014)
BindingDB Entry DOI: 10.7270/Q25H7F0C |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50128809
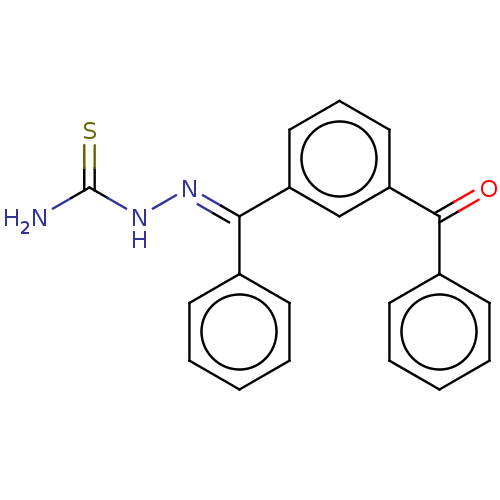
(CHEMBL3629191)Show SMILES NC(=S)N\N=C(/c1ccccc1)c1cccc(c1)C(=O)c1ccccc1 Show InChI InChI=1S/C21H17N3OS/c22-21(26)24-23-19(15-8-3-1-4-9-15)17-12-7-13-18(14-17)20(25)16-10-5-2-6-11-16/h1-14H,(H3,22,24,26)/b23-19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L using Z-FR-AMC as substrate measured every 15 sec for 5 mins by fluorescence assay |
Bioorg Med Chem 23: 6974-92 (2015)
Article DOI: 10.1016/j.bmc.2015.09.036
BindingDB Entry DOI: 10.7270/Q2T43VX1 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM138142

(US8877967, 3)Show SMILES CN(N=C(c1ccccc1)c1cccc(c1)C(=O)c1ccccc1)C(N)=S |w:2.1| Show InChI InChI=1S/C22H19N3OS/c1-25(22(23)27)24-20(16-9-4-2-5-10-16)18-13-8-14-19(15-18)21(26)17-11-6-3-7-12-17/h2-15H,1H3,(H2,23,27)/b24-20+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 10.5 | n/a | n/a | n/a | n/a | 5.5 | n/a |
OXiGENE, Inc.; Baylor University
US Patent
| Assay Description
Human liver Cathepsin L (Sigma) was preincubated with test compounds at various concentrations for 5 minutes at 25C. The assay was initiated by addit... |
US Patent US8877967 (2014)
BindingDB Entry DOI: 10.7270/Q25H7F0C |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50128812

(CHEMBL3629194)Show SMILES NC(=S)N\N=C(/c1ccc(F)cc1)c1cccc(c1)C(=O)c1ccc(F)cc1 Show InChI InChI=1S/C21H15F2N3OS/c22-17-8-4-13(5-9-17)19(25-26-21(24)28)15-2-1-3-16(12-15)20(27)14-6-10-18(23)11-7-14/h1-12H,(H3,24,26,28)/b25-19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L using Z-FR-AMC as substrate measured every 15 sec for 5 mins by fluorescence assay |
Bioorg Med Chem 23: 6974-92 (2015)
Article DOI: 10.1016/j.bmc.2015.09.036
BindingDB Entry DOI: 10.7270/Q2T43VX1 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50189273
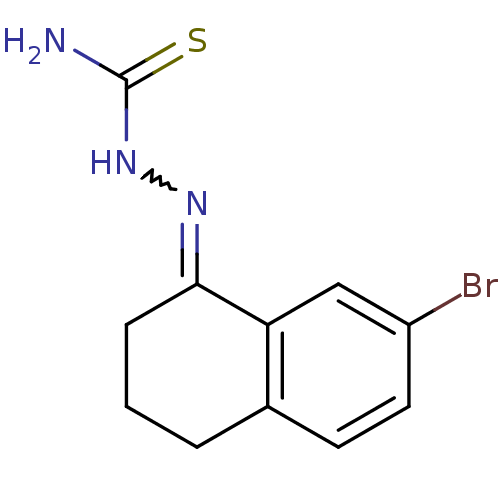
(1-(7-bromo-3,4-dihydronaphthalen-1(2H)-ylidene)thi...)Show InChI InChI=1S/C11H12BrN3S/c12-8-5-4-7-2-1-3-10(9(7)6-8)14-15-11(13)16/h4-6H,1-3H2,(H3,13,15,16) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi recombinant cruzain |
Bioorg Med Chem Lett 16: 4405-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.041
BindingDB Entry DOI: 10.7270/Q23R0SHC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM138142

(US8877967, 3)Show SMILES CN(N=C(c1ccccc1)c1cccc(c1)C(=O)c1ccccc1)C(N)=S |w:2.1| Show InChI InChI=1S/C22H19N3OS/c1-25(22(23)27)24-20(16-9-4-2-5-10-16)18-13-8-14-19(15-18)21(26)17-11-6-3-7-12-17/h2-15H,1H3,(H2,23,27)/b24-20+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 17.4 | n/a | n/a | n/a | n/a | 3.5 | 25 |
OXiGENE, Inc.; Baylor University
US Patent
| Assay Description
Recombinant human procathepsin K was obtained from Enzo Life Sciences. Activation of the proenzyme was performed in 32.5 mM sodium acetate pH 3.5, ED... |
US Patent US8877967 (2014)
BindingDB Entry DOI: 10.7270/Q25H7F0C |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM138143
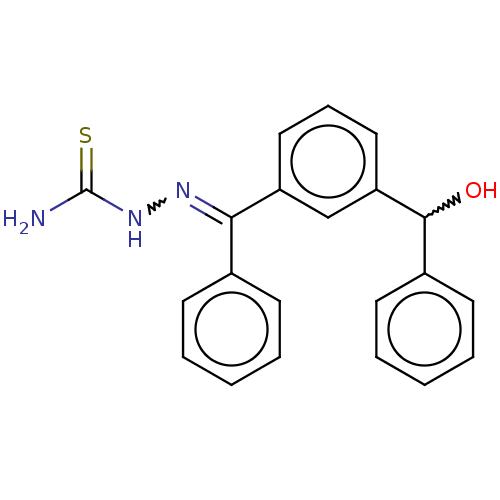
(US8877967, 6)Show SMILES NC(=S)NN=C(c1ccccc1)c1cccc(c1)C(O)c1ccccc1 |w:18.20,4.3| Show InChI InChI=1S/C21H19N3OS/c22-21(26)24-23-19(15-8-3-1-4-9-15)17-12-7-13-18(14-17)20(25)16-10-5-2-6-11-16/h1-14,20,25H,(H3,22,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 23.8 | n/a | n/a | n/a | n/a | 5.5 | n/a |
