 Found 184 hits with Last Name = 'pohl' and Initial = 'r'
Found 184 hits with Last Name = 'pohl' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50467705
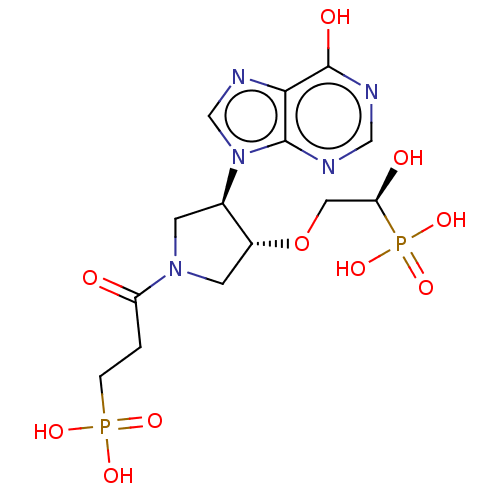
(CHEMBL4282830)Show SMILES O[C@H](CO[C@@H]1CN(C[C@H]1n1cnc2c(O)ncnc12)C(=O)CCP(O)(O)=O)P(O)(O)=O |r| Show InChI InChI=1S/C14H21N5O10P2/c20-10(1-2-30(23,24)25)18-3-8(9(4-18)29-5-11(21)31(26,27)28)19-7-17-12-13(19)15-6-16-14(12)22/h6-9,11,21H,1-5H2,(H,15,16,22)(H2,23,24,25)(H2,26,27,28)/t8-,9-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using fixed concentration of guanine and variable concentrations of PRib-PP as substrate by spectrophotometric analysis |
Eur J Med Chem 159: 10-22 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.039
BindingDB Entry DOI: 10.7270/Q2D22197 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50467710
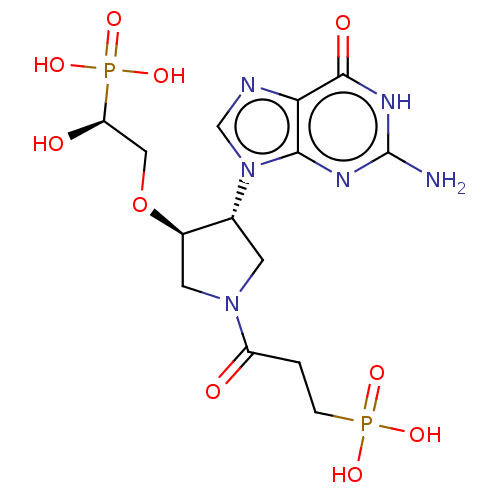
(CHEMBL4290716)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@H]1OC[C@@H](O)P(O)(O)=O)C(=O)CCP(O)(O)=O |r| Show InChI InChI=1S/C14H22N6O10P2/c15-14-17-12-11(13(23)18-14)16-6-20(12)7-3-19(9(21)1-2-31(24,25)26)4-8(7)30-5-10(22)32(27,28)29/h6-8,10,22H,1-5H2,(H2,24,25,26)(H2,27,28,29)(H3,15,17,18,23)/t7-,8-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HGPRT using fixed concentration of guanine and variable concentrations of PRib-PP as substrate by Hanes plot analysis |
Eur J Med Chem 159: 10-22 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.039
BindingDB Entry DOI: 10.7270/Q2D22197 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382672

(CHEMBL2024005)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@@H]1O)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O6P/c11-9-13-7-6(8(18)14-9)12-3-16(7)4-1-15(2-5(4)17)10(19)23(20,21)22/h3-5,17H,1-2H2,(H2,20,21,22)(H3,11,13,14,18)/t4-,5+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human DoHH2 cells using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382672

(CHEMBL2024005)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@@H]1O)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O6P/c11-9-13-7-6(8(18)14-9)12-3-16(7)4-1-15(2-5(4)17)10(19)23(20,21)22/h3-5,17H,1-2H2,(H2,20,21,22)(H3,11,13,14,18)/t4-,5+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant PNP using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50467708

