

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582801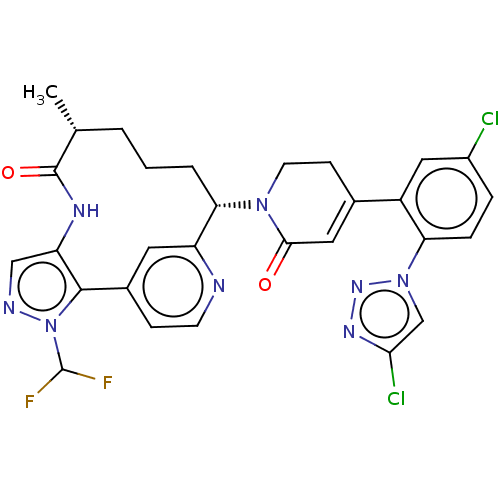 (CHEMBL5076656) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250493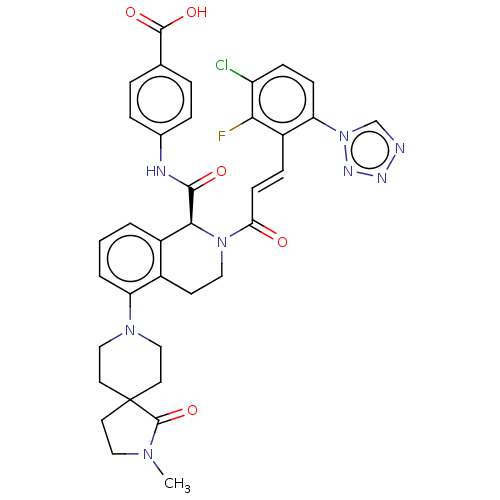 (CHEMBL4068445) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250492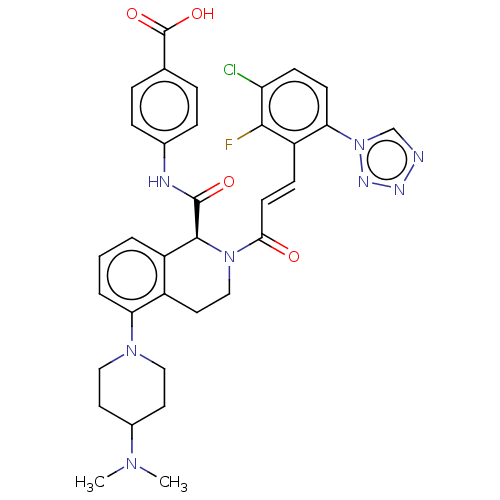 (CHEMBL4097304) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM247411 (US10336754, Example 353 | US11053247, Example 353 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582799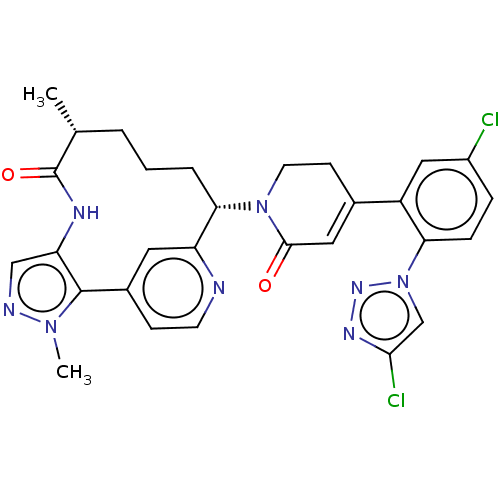 (CHEMBL5094166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230322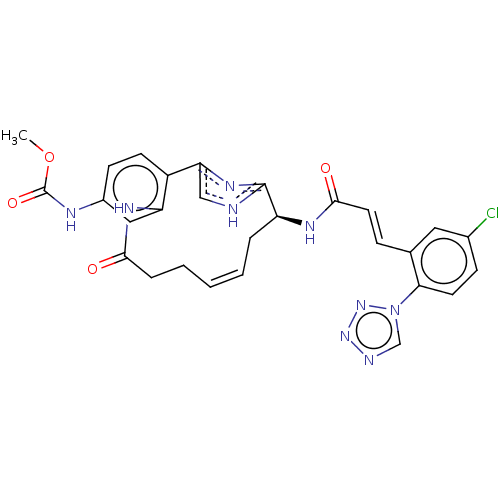 (CHEMBL4071545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582800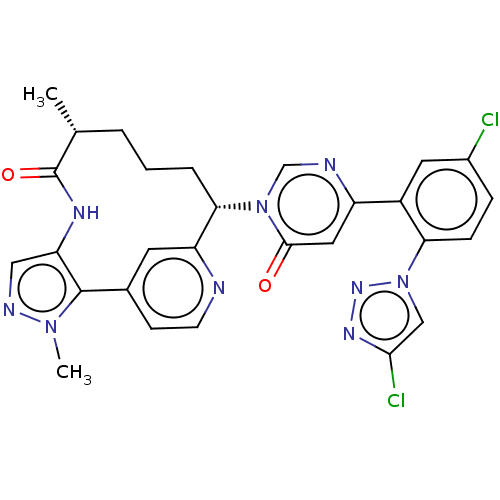 (CHEMBL5093567) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50514438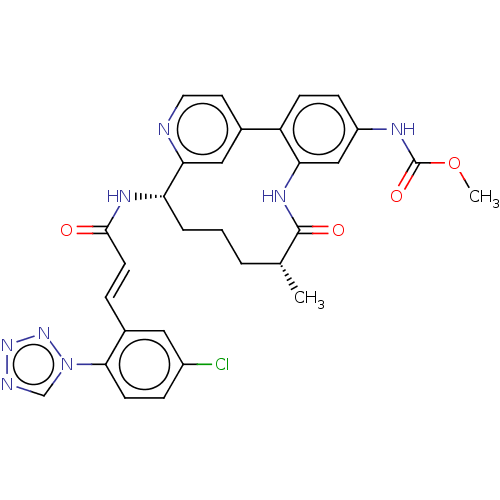 (CHEMBL4439729) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50269186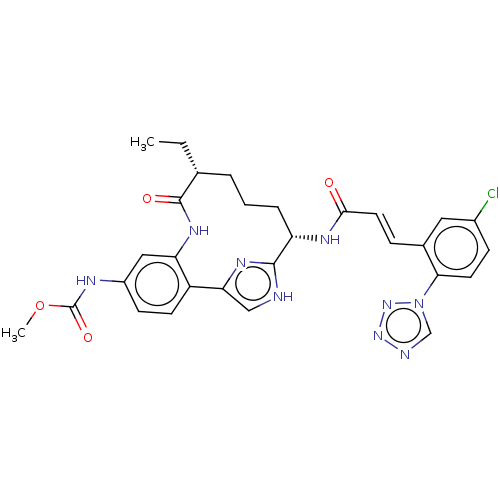 (CHEMBL4089185) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50514437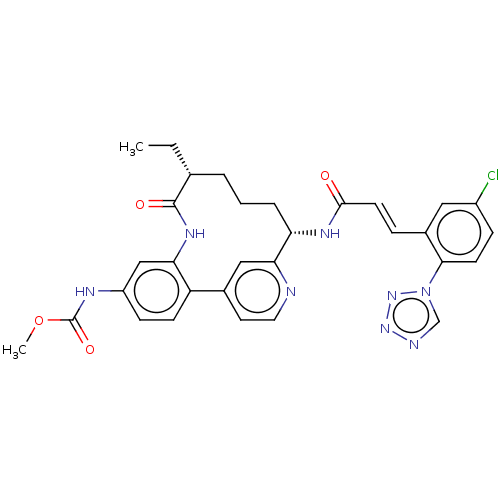 (CHEMBL4444690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50541586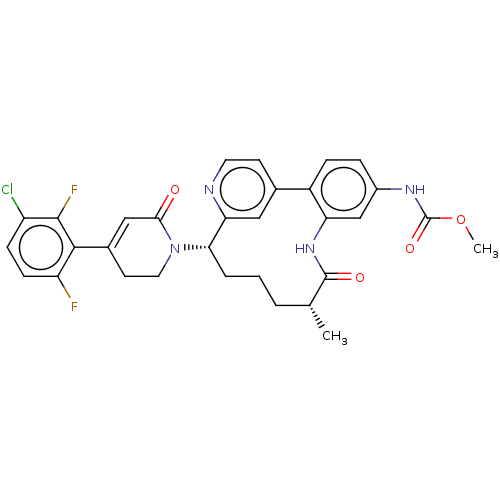 (CHEMBL4638245) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525762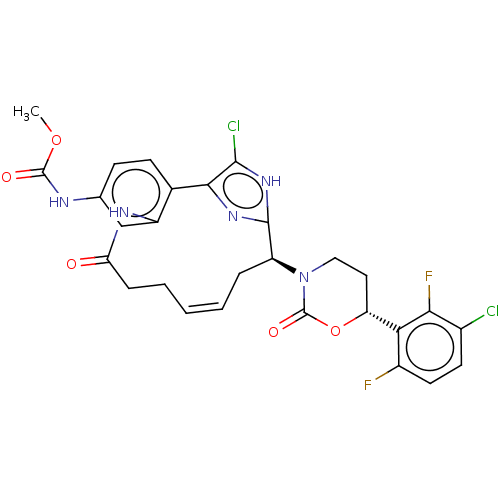 (CHEMBL4467360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50458535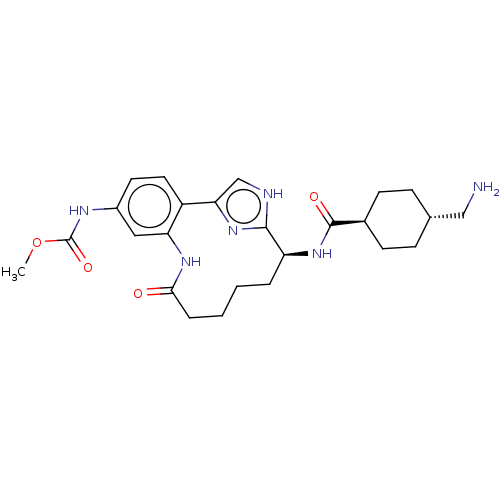 (CHEMBL4203066) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a incubated for 60 mins by Cheng-Prusoff equation analysis | J Med Chem 61: 7425-7447 (2018) Article DOI: 10.1021/acs.jmedchem.8b00173 BindingDB Entry DOI: 10.7270/Q2QJ7KW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250501 (CHEMBL4078562) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50269195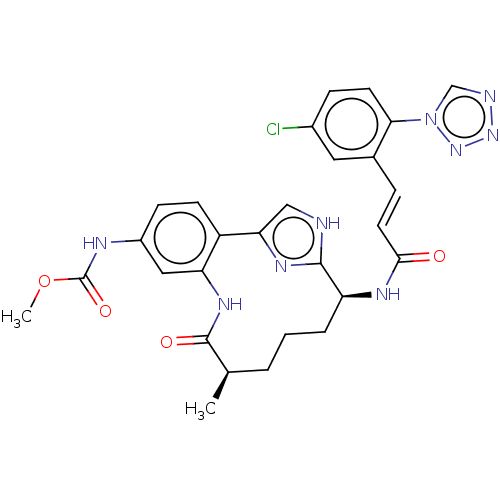 (CHEMBL4101766) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250490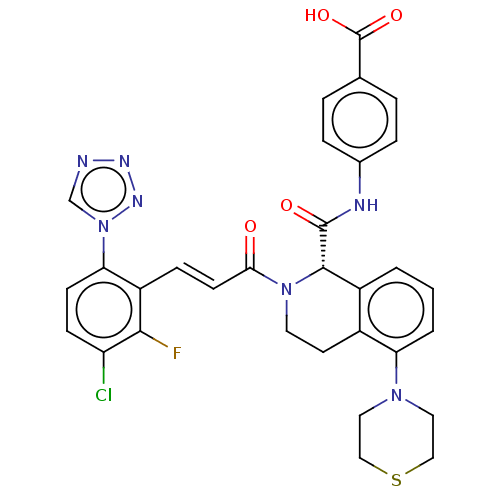 (CHEMBL4087166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM349975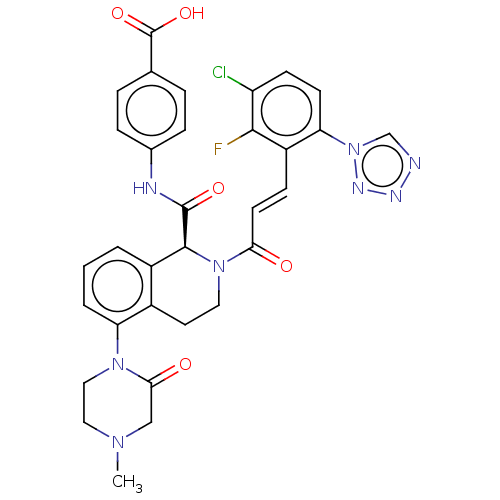 ((S,E)-4-(2-(3-(3-chloro-2-fluoro-6-(1H-tetrazol-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582798 (CHEMBL5082323) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525762 (CHEMBL4467360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582795 (CHEMBL5081455) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50514439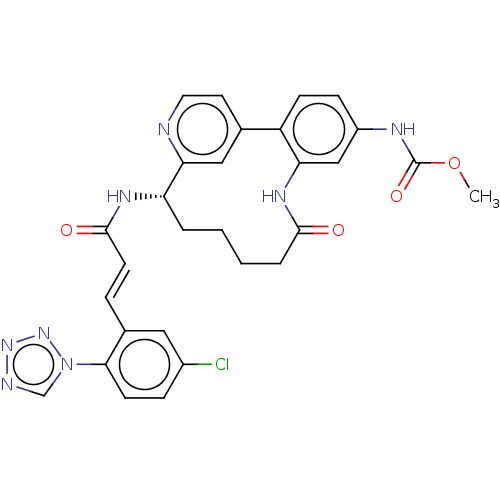 (CHEMBL4456818) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582794 (CHEMBL5077709) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250494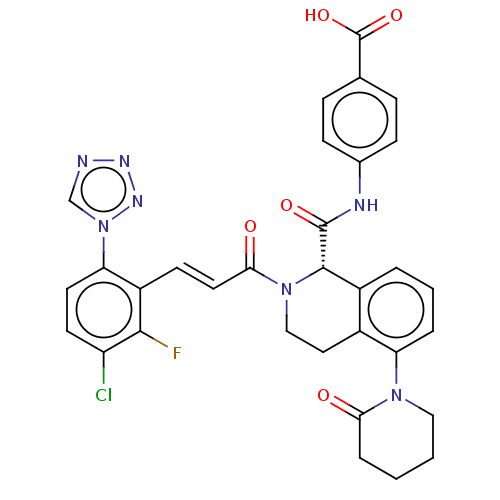 (CHEMBL4089581) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250495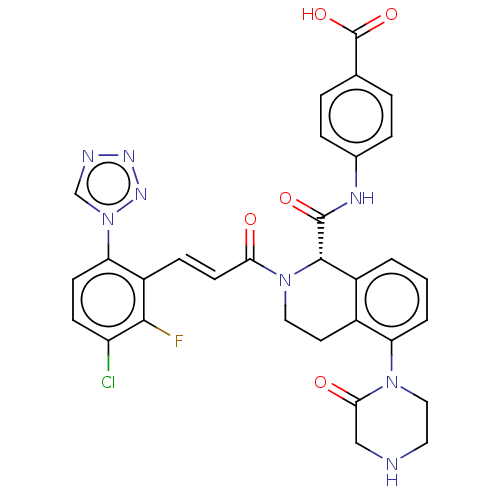 (CHEMBL4081710) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582793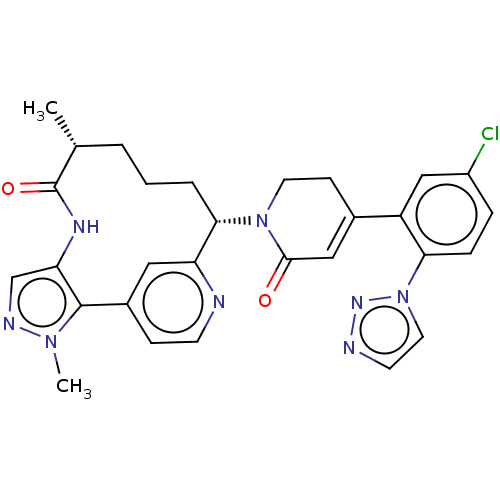 (CHEMBL5082956) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525770 (CHEMBL4452300) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525779 (CHEMBL4550408) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250512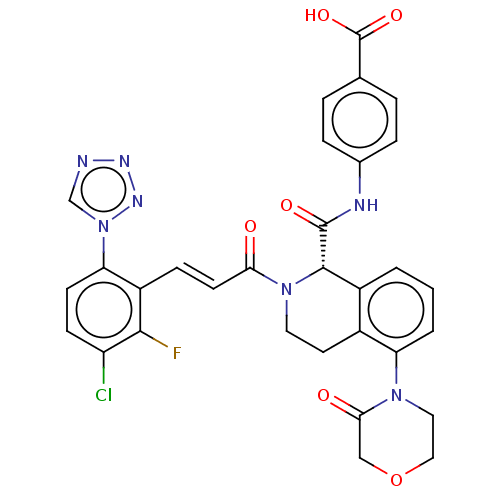 (CHEMBL4059788) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250491 (CHEMBL4105006) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM350469 (US10208068, Example 40) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525772 (CHEMBL4475559) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525773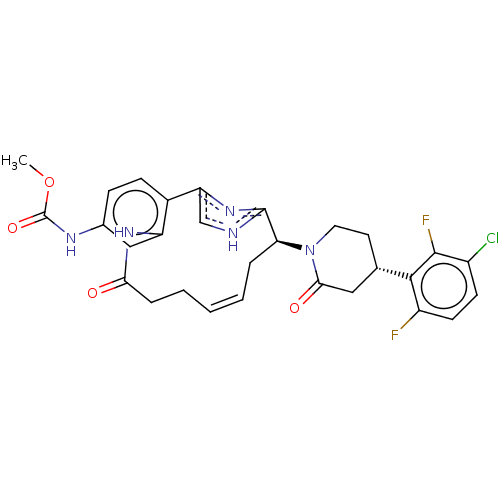 (CHEMBL4460266) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM350476 (US10208068, Example 78) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250511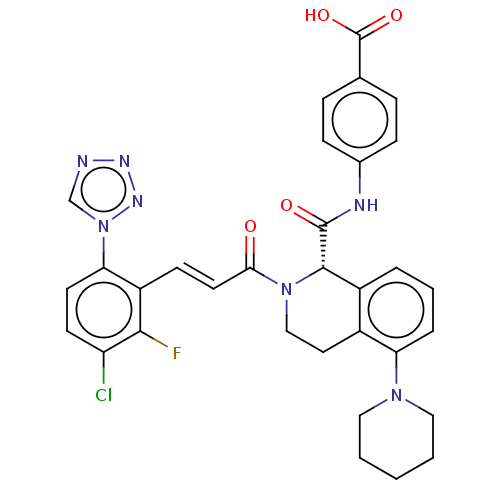 (CHEMBL4079281) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50582791 (CHEMBL5094070) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human coagulation factor 11a using L-Pyroglutamyl-L-prolyl-L-arginine p-Nitroaniline as substrate assessed as inhibition constant... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00613 BindingDB Entry DOI: 10.7270/Q20005Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50063669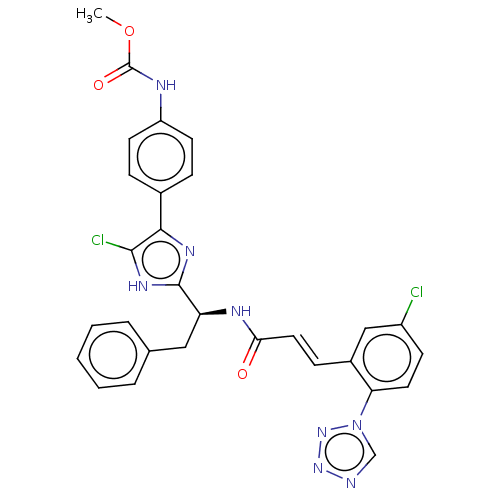 (CHEMBL3398641) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated coagulation factor 11 (unknown origin) assessed as inhibitory constant | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50514435 (CHEMBL4437236) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM161055 (US9108951, 10 | US9394276, 10 | US9725435, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50514432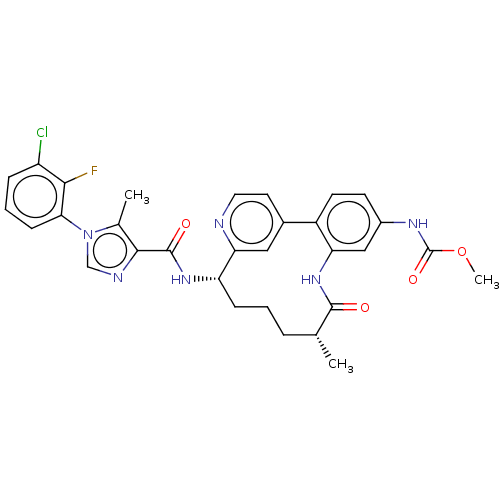 (CHEMBL4438385) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525771 (CHEMBL4465144) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525769 (CHEMBL4551053) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525761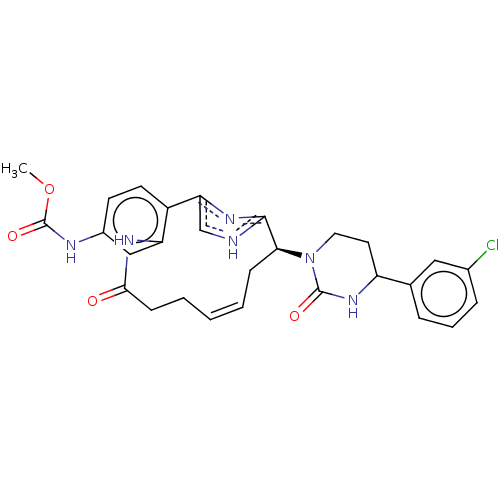 (CHEMBL4463167) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250489 (CHEMBL4090783) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM349974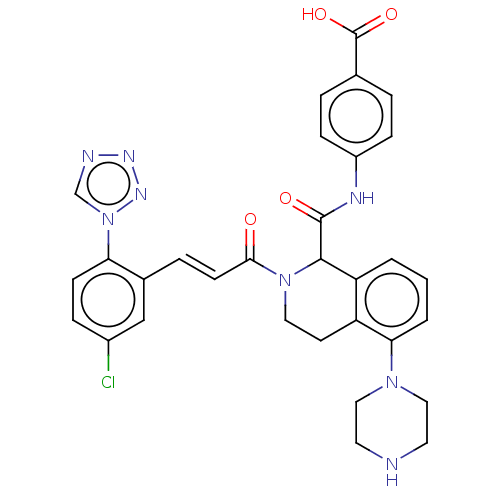 ((E)-4-(2-(3-(5-chloro-2-(1H-tetrazol-1-yl)phenyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525763 (CHEMBL4438562) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50514441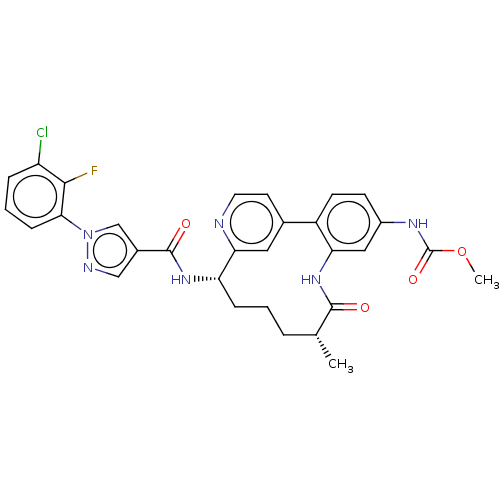 (CHEMBL4476061) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM349979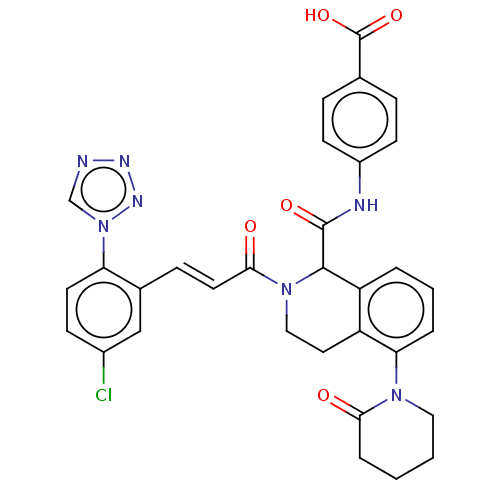 ((E)-4-(2-(3-(5-Chloro-2-(1H-tetrazol-1-yl)phenyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250496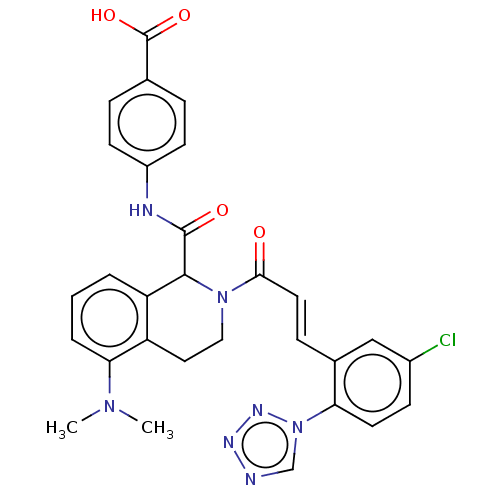 (CHEMBL4074535) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250485 (CHEMBL4063912) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50514432 (CHEMBL4438385) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human kallikrein using H-(D)-Pro-Phe-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 179 total ) | Next | Last >> |