OXiGENE, Inc.; Baylor University
US Patent
| Assay Description
Human liver Cathepsin L (Sigma) was preincubated with test compounds at various concentrations for 5 minutes at 25C. The assay was initiated by addit... |
US Patent US8877967 (2014)
BindingDB Entry DOI: 10.7270/Q25H7F0C |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50189279
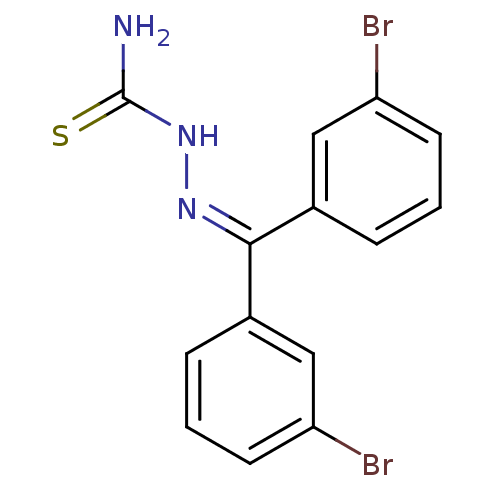
(1-(bis(3-bromophenyl)methylene)thiosemicarbazide |...)Show SMILES [#7]-[#6](=S)-[#7]\[#7]=[#6](\c1cccc(Br)c1)-c1cccc(Br)c1 Show InChI InChI=1S/C14H11Br2N3S/c15-11-5-1-3-9(7-11)13(18-19-14(17)20)10-4-2-6-12(16)8-10/h1-8H,(H3,17,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi recombinant cruzain |
Bioorg Med Chem Lett 16: 4405-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.041
BindingDB Entry DOI: 10.7270/Q23R0SHC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50128811

(CHEMBL3629193)Show SMILES NC(=S)N\N=C(/c1ccccc1)c1cccc(c1)C(O)c1ccccc1 Show InChI InChI=1S/C21H19N3OS/c22-21(26)24-23-19(15-8-3-1-4-9-15)17-12-7-13-18(14-17)20(25)16-10-5-2-6-11-16/h1-14,20,25H,(H3,22,24,26)/b23-19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L using Z-FR-AMC as substrate measured every 15 sec for 5 mins by fluorescence assay |
Bioorg Med Chem 23: 6974-92 (2015)
Article DOI: 10.1016/j.bmc.2015.09.036
BindingDB Entry DOI: 10.7270/Q2T43VX1 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM138146
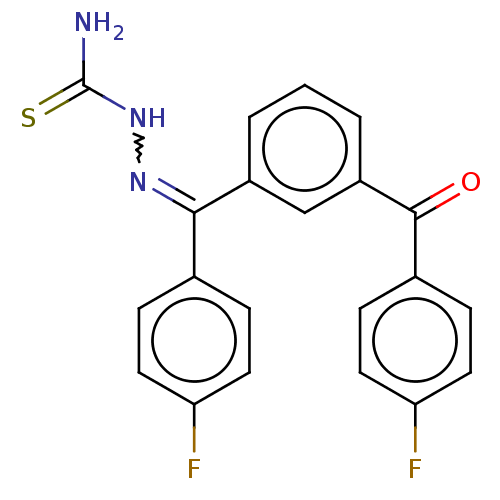
(US8877967, 13)Show SMILES NC(=S)NN=C(c1ccc(F)cc1)c1cccc(c1)C(=O)c1ccc(F)cc1 |w:4.3| Show InChI InChI=1S/C21H15F2N3OS/c22-17-8-4-13(5-9-17)19(25-26-21(24)28)15-2-1-3-16(12-15)20(27)14-6-10-18(23)11-7-14/h1-12H,(H3,24,26,28)/b25-19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 24.3 | n/a | n/a | n/a | n/a | 5.5 | n/a |
OXiGENE, Inc.; Baylor University
US Patent
| Assay Description
Human liver Cathepsin L (Sigma) was preincubated with test compounds at various concentrations for 5 minutes at 25C. The assay was initiated by addit... |
US Patent US8877967 (2014)
BindingDB Entry DOI: 10.7270/Q25H7F0C |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM138154

(US8877967, 25)Show SMILES NC(=S)NN=C(c1ccccc1)c1cc(cc(c1)C(=O)c1ccccc1)C(=O)c1ccccc1 |w:4.3| Show InChI InChI=1S/C28H21N3O2S/c29-28(34)31-30-25(19-10-4-1-5-11-19)22-16-23(26(32)20-12-6-2-7-13-20)18-24(17-22)27(33)21-14-8-3-9-15-21/h1-18H,(H3,29,31,34)/b30-25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | 5.5 | n/a |
OXiGENE, Inc.; Baylor University
US Patent
| Assay Description
Human liver Cathepsin L (Sigma) was preincubated with test compounds at various concentrations for 5 minutes at 25C. The assay was initiated by addit... |
US Patent US8877967 (2014)
BindingDB Entry DOI: 10.7270/Q25H7F0C |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50306641
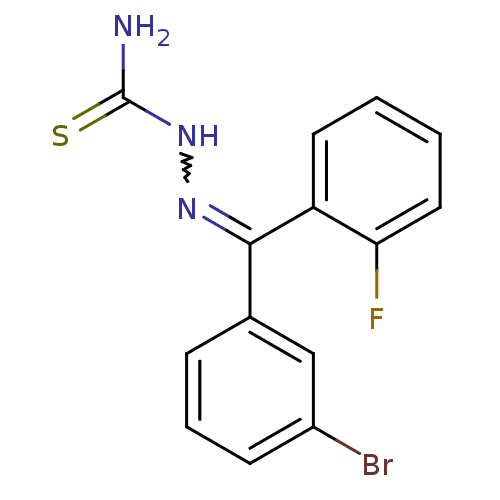
(CHEMBL589280 | [(3-Bromophenyl)-(2-fluorophenyl)-k...)Show InChI InChI=1S/C14H11BrFN3S/c15-10-5-3-4-9(8-10)13(18-19-14(17)20)11-6-1-2-7-12(11)16/h1-8H,(H3,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L |