(CHEMBL4277753)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@H]1OC[C@H](O)P(O)(O)=O)C(=O)CCP(O)(O)=O |r| Show InChI InChI=1S/C14H22N6O10P2/c15-14-17-12-11(13(23)18-14)16-6-20(12)7-3-19(9(21)1-2-31(24,25)26)4-8(7)30-5-10(22)32(27,28)29/h6-8,10,22H,1-5H2,(H2,24,25,26)(H2,27,28,29)(H3,15,17,18,23)/t7-,8-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HGPRT using fixed concentration of guanine and variable concentrations of PRib-PP as substrate by Hanes plot analysis |
Eur J Med Chem 159: 10-22 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.039
BindingDB Entry DOI: 10.7270/Q2D22197 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50467712

(CHEMBL4285691)Show SMILES O[C@@H](CO[C@@H]1CN(C[C@H]1n1cnc2c(O)ncnc12)C(=O)CCP(O)(O)=O)P(O)(O)=O |r| Show InChI InChI=1S/C14H21N5O10P2/c20-10(1-2-30(23,24)25)18-3-8(9(4-18)29-5-11(21)31(26,27)28)19-7-17-12-13(19)15-6-16-14(12)22/h6-9,11,21H,1-5H2,(H,15,16,22)(H2,23,24,25)(H2,26,27,28)/t8-,9-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using fixed concentration of guanine and variable concentrations of PRib-PP as substrate by spectrophotometric analysis |
Eur J Med Chem 159: 10-22 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.039
BindingDB Entry DOI: 10.7270/Q2D22197 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382672

(CHEMBL2024005)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@@H]1O)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O6P/c11-9-13-7-6(8(18)14-9)12-3-16(7)4-1-15(2-5(4)17)10(19)23(20,21)22/h3-5,17H,1-2H2,(H2,20,21,22)(H3,11,13,14,18)/t4-,5+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human Jurkat cells using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382672

(CHEMBL2024005)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@@H]1O)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O6P/c11-9-13-7-6(8(18)14-9)12-3-16(7)4-1-15(2-5(4)17)10(19)23(20,21)22/h3-5,17H,1-2H2,(H2,20,21,22)(H3,11,13,14,18)/t4-,5+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human K562 cells using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382672

(CHEMBL2024005)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@@H]1O)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O6P/c11-9-13-7-6(8(18)14-9)12-3-16(7)4-1-15(2-5(4)17)10(19)23(20,21)22/h3-5,17H,1-2H2,(H2,20,21,22)(H3,11,13,14,18)/t4-,5+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human MINO cells using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382672

(CHEMBL2024005)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@@H]1O)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O6P/c11-9-13-7-6(8(18)14-9)12-3-16(7)4-1-15(2-5(4)17)10(19)23(20,21)22/h3-5,17H,1-2H2,(H2,20,21,22)(H3,11,13,14,18)/t4-,5+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human PBMC using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382672

(CHEMBL2024005)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@@H]1O)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O6P/c11-9-13-7-6(8(18)14-9)12-3-16(7)4-1-15(2-5(4)17)10(19)23(20,21)22/h3-5,17H,1-2H2,(H2,20,21,22)(H3,11,13,14,18)/t4-,5+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human MC116 cells using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382672

(CHEMBL2024005)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@@H]1O)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O6P/c11-9-13-7-6(8(18)14-9)12-3-16(7)4-1-15(2-5(4)17)10(19)23(20,21)22/h3-5,17H,1-2H2,(H2,20,21,22)(H3,11,13,14,18)/t4-,5+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human SU-DHL-1 cells using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382672

(CHEMBL2024005)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@@H]1O)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O6P/c11-9-13-7-6(8(18)14-9)12-3-16(7)4-1-15(2-5(4)17)10(19)23(20,21)22/h3-5,17H,1-2H2,(H2,20,21,22)(H3,11,13,14,18)/t4-,5+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human AML cells obtained from patients using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382672

(CHEMBL2024005)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@@H]1O)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O6P/c11-9-13-7-6(8(18)14-9)12-3-16(7)4-1-15(2-5(4)17)10(19)23(20,21)22/h3-5,17H,1-2H2,(H2,20,21,22)(H3,11,13,14,18)/t4-,5+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human CML cells obtained from patients using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382673
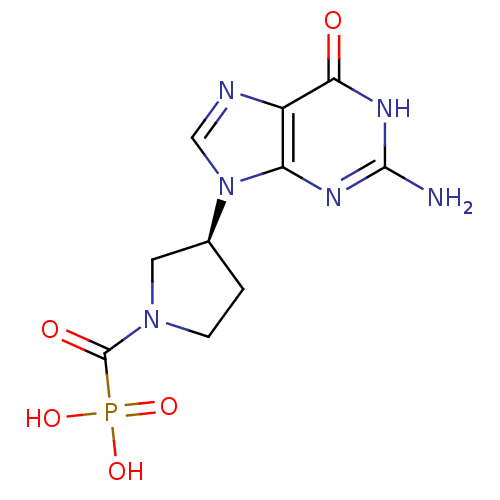
(CHEMBL2024003)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@H]1CCN(C1)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O5P/c11-9-13-7-6(8(17)14-9)12-4-16(7)5-1-2-15(3-5)10(18)22(19,20)21/h4-5H,1-3H2,(H2,19,20,21)(H3,11,13,14,17)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human Jurkat cells using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382672

(CHEMBL2024005)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@@H]1O)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O6P/c11-9-13-7-6(8(18)14-9)12-3-16(7)4-1-15(2-5(4)17)10(19)23(20,21)22/h3-5,17H,1-2H2,(H2,20,21,22)(H3,11,13,14,18)/t4-,5+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human OPM2 cells using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382672

(CHEMBL2024005)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@@H]1O)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O6P/c11-9-13-7-6(8(18)14-9)12-3-16(7)4-1-15(2-5(4)17)10(19)23(20,21)22/h3-5,17H,1-2H2,(H2,20,21,22)(H3,11,13,14,18)/t4-,5+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human HBL2 cells using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20079
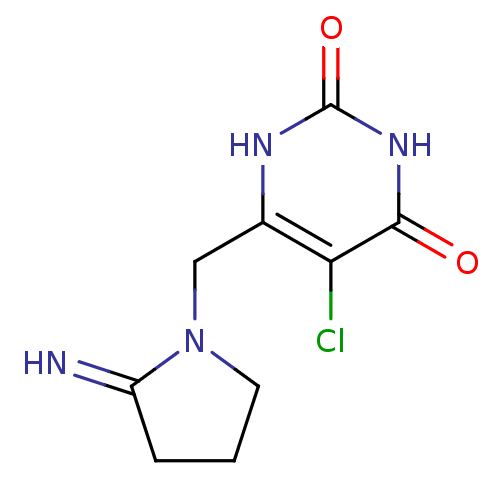
(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant thymidine phosphorylase |
Bioorg Med Chem Lett 20: 862-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.081
BindingDB Entry DOI: 10.7270/Q2930V4K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382673

(CHEMBL2024003)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@H]1CCN(C1)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O5P/c11-9-13-7-6(8(17)14-9)12-4-16(7)5-1-2-15(3-5)10(18)22(19,20)21/h4-5H,1-3H2,(H2,19,20,21)(H3,11,13,14,17)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human PBMC using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382672

(CHEMBL2024005)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@@H]1O)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O6P/c11-9-13-7-6(8(18)14-9)12-3-16(7)4-1-15(2-5(4)17)10(19)23(20,21)22/h3-5,17H,1-2H2,(H2,20,21,22)(H3,11,13,14,18)/t4-,5+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human RPMI8226 cells using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382672

(CHEMBL2024005)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@@H]1O)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O6P/c11-9-13-7-6(8(18)14-9)12-3-16(7)4-1-15(2-5(4)17)10(19)23(20,21)22/h3-5,17H,1-2H2,(H2,20,21,22)(H3,11,13,14,18)/t4-,5+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human HBL2 tumor cells using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382673

(CHEMBL2024003)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@H]1CCN(C1)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O5P/c11-9-13-7-6(8(17)14-9)12-4-16(7)5-1-2-15(3-5)10(18)22(19,20)21/h4-5H,1-3H2,(H2,19,20,21)(H3,11,13,14,17)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant PNP using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382673

(CHEMBL2024003)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@H]1CCN(C1)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O5P/c11-9-13-7-6(8(17)14-9)12-4-16(7)5-1-2-15(3-5)10(18)22(19,20)21/h4-5H,1-3H2,(H2,19,20,21)(H3,11,13,14,17)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human MINO cells using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50467707