Bioorg Med Chem Lett 20: 1415-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.090
BindingDB Entry DOI: 10.7270/Q2NG4QQZ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50388838

(CHEMBL2062897)Show InChI InChI=1S/C10H9F2N3S2/c11-6-3-5-8(14-15-10(13)16)1-2-17-9(5)4-7(6)12/h3-4H,1-2H2,(H3,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L using Z-R-R-AMC as substrate preincubated with compound for 5 mins measured after 20 mins by fluorescence analy... |
ACS Med Chem Lett 3: 450-453 (2012)
Article DOI: 10.1021/ml200299g
BindingDB Entry DOI: 10.7270/Q2QC04JR |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50306647
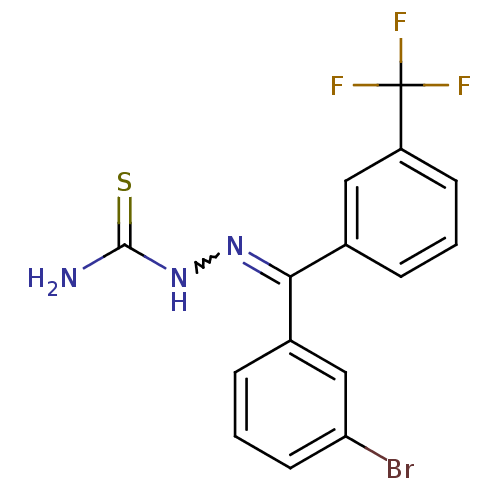
(CHEMBL601890 | [(3-Bromophenyl)-(3-trifluoromethyl...)Show SMILES NC(=S)NN=C(c1cccc(Br)c1)c1cccc(c1)C(F)(F)F |w:4.3| Show InChI InChI=1S/C15H11BrF3N3S/c16-12-6-2-4-10(8-12)13(21-22-14(20)23)9-3-1-5-11(7-9)15(17,18)19/h1-8H,(H3,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L |
Bioorg Med Chem Lett 20: 1415-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.090
BindingDB Entry DOI: 10.7270/Q2NG4QQZ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50128810
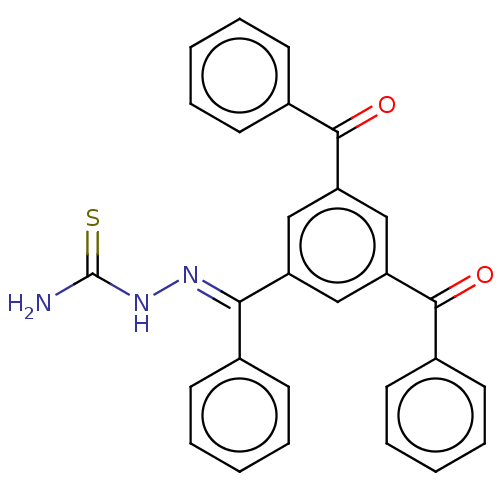
(CHEMBL3629192)Show SMILES NC(=S)N\N=C(/c1ccccc1)c1cc(cc(c1)C(=O)c1ccccc1)C(=O)c1ccccc1 Show InChI InChI=1S/C28H21N3O2S/c29-28(34)31-30-25(19-10-4-1-5-11-19)22-16-23(26(32)20-12-6-2-7-13-20)18-24(17-22)27(33)21-14-8-3-9-15-21/h1-18H,(H3,29,31,34)/b30-25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L using Z-FR-AMC as substrate measured every 15 sec for 5 mins by fluorescence assay |
Bioorg Med Chem 23: 6974-92 (2015)
Article DOI: 10.1016/j.bmc.2015.09.036
BindingDB Entry DOI: 10.7270/Q2T43VX1 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50306656
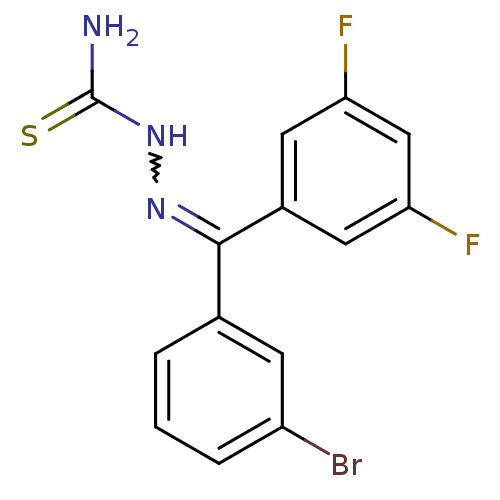
(CHEMBL600860 | [(3-Bromophenyl)-(2,4-difluoropheny...)Show SMILES NC(=S)NN=C(c1cccc(Br)c1)c1cc(F)cc(F)c1 |w:4.3| Show InChI InChI=1S/C14H10BrF2N3S/c15-10-3-1-2-8(4-10)13(19-20-14(18)21)9-5-11(16)7-12(17)6-9/h1-7H,(H3,18,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L |
Bioorg Med Chem Lett 20: 1415-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.090
BindingDB Entry DOI: 10.7270/Q2NG4QQZ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50306662
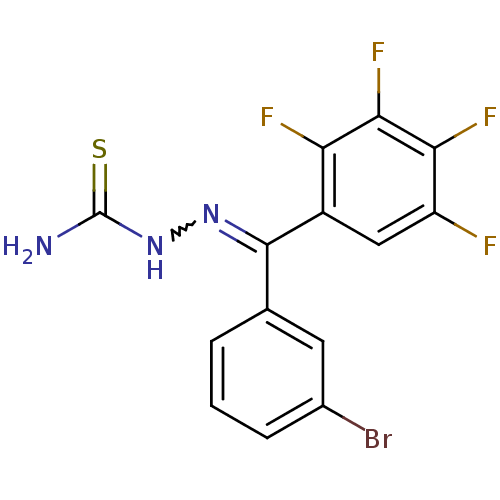
(CHEMBL602934 | [(3-Bromophenyl)-(2,3,4,5-tetrafluo...)Show SMILES NC(=S)NN=C(c1cccc(Br)c1)c1cc(F)c(F)c(F)c1F |w:4.3| Show InChI InChI=1S/C14H8BrF4N3S/c15-7-3-1-2-6(4-7)13(21-22-14(20)23)8-5-9(16)11(18)12(19)10(8)17/h1-5H,(H3,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L |
Bioorg Med Chem Lett 20: 1415-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.090
BindingDB Entry DOI: 10.7270/Q2NG4QQZ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50388851
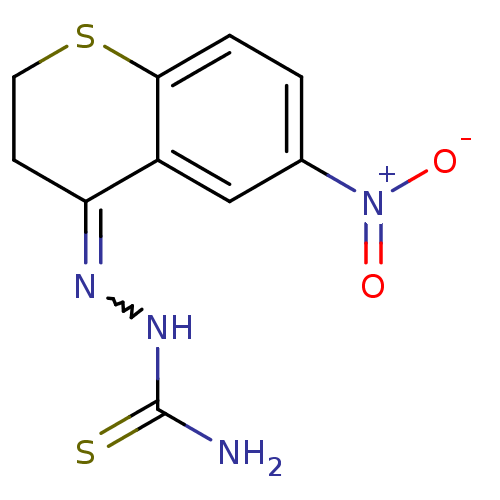
(CHEMBL2062909)Show SMILES NC(=S)NN=C1CCSc2ccc(cc12)[N+]([O-])=O |w:4.3| Show InChI InChI=1S/C10H10N4O2S2/c11-10(17)13-12-8-3-4-18-9-2-1-6(14(15)16)5-7(8)9/h1-2,5H,3-4H2,(H3,11,13,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L using Z-R-R-AMC as substrate preincubated with compound for 5 mins measured after 20 mins by fluorescence analy... |
ACS Med Chem Lett 3: 450-453 (2012)
Article DOI: 10.1021/ml200299g