(CHEMBL4280490)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@@H]1OCCP(O)(O)=O)C(=O)CCP(O)(O)=O |r| Show InChI InChI=1S/C14H22N6O9P2/c15-14-17-12-11(13(22)18-14)16-7-20(12)8-5-19(10(21)1-3-30(23,24)25)6-9(8)29-2-4-31(26,27)28/h7-9H,1-6H2,(H2,23,24,25)(H2,26,27,28)(H3,15,17,18,22)/t8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using fixed concentration of guanine and variable concentrations of PRib-PP as substrate by spectrophotometric analysis |
Eur J Med Chem 159: 10-22 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.039
BindingDB Entry DOI: 10.7270/Q2D22197 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382673

(CHEMBL2024003)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@H]1CCN(C1)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O5P/c11-9-13-7-6(8(17)14-9)12-4-16(7)5-1-2-15(3-5)10(18)22(19,20)21/h4-5H,1-3H2,(H2,19,20,21)(H3,11,13,14,17)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human SU-DHL-1 cells using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50467707

(CHEMBL4280490)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@@H]1OCCP(O)(O)=O)C(=O)CCP(O)(O)=O |r| Show InChI InChI=1S/C14H22N6O9P2/c15-14-17-12-11(13(22)18-14)16-7-20(12)8-5-19(10(21)1-3-30(23,24)25)6-9(8)29-2-4-31(26,27)28/h7-9H,1-6H2,(H2,23,24,25)(H2,26,27,28)(H3,15,17,18,22)/t8-,9+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant N-terminal 6His-tagged HGPRT expressed in Escherichia coli BL21 (DE3) using fixed concentration ... |
Eur J Med Chem 159: 10-22 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.039
BindingDB Entry DOI: 10.7270/Q2D22197 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382673

(CHEMBL2024003)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@H]1CCN(C1)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O5P/c11-9-13-7-6(8(17)14-9)12-4-16(7)5-1-2-15(3-5)10(18)22(19,20)21/h4-5H,1-3H2,(H2,19,20,21)(H3,11,13,14,17)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human DoHH2 cells using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382673

(CHEMBL2024003)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@H]1CCN(C1)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O5P/c11-9-13-7-6(8(17)14-9)12-4-16(7)5-1-2-15(3-5)10(18)22(19,20)21/h4-5H,1-3H2,(H2,19,20,21)(H3,11,13,14,17)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human RPMI8226 cells using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382673

(CHEMBL2024003)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@H]1CCN(C1)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O5P/c11-9-13-7-6(8(17)14-9)12-4-16(7)5-1-2-15(3-5)10(18)22(19,20)21/h4-5H,1-3H2,(H2,19,20,21)(H3,11,13,14,17)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human MC116 cells using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382673

(CHEMBL2024003)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@H]1CCN(C1)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O5P/c11-9-13-7-6(8(17)14-9)12-4-16(7)5-1-2-15(3-5)10(18)22(19,20)21/h4-5H,1-3H2,(H2,19,20,21)(H3,11,13,14,17)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human AML cells obtained from patients using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50467705

(CHEMBL4282830)Show SMILES O[C@H](CO[C@@H]1CN(C[C@H]1n1cnc2c(O)ncnc12)C(=O)CCP(O)(O)=O)P(O)(O)=O |r| Show InChI InChI=1S/C14H21N5O10P2/c20-10(1-2-30(23,24)25)18-3-8(9(4-18)29-5-11(21)31(26,27)28)19-7-17-12-13(19)15-6-16-14(12)22/h6-9,11,21H,1-5H2,(H,15,16,22)(H2,23,24,25)(H2,26,27,28)/t8-,9-,11+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant N-terminal 6His-tagged HGPRT expressed in Escherichia coli BL21 (DE3) using fixed concentration ... |
Eur J Med Chem 159: 10-22 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.039
BindingDB Entry DOI: 10.7270/Q2D22197 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382673

(CHEMBL2024003)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@H]1CCN(C1)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O5P/c11-9-13-7-6(8(17)14-9)12-4-16(7)5-1-2-15(3-5)10(18)22(19,20)21/h4-5H,1-3H2,(H2,19,20,21)(H3,11,13,14,17)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human K562 cells using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50467710