BindingDB Entry DOI: 10.7270/Q2QC04JR |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50128820
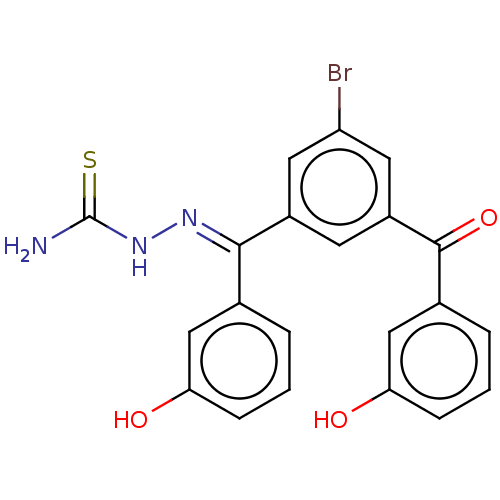
(CHEMBL3629202)Show SMILES NC(=S)N\N=C(/c1cccc(O)c1)c1cc(Br)cc(c1)C(=O)c1cccc(O)c1 Show InChI InChI=1S/C21H16BrN3O3S/c22-16-8-14(7-15(9-16)20(28)13-4-2-6-18(27)11-13)19(24-25-21(23)29)12-3-1-5-17(26)10-12/h1-11,26-27H,(H3,23,25,29)/b24-19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L using Z-FR-AMC as substrate measured every 15 sec for 5 mins by fluorescence assay |
Bioorg Med Chem 23: 6974-92 (2015)
Article DOI: 10.1016/j.bmc.2015.09.036
BindingDB Entry DOI: 10.7270/Q2T43VX1 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50306649
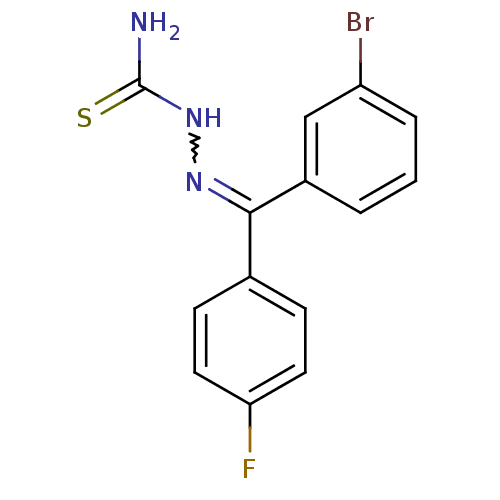
(CHEMBL602093 | [(3-Bromophenyl)-(4-fluorophenyl)-k...)Show SMILES NC(=S)NN=C(c1ccc(F)cc1)c1cccc(Br)c1 |w:4.3| Show InChI InChI=1S/C14H11BrFN3S/c15-11-3-1-2-10(8-11)13(18-19-14(17)20)9-4-6-12(16)7-5-9/h1-8H,(H3,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L |
Bioorg Med Chem Lett 20: 1415-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.090
BindingDB Entry DOI: 10.7270/Q2NG4QQZ |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50189280
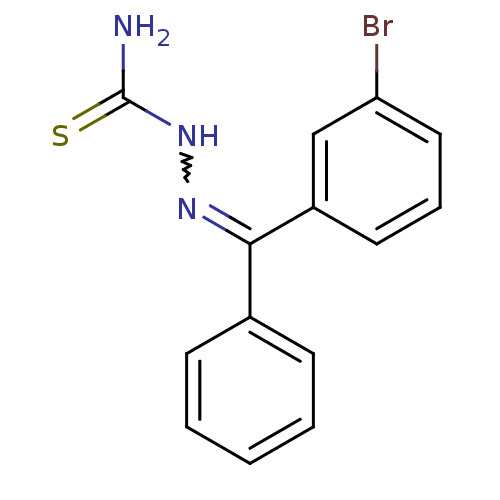
(1-((3-bromophenyl)(phenyl)methylene)thiosemicarbaz...)Show InChI InChI=1S/C14H12BrN3S/c15-12-8-4-7-11(9-12)13(17-18-14(16)19)10-5-2-1-3-6-10/h1-9H,(H3,16,18,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi recombinant cruzain |
Bioorg Med Chem Lett 16: 4405-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.041
BindingDB Entry DOI: 10.7270/Q23R0SHC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50306654
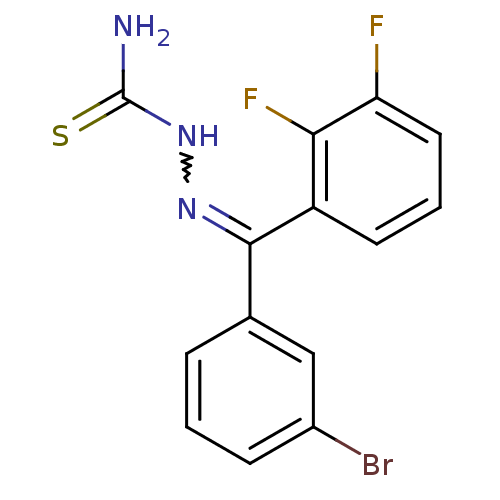
(CHEMBL601644 | [(3-Bromophenyl)-(2,3-difluoropheny...)Show SMILES NC(=S)NN=C(c1cccc(Br)c1)c1cccc(F)c1F |w:4.3| Show InChI InChI=1S/C14H10BrF2N3S/c15-9-4-1-3-8(7-9)13(19-20-14(18)21)10-5-2-6-11(16)12(10)17/h1-7H,(H3,18,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 83.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L |
Bioorg Med Chem Lett 20: 1415-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.090
BindingDB Entry DOI: 10.7270/Q2NG4QQZ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50306658
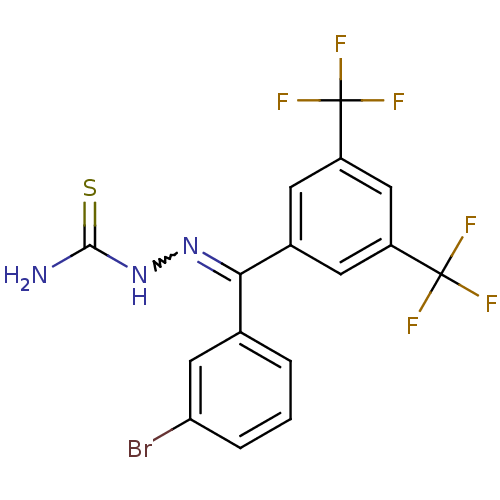
(CHEMBL603084 | [(3-Bromophenyl)-(2,4-ditrifluorome...)Show SMILES NC(=S)NN=C(c1cccc(Br)c1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |w:4.3| Show InChI InChI=1S/C16H10BrF6N3S/c17-12-3-1-2-8(6-12)13(25-26-14(24)27)9-4-10(15(18,19)20)7-11(5-9)16(21,22)23/h1-7H,(H3,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L |
Bioorg Med Chem Lett 20: 1415-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.090
BindingDB Entry DOI: 10.7270/Q2NG4QQZ |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50189271
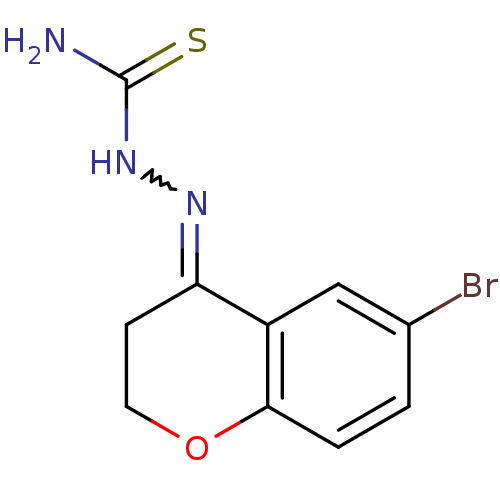
(1-(6-bromo-2,3-dihydrochromen-4-ylidene)thiosemica...)Show InChI InChI=1S/C10H10BrN3OS/c11-6-1-2-9-7(5-6)8(3-4-15-9)13-14-10(12)16/h1-2,5H,3-4H2,(H3,12,14,16) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi recombinant cruzain |
Bioorg Med Chem Lett 16: 4405-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.041
BindingDB Entry DOI: 10.7270/Q23R0SHC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50388835

(CHEMBL2062926)Show SMILES NC(=S)NN=C1CCS(=O)(=O)c2ccc(cc12)[N+]([O-])=O |w:4.3| Show InChI InChI=1S/C10H10N4O4S2/c11-10(19)13-12-8-3-4-20(17,18)9-2-1-6(14(15)16)5-7(8)9/h1-2,5H,3-4H2,(H3,11,13,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L using Z-R-R-AMC as substrate preincubated with compound for 5 mins measured after 20 mins by fluorescence analy... |
ACS Med Chem Lett 3: 450-453 (2012)
Article DOI: 10.1021/ml200299g
BindingDB Entry DOI: 10.7270/Q2QC04JR |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50306660
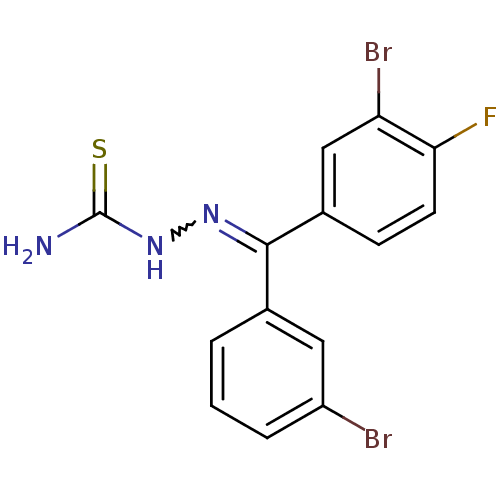
(CHEMBL603085 | [(3-Bromophenyl)-(3-bromo,4-fluorop...)Show SMILES NC(=S)NN=C(c1cccc(Br)c1)c1ccc(F)c(Br)c1 |w:4.3| Show InChI InChI=1S/C14H10Br2FN3S/c15-10-3-1-2-8(6-10)13(19-20-14(18)21)9-4-5-12(17)11(16)7-9/h1-7H,(H3,18,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L |
Bioorg Med Chem Lett 20: 1415-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.090
BindingDB Entry DOI: 10.7270/Q2NG4QQZ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50306661
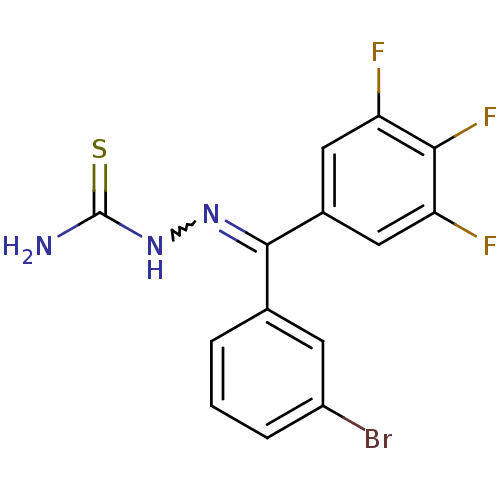
(CHEMBL599998 | [(3-Bromophenyl)-(3,4,5-trifluoroph...)Show SMILES NC(=S)NN=C(c1cccc(Br)c1)c1cc(F)c(F)c(F)c1 |w:4.3| Show InChI InChI=1S/C14H9BrF3N3S/c15-9-3-1-2-7(4-9)13(20-21-14(19)22)8-5-10(16)12(18)11(17)6-8/h1-6H,(H3,19,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L |
Bioorg Med Chem Lett 20: 1415-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.090
BindingDB Entry DOI: 10.7270/Q2NG4QQZ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50330034
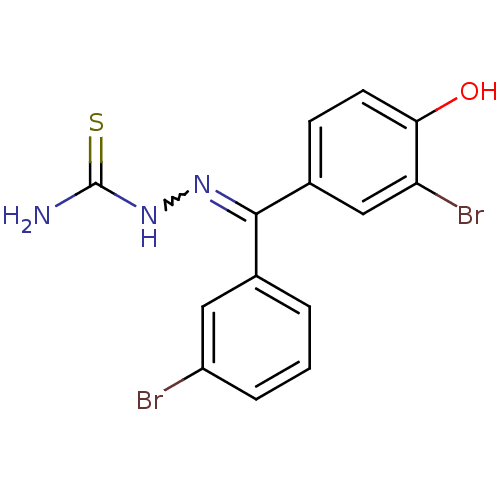
(Bis(3-bromophenyl)(4-hydroxy)thiosemicarbazone | C...)Show SMILES NC(=S)NN=C(c1cccc(Br)c1)c1ccc(O)c(Br)c1 |w:4.3| Show InChI InChI=1S/C14H11Br2N3OS/c15-10-3-1-2-8(6-10)13(18-19-14(17)21)9-4-5-12(20)11(16)7-9/h1-7,20H,(H3,17,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L after 5 mins by microplate reader analysis |
Bioorg Med Chem Lett 20: 6610-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.026
BindingDB Entry DOI: 10.7270/Q2MP53HZ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50306646
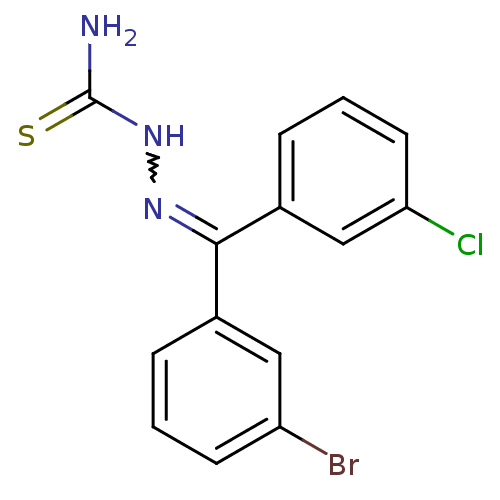
(CHEMBL606452 | [(3-Bromophenyl)-(3-chlorophenyl)-k...)Show SMILES NC(=S)NN=C(c1cccc(Cl)c1)c1cccc(Br)c1 |w:4.3| Show InChI InChI=1S/C14H11BrClN3S/c15-11-5-1-3-9(7-11)13(18-19-14(17)20)10-4-2-6-12(16)8-10/h1-8H,(H3,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L |
Bioorg Med Chem Lett 20: 1415-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.090
BindingDB Entry DOI: 10.7270/Q2NG4QQZ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50330030

(CHEMBL1269632 | [(3-Bromophenyl)-(3-hydroxyphenyl)...)Show SMILES NC(=S)NN=C(c1cccc(O)c1)c1cccc(Br)c1 |w:4.3| Show InChI InChI=1S/C14H12BrN3OS/c15-11-5-1-3-9(7-11)13(17-18-14(16)20)10-4-2-6-12(19)8-10/h1-8,19H,(H3,16,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L after 5 mins by microplate reader analysis |
Bioorg Med Chem Lett 20: 6610-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.026
BindingDB Entry DOI: 10.7270/Q2MP53HZ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50330031
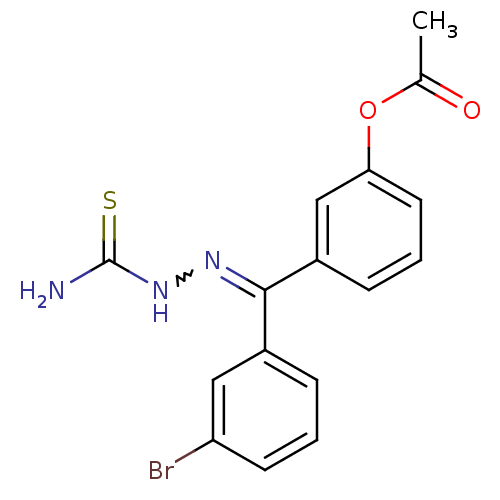