(CHEMBL4290716)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@H]1OC[C@@H](O)P(O)(O)=O)C(=O)CCP(O)(O)=O |r| Show InChI InChI=1S/C14H22N6O10P2/c15-14-17-12-11(13(23)18-14)16-6-20(12)7-3-19(9(21)1-2-31(24,25)26)4-8(7)30-5-10(22)32(27,28)29/h6-8,10,22H,1-5H2,(H2,24,25,26)(H2,27,28,29)(H3,15,17,18,23)/t7-,8-,10+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant N-terminal 6His-tagged HGPRT expressed in Escherichia coli BL21 (DE3) using fixed concentration ... |
Eur J Med Chem 159: 10-22 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.039
BindingDB Entry DOI: 10.7270/Q2D22197 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382673

(CHEMBL2024003)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@H]1CCN(C1)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O5P/c11-9-13-7-6(8(17)14-9)12-4-16(7)5-1-2-15(3-5)10(18)22(19,20)21/h4-5H,1-3H2,(H2,19,20,21)(H3,11,13,14,17)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human CML cells obtained from patients using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382673

(CHEMBL2024003)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@H]1CCN(C1)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O5P/c11-9-13-7-6(8(17)14-9)12-4-16(7)5-1-2-15(3-5)10(18)22(19,20)21/h4-5H,1-3H2,(H2,19,20,21)(H3,11,13,14,17)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human OPM2 cells using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382673

(CHEMBL2024003)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@H]1CCN(C1)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O5P/c11-9-13-7-6(8(17)14-9)12-4-16(7)5-1-2-15(3-5)10(18)22(19,20)21/h4-5H,1-3H2,(H2,19,20,21)(H3,11,13,14,17)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human HBL2 cells using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382673

(CHEMBL2024003)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@H]1CCN(C1)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O5P/c11-9-13-7-6(8(17)14-9)12-4-16(7)5-1-2-15(3-5)10(18)22(19,20)21/h4-5H,1-3H2,(H2,19,20,21)(H3,11,13,14,17)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Competitive inhibition of PNP in human HBL2 tumor cells using MSEG as substrate by Dixon-plot analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50467708

(CHEMBL4277753)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@H]1OC[C@H](O)P(O)(O)=O)C(=O)CCP(O)(O)=O |r| Show InChI InChI=1S/C14H22N6O10P2/c15-14-17-12-11(13(23)18-14)16-6-20(12)7-3-19(9(21)1-2-31(24,25)26)4-8(7)30-5-10(22)32(27,28)29/h6-8,10,22H,1-5H2,(H2,24,25,26)(H2,27,28,29)(H3,15,17,18,23)/t7-,8-,10-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant N-terminal 6His-tagged HGPRT expressed in Escherichia coli BL21 (DE3) using fixed concentration ... |
Eur J Med Chem 159: 10-22 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.039
BindingDB Entry DOI: 10.7270/Q2D22197 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50467712