(CHEMBL1272261 | [(3-Bromophenyl)-(3-acetoxyphenyl)...)Show SMILES CC(=O)Oc1cccc(c1)C(=NNC(N)=S)c1cccc(Br)c1 |w:11.12| Show InChI InChI=1S/C16H14BrN3O2S/c1-10(21)22-14-7-3-5-12(9-14)15(19-20-16(18)23)11-4-2-6-13(17)8-11/h2-9H,1H3,(H3,18,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L after 5 mins by microplate reader analysis |
Bioorg Med Chem Lett 20: 6610-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.026
BindingDB Entry DOI: 10.7270/Q2MP53HZ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50388837
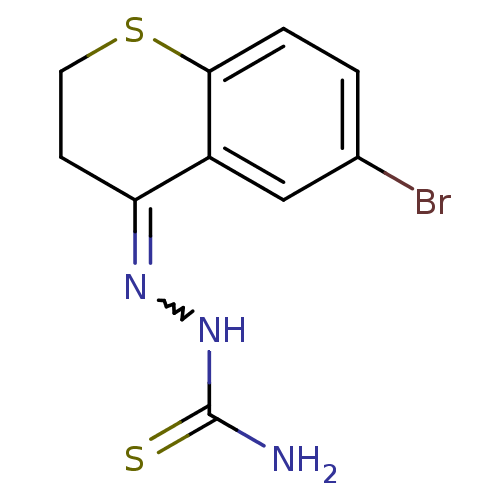
(CHEMBL2062896)Show InChI InChI=1S/C10H10BrN3S2/c11-6-1-2-9-7(5-6)8(3-4-16-9)13-14-10(12)15/h1-2,5H,3-4H2,(H3,12,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L using Z-R-R-AMC as substrate preincubated with compound for 5 mins measured after 20 mins by fluorescence analy... |
ACS Med Chem Lett 3: 450-453 (2012)
Article DOI: 10.1021/ml200299g
BindingDB Entry DOI: 10.7270/Q2QC04JR |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50491138

(CHEMBL2376019)Show InChI InChI=1S/C10H11BrN4S/c11-6-1-2-8-7(5-6)9(3-4-13-8)14-15-10(12)16/h1-2,5,13H,3-4H2,(H3,12,15,16)/b14-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 164 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver Cathepsin L using Z-Phe-Arg-aminomethylcoumarin as substrate incubated for 5 mins prior to substrate addition measured for ... |
Bioorg Med Chem Lett 23: 2801-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.025
BindingDB Entry DOI: 10.7270/Q2NS0XSX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50232473

(CHEMBL4087627)Show InChI InChI=1S/C14H11Br2N3OS/c15-10-4-9(5-11(16)7-10)13(18-19-14(17)21)8-2-1-3-12(20)6-8/h1-7,20H,(H3,17,19,21)/b18-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 202 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin-L using Z-FR-AMC as substrate preincubated for 5 mins followed by substrate addition measured at 15 sec interval ... |
Bioorg Med Chem Lett 27: 1304-1310 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.039
BindingDB Entry DOI: 10.7270/Q23B62C1 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50189278
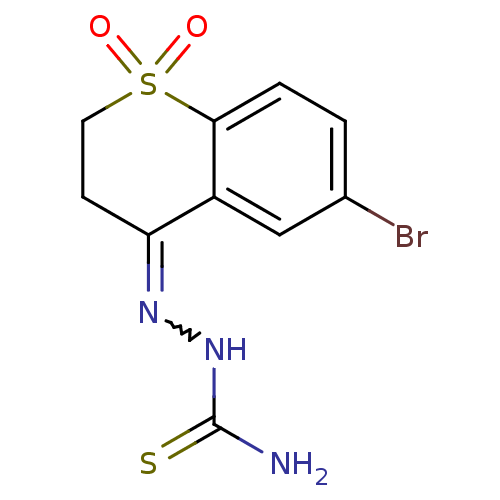
(6-bromo-2,3-dihydro-4H-thiochromen-4-one thiosemic...)Show SMILES NC(=S)NN=C1CCS(=O)(=O)c2ccc(Br)cc12 |w:4.3| Show InChI InChI=1S/C10H10BrN3O2S2/c11-6-1-2-9-7(5-6)8(13-14-10(12)17)3-4-18(9,15)16/h1-2,5H,3-4H2,(H3,12,14,17) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi recombinant cruzain |
Bioorg Med Chem Lett 16: 4405-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.041
BindingDB Entry DOI: 10.7270/Q23R0SHC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50388842
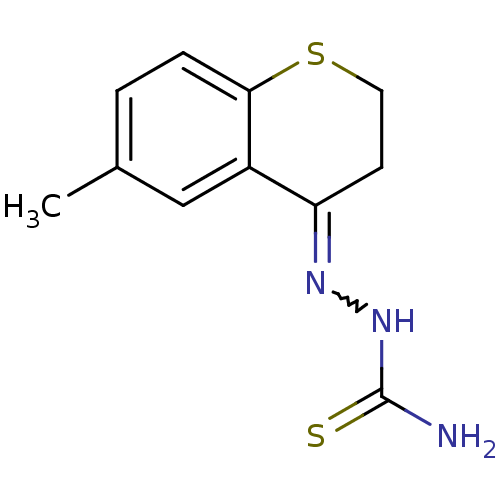
(CHEMBL2062901)Show InChI InChI=1S/C11H13N3S2/c1-7-2-3-10-8(6-7)9(4-5-16-10)13-14-11(12)15/h2-3,6H,4-5H2,1H3,(H3,12,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 214 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L using Z-R-R-AMC as substrate preincubated with compound for 5 mins measured after 20 mins by fluorescence analy... |
ACS Med Chem Lett 3: 450-453 (2012)
Article DOI: 10.1021/ml200299g
BindingDB Entry DOI: 10.7270/Q2QC04JR |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50306648
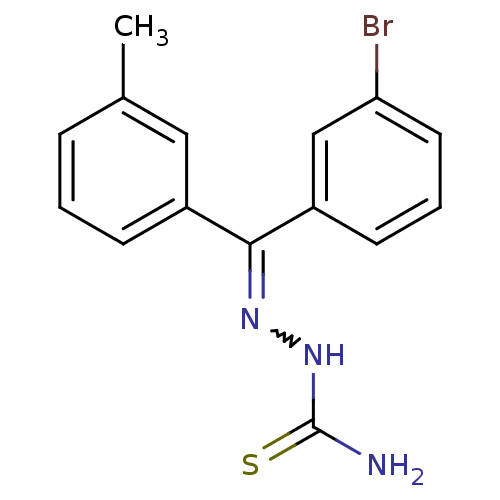