(CHEMBL4285691)Show SMILES O[C@@H](CO[C@@H]1CN(C[C@H]1n1cnc2c(O)ncnc12)C(=O)CCP(O)(O)=O)P(O)(O)=O |r| Show InChI InChI=1S/C14H21N5O10P2/c20-10(1-2-30(23,24)25)18-3-8(9(4-18)29-5-11(21)31(26,27)28)19-7-17-12-13(19)15-6-16-14(12)22/h6-9,11,21H,1-5H2,(H,15,16,22)(H2,23,24,25)(H2,26,27,28)/t8-,9-,11-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant N-terminal 6His-tagged HGPRT expressed in Escherichia coli BL21 (DE3) using fixed concentration ... |
Eur J Med Chem 159: 10-22 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.039
BindingDB Entry DOI: 10.7270/Q2D22197 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50504220
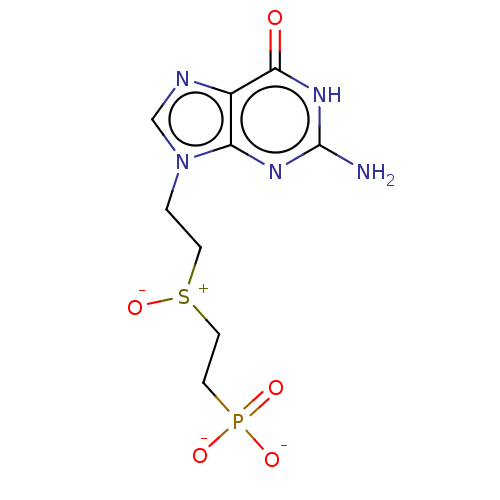
(CHEMBL4454497)Show SMILES Nc1nc2n(CC[S+]([O-])CCP([O-])([O-])=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C9H14N5O5PS.2Na/c10-9-12-7-6(8(15)13-9)11-5-14(7)1-3-21(19)4-2-20(16,17)18;;/h5H,1-4H2,(H2,16,17,18)(H3,10,12,13,15);;/q;2*+1/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by Hanes-plot based method |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111667
BindingDB Entry DOI: 10.7270/Q2JS9TPT |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50310202
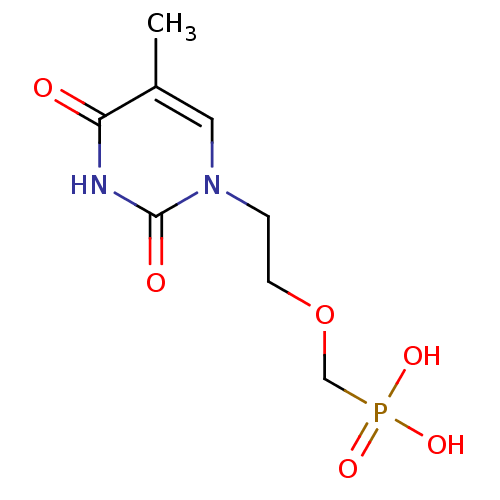
((2-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-...)Show InChI InChI=1S/C8H13N2O6P/c1-6-4-10(8(12)9-7(6)11)2-3-16-5-17(13,14)15/h4H,2-3,5H2,1H3,(H,9,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant thymidine phosphorylase |
Bioorg Med Chem Lett 20: 862-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.081
BindingDB Entry DOI: 10.7270/Q2930V4K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50310203
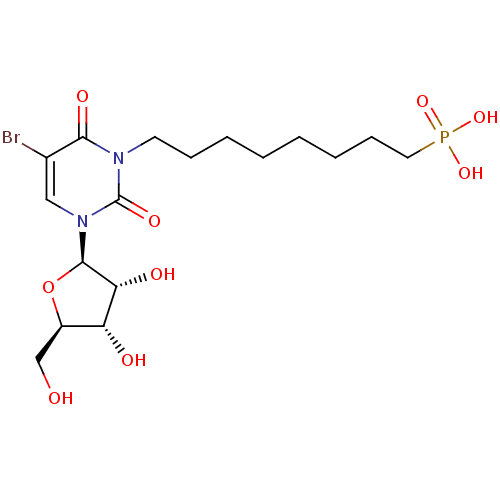
(8-(5-bromo-3-((2R,3R,4S,5R)-3,4-dihydroxy-5-(hydro...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(Br)c(=O)n(CCCCCCCCP(O)(O)=O)c1=O |r| Show InChI InChI=1S/C17H28BrN2O9P/c18-11-9-20(16-14(23)13(22)12(10-21)29-16)17(25)19(15(11)24)7-5-3-1-2-4-6-8-30(26,27)28/h9,12-14,16,21-23H,1-8,10H2,(H2,26,27,28)/t12-,13-,14-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant thymidine phosphorylase |
Bioorg Med Chem Lett 20: 862-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.081
BindingDB Entry DOI: 10.7270/Q2930V4K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50310200
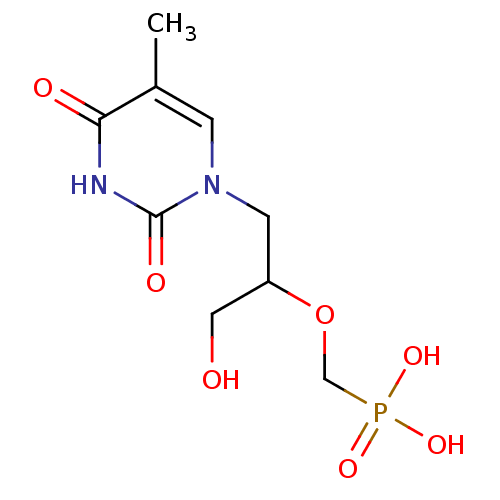
((1-hydroxy-3-(5-methyl-2,4-dioxo-3,4-dihydropyrimi...)Show InChI InChI=1S/C9H15N2O7P/c1-6-2-11(9(14)10-8(6)13)3-7(4-12)18-5-19(15,16)17/h2,7,12H,3-5H2,1H3,(H,10,13,14)(H2,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant thymidine phosphorylase |
Bioorg Med Chem Lett 20: 862-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.081
BindingDB Entry DOI: 10.7270/Q2930V4K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50310199
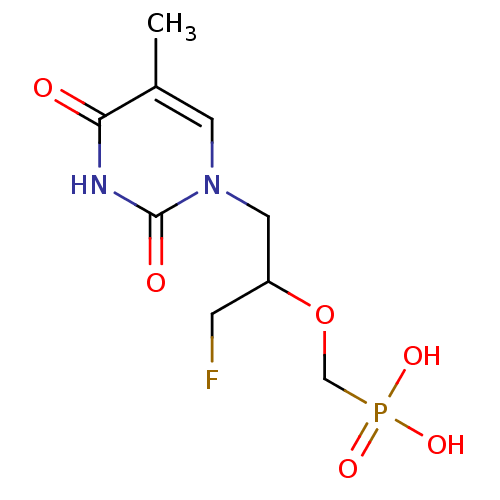
((1-fluoro-3-(5-methyl-2,4-dioxo-3,4-dihydropyrimid...)Show InChI InChI=1S/C9H14FN2O6P/c1-6-3-12(9(14)11-8(6)13)4-7(2-10)18-5-19(15,16)17/h3,7H,2,4-5H2,1H3,(H,11,13,14)(H2,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant thymidine phosphorylase |
Bioorg Med Chem Lett 20: 862-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.081
BindingDB Entry DOI: 10.7270/Q2930V4K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50310201
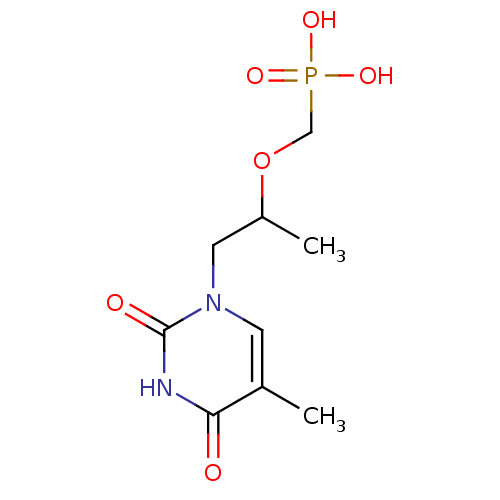
((1-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-...)Show InChI InChI=1S/C9H15N2O6P/c1-6-3-11(9(13)10-8(6)12)4-7(2)17-5-18(14,15)16/h3,7H,4-5H2,1-2H3,(H,10,12,13)(H2,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant thymidine phosphorylase |
Bioorg Med Chem Lett 20: 862-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.081
BindingDB Entry DOI: 10.7270/Q2930V4K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50201010
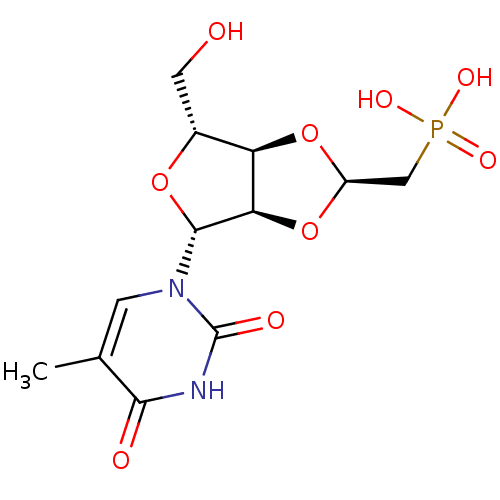
(((2R,3aR,4R,6R,6aR)-4-(hydroxymethyl)-6-(5-methyl-...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)[C@H]3O[C@@H](CP(O)(O)=O)O[C@@H]23)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C12H17N2O9P/c1-5-2-14(12(17)13-10(5)16)11-9-8(6(3-15)21-11)22-7(23-9)4-24(18,19)20/h2,6-9,11,15H,3-4H2,1H3,(H,13,16,17)(H2,18,19,20)/t6-,7-,8-,9-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant thymidine phosphorylase |
Bioorg Med Chem Lett 20: 862-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.081
BindingDB Entry DOI: 10.7270/Q2930V4K |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50504218