(CHEMBL602092 | [(3-Bromophenyl)-m-tolyl-ketone]thi...)Show SMILES Cc1cccc(c1)C(=NNC(N)=S)c1cccc(Br)c1 |w:8.9| Show InChI InChI=1S/C15H14BrN3S/c1-10-4-2-5-11(8-10)14(18-19-15(17)20)12-6-3-7-13(16)9-12/h2-9H,1H3,(H3,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L |
Bioorg Med Chem Lett 20: 1415-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.090
BindingDB Entry DOI: 10.7270/Q2NG4QQZ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM87123
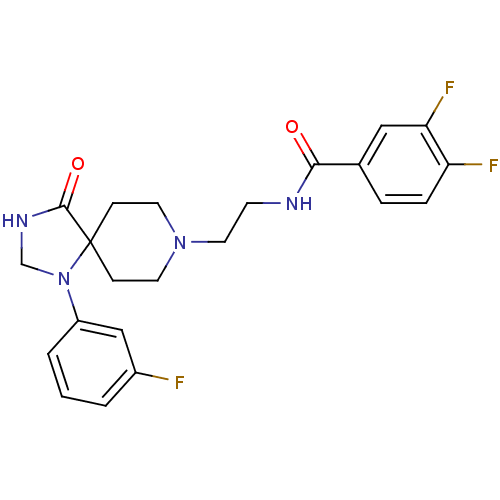
(3,4-bis(fluoranyl)-N-[2-[1-(3-fluorophenyl)-4-oxid...)Show SMILES Fc1cccc(c1)N1CNC(=O)C11CCN(CCNC(=O)c2ccc(F)c(F)c2)CC1 Show InChI InChI=1S/C22H23F3N4O2/c23-16-2-1-3-17(13-16)29-14-27-21(31)22(29)6-9-28(10-7-22)11-8-26-20(30)15-4-5-18(24)19(25)12-15/h1-5,12-13H,6-11,14H2,(H,26,30)(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 228 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L using Z-R-R-AMC as substrate preincubated with compound for 5 mins measured after 20 mins by fluorescence analy... |
ACS Med Chem Lett 3: 450-453 (2012)
Article DOI: 10.1021/ml200299g
BindingDB Entry DOI: 10.7270/Q2QC04JR |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50491134
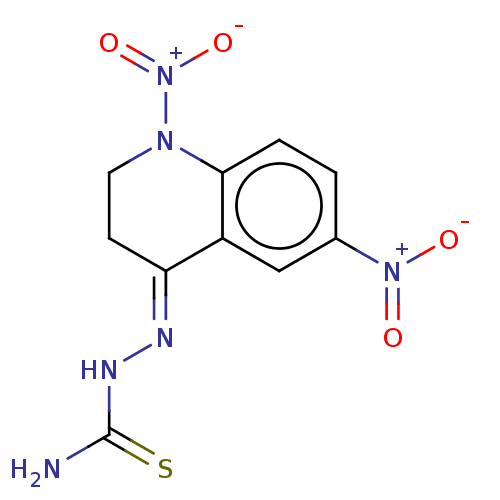
(CHEMBL2376003)Show SMILES NC(=S)N\N=C1/CCN(c2ccc(cc12)[N+]([O-])=O)[N+]([O-])=O Show InChI InChI=1S/C10H10N6O4S/c11-10(21)13-12-8-3-4-14(16(19)20)9-2-1-6(15(17)18)5-7(8)9/h1-2,5H,3-4H2,(H3,11,13,21)/b12-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 228 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver Cathepsin L using Z-Phe-Arg-aminomethylcoumarin as substrate incubated for 5 mins prior to substrate addition measured for ... |
Bioorg Med Chem Lett 23: 2801-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.025
BindingDB Entry DOI: 10.7270/Q2NS0XSX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50330036
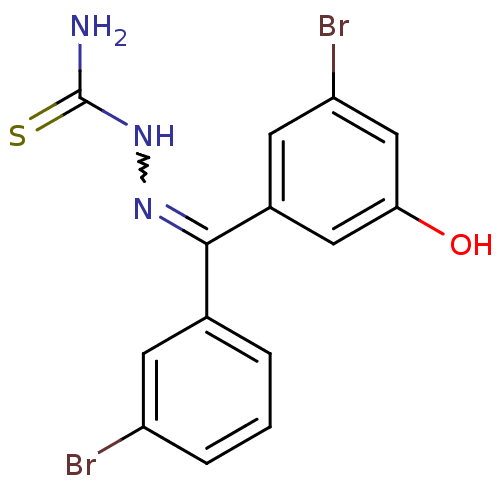
(Bis(3-bromophenyl)(5-hydroxy)thiosemicarbazone | C...)Show SMILES NC(=S)NN=C(c1cccc(Br)c1)c1cc(O)cc(Br)c1 |w:4.3| Show InChI InChI=1S/C14H11Br2N3OS/c15-10-3-1-2-8(4-10)13(18-19-14(17)21)9-5-11(16)7-12(20)6-9/h1-7,20H,(H3,17,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 232 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L after 5 mins by microplate reader analysis |
Bioorg Med Chem Lett 20: 6610-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.026
BindingDB Entry DOI: 10.7270/Q2MP53HZ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50306659
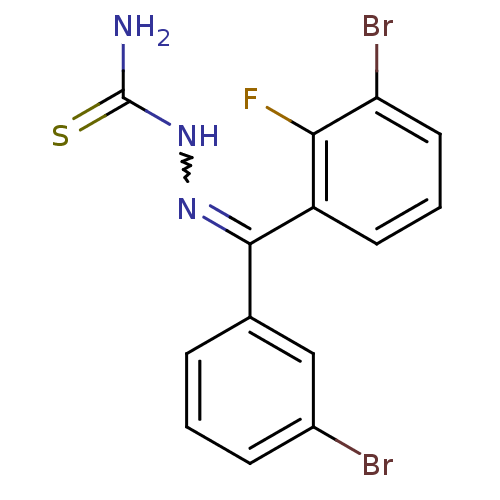
(CHEMBL602683 | [(3-Bromophenyl)-(3-bromo,2-fluorop...)Show SMILES NC(=S)NN=C(c1cccc(Br)c1)c1cccc(Br)c1F |w:4.3| Show InChI InChI=1S/C14H10Br2FN3S/c15-9-4-1-3-8(7-9)13(19-20-14(18)21)10-5-2-6-11(16)12(10)17/h1-7H,(H3,18,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L |
Bioorg Med Chem Lett 20: 1415-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.090
BindingDB Entry DOI: 10.7270/Q2NG4QQZ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50306645
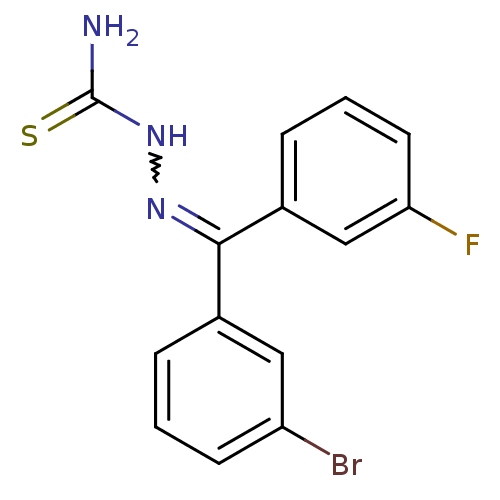
(CHEMBL605998 | [(3-Bromophenyl)-(3-fluorophenyl)-k...)Show SMILES NC(=S)NN=C(c1cccc(F)c1)c1cccc(Br)c1 |w:4.3| Show InChI InChI=1S/C14H11BrFN3S/c15-11-5-1-3-9(7-11)13(18-19-14(17)20)10-4-2-6-12(16)8-10/h1-8H,(H3,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L |
Bioorg Med Chem Lett 20: 1415-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.090
BindingDB Entry DOI: 10.7270/Q2NG4QQZ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50388848
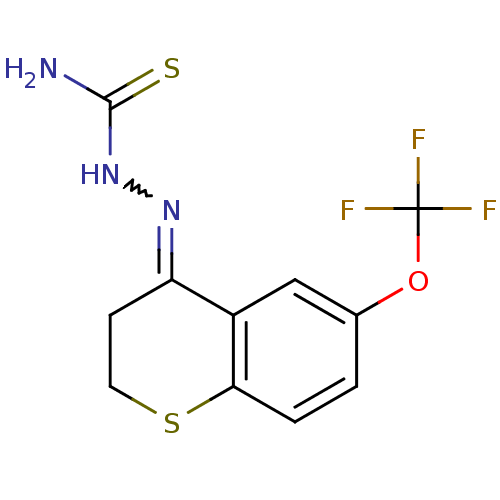
(CHEMBL2062907)Show InChI InChI=1S/C11H10F3N3OS2/c12-11(13,14)18-6-1-2-9-7(5-6)8(3-4-20-9)16-17-10(15)19/h1-2,5H,3-4H2,(H3,15,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 256 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L using Z-R-R-AMC as substrate preincubated with compound for 5 mins measured after 20 mins by fluorescence analy... |
ACS Med Chem Lett 3: 450-453 (2012)
Article DOI: 10.1021/ml200299g
BindingDB Entry DOI: 10.7270/Q2QC04JR |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50388832
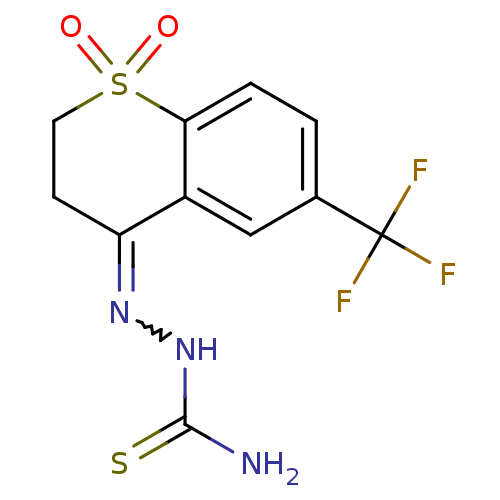
(CHEMBL2062923)Show SMILES NC(=S)NN=C1CCS(=O)(=O)c2ccc(cc12)C(F)(F)F |w:4.3| Show InChI InChI=1S/C11H10F3N3O2S2/c12-11(13,14)6-1-2-9-7(5-6)8(16-17-10(15)20)3-4-21(9,18)19/h1-2,5H,3-4H2,(H3,15,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L using Z-R-R-AMC as substrate preincubated with compound for 5 mins measured after 20 mins by fluorescence analy... |
ACS Med Chem Lett 3: 450-453 (2012)
Article DOI: 10.1021/ml200299g
BindingDB Entry DOI: 10.7270/Q2QC04JR |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50388847
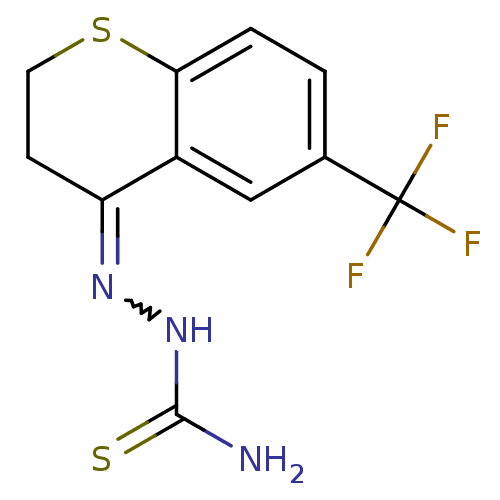
(CHEMBL2062906)Show InChI InChI=1S/C11H10F3N3S2/c12-11(13,14)6-1-2-9-7(5-6)8(3-4-19-9)16-17-10(15)18/h1-2,5H,3-4H2,(H3,15,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 284 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L using Z-R-R-AMC as substrate preincubated with compound for 5 mins measured after 20 mins by fluorescence analy... |
ACS Med Chem Lett 3: 450-453 (2012)
Article DOI: 10.1021/ml200299g
BindingDB Entry DOI: 10.7270/Q2QC04JR |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50306650
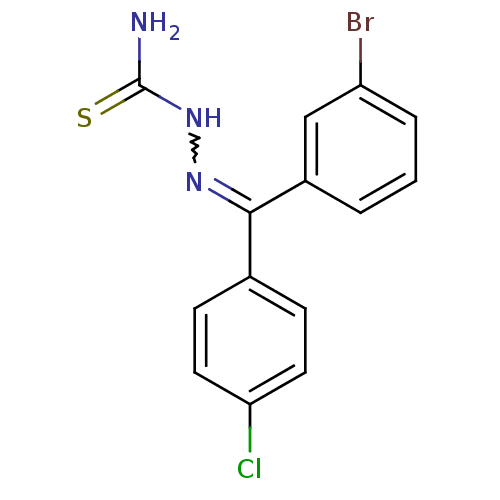
(CHEMBL602094 | [(3-Bromophenyl)-(4-chlorophenyl)-k...)Show SMILES NC(=S)NN=C(c1ccc(Cl)cc1)c1cccc(Br)c1 |w:4.3| Show InChI InChI=1S/C14H11BrClN3S/c15-11-3-1-2-10(8-11)13(18-19-14(17)20)9-4-6-12(16)7-5-9/h1-8H,(H3,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 327 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L |
Bioorg Med Chem Lett 20: 1415-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.090
BindingDB Entry DOI: 10.7270/Q2NG4QQZ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50491155
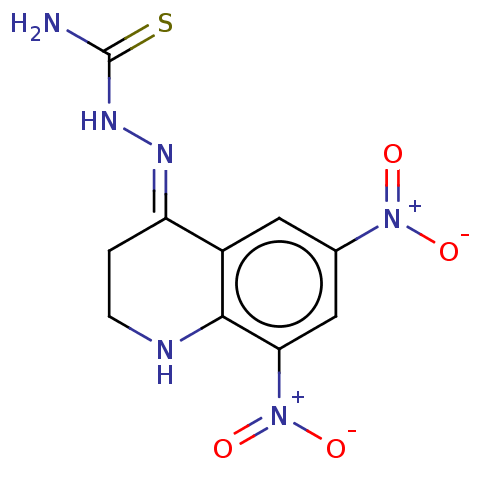
(CHEMBL2376004)Show SMILES NC(=S)N\N=C1/CCNc2c1cc(cc2[N+]([O-])=O)[N+]([O-])=O Show InChI InChI=1S/C10H10N6O4S/c11-10(21)14-13-7-1-2-12-9-6(7)3-5(15(17)18)4-8(9)16(19)20/h3-4,12H,1-2H2,(H3,11,14,21)/b13-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 338 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University
Curated by ChEMBL
| Assay Description
Inhibition of human liver Cathepsin L using Z-Phe-Arg-aminomethylcoumarin as substrate incubated for 5 mins prior to substrate addition measured for ... |
Bioorg Med Chem Lett 23: 2801-7 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.025
BindingDB Entry DOI: 10.7270/Q2NS0XSX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data