(CHEMBL4437088)Show SMILES Nc1nc2n(CCSCCP([O-])([O-])=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C9H14N5O4PS/c10-9-12-7-6(8(15)13-9)11-5-14(7)1-3-20-4-2-19(16,17)18/h5H,1-4H2,(H2,16,17,18)(H3,10,12,13,15)/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by Hanes-plot based method |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111667
BindingDB Entry DOI: 10.7270/Q2JS9TPT |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50467709

(CHEMBL4291117)Show SMILES Oc1ncnc2n(cnc12)[C@@H]1CN(C[C@@H]1OCCP(O)(O)=O)C(=O)CCP(O)(O)=O |r| Show InChI InChI=1S/C14H21N5O9P2/c20-11(1-3-29(22,23)24)18-5-9(10(6-18)28-2-4-30(25,26)27)19-8-17-12-13(19)15-7-16-14(12)21/h7-10H,1-6H2,(H,15,16,21)(H2,22,23,24)(H2,25,26,27)/t9-,10+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant N-terminal 6His-tagged HGPRT expressed in Escherichia coli BL21 (DE3) using fixed concentration ... |
Eur J Med Chem 159: 10-22 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.039
BindingDB Entry DOI: 10.7270/Q2D22197 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50467709

(CHEMBL4291117)Show SMILES Oc1ncnc2n(cnc12)[C@@H]1CN(C[C@@H]1OCCP(O)(O)=O)C(=O)CCP(O)(O)=O |r| Show InChI InChI=1S/C14H21N5O9P2/c20-11(1-3-29(22,23)24)18-5-9(10(6-18)28-2-4-30(25,26)27)19-8-17-12-13(19)15-7-16-14(12)21/h7-10H,1-6H2,(H,15,16,21)(H2,22,23,24)(H2,25,26,27)/t9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using fixed concentration of guanine and variable concentrations of PRib-PP as substrate by spectrophotometric analysis |
Eur J Med Chem 159: 10-22 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.039
BindingDB Entry DOI: 10.7270/Q2D22197 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50467711
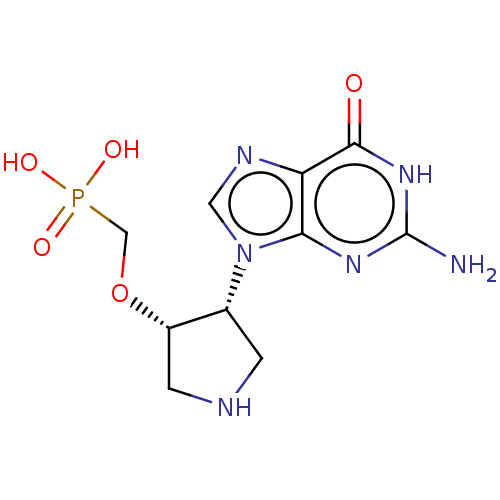
(CHEMBL4283940)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CNC[C@@H]1OCP(O)(O)=O |r| Show InChI InChI=1S/C10H15N6O5P/c11-10-14-8-7(9(17)15-10)13-3-16(8)5-1-12-2-6(5)21-4-22(18,19)20/h3,5-6,12H,1-2,4H2,(H2,18,19,20)(H3,11,14,15,17)/t5-,6+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant N-terminal 6His-tagged HGPRT expressed in Escherichia coli BL21 (DE3) using fixed concentration ... |
Eur J Med Chem 159: 10-22 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.039
BindingDB Entry DOI: 10.7270/Q2D22197 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data