

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146832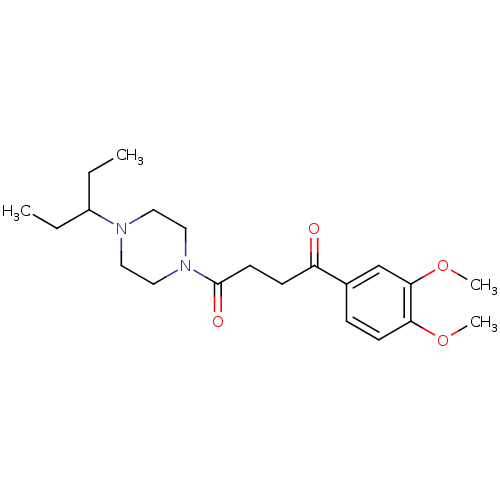 (1-(3,4-Dimethoxy-phenyl)-4-[4-(1-ethyl-propyl)-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370330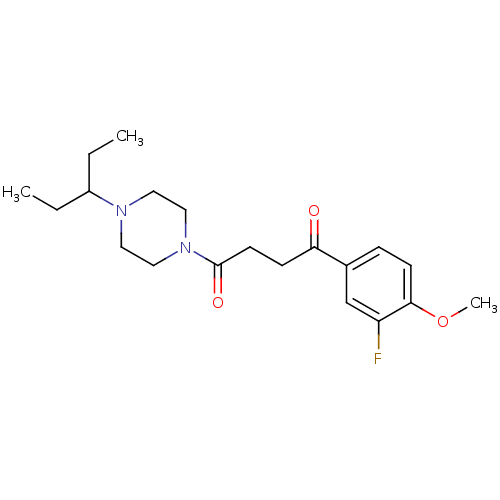 (CHEMBL1202893) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146836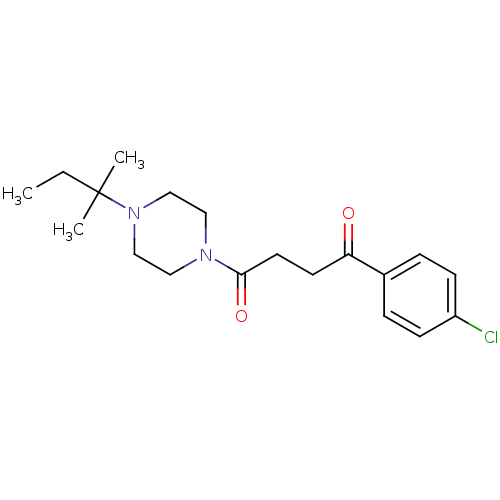 (1-(4-Chloro-phenyl)-4-[4-(1,1-dimethyl-propyl)-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370328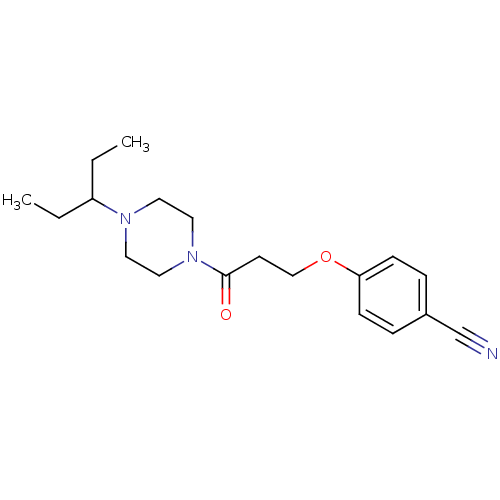 (CHEMBL1202892) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146838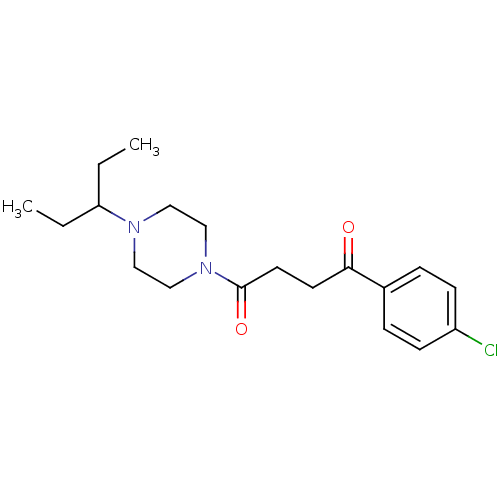 (1-(4-Chloro-phenyl)-4-[4-(1-ethyl-propyl)-piperazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370331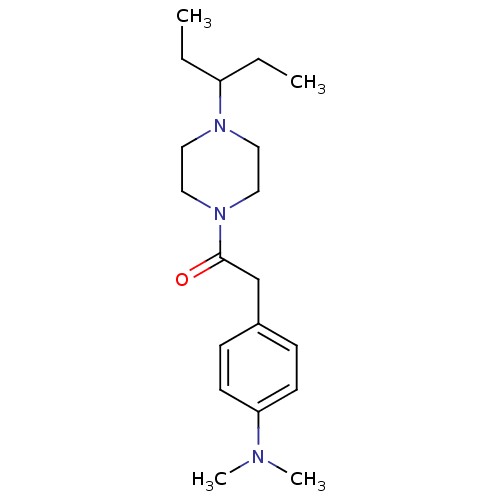 (CHEMBL1202896) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146840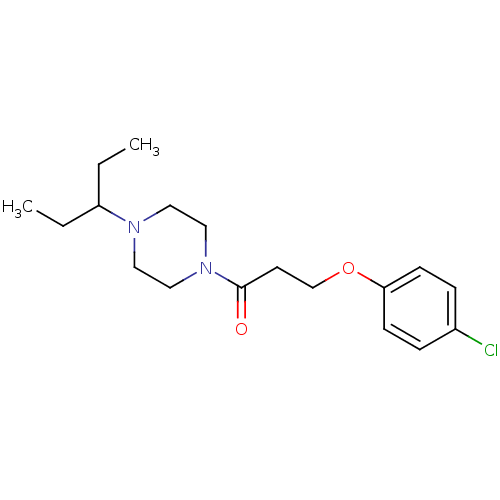 (3-(4-Chloro-phenoxy)-1-[4-(1-ethyl-propyl)-piperaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146835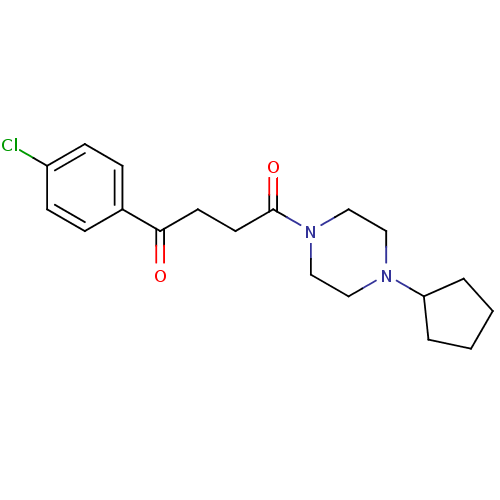 (1-(4-Chloro-phenyl)-4-(4-cyclopentyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146826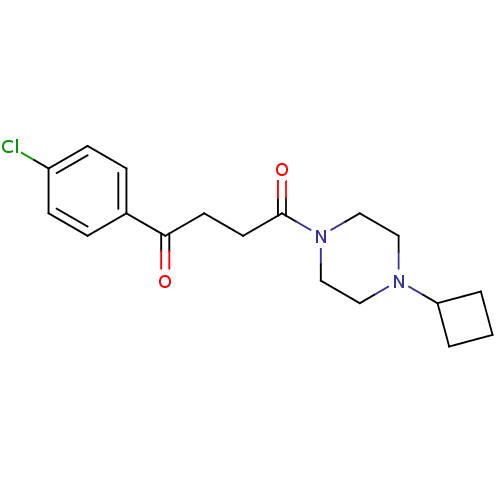 (1-(4-Chloro-phenyl)-4-(4-cyclobutyl-piperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370336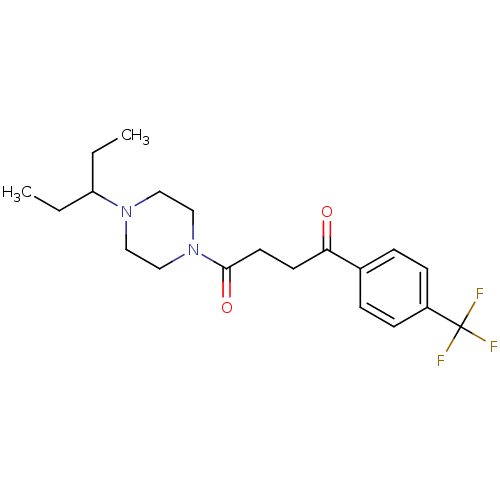 (CHEMBL1202903) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370338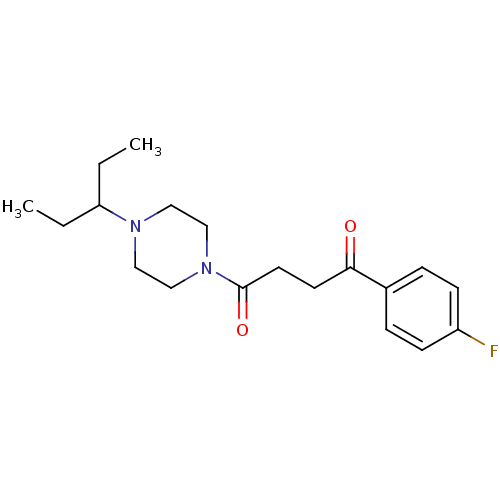 (CHEMBL1202904) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370327 (CHEMBL1202901) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370326 (CHEMBL1202900) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370329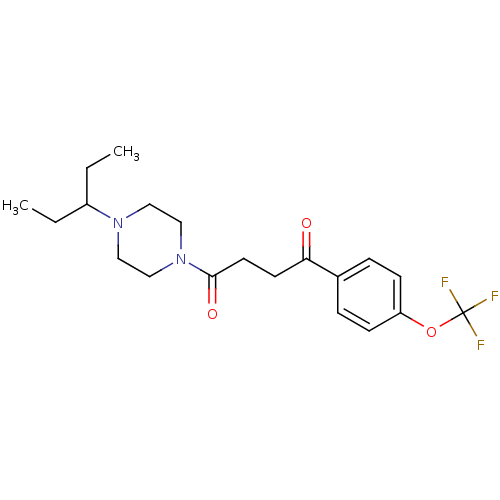 (CHEMBL1202894) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22904 ((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370339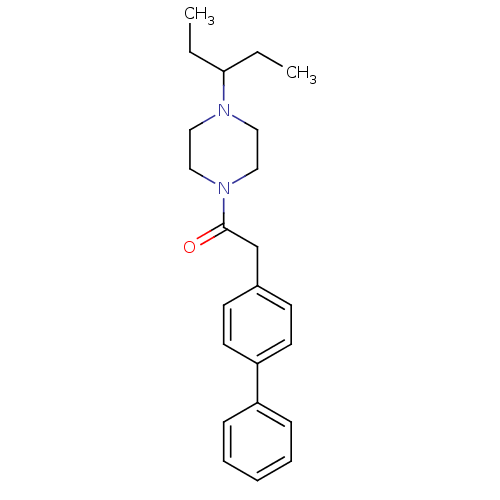 (CHEMBL1202899) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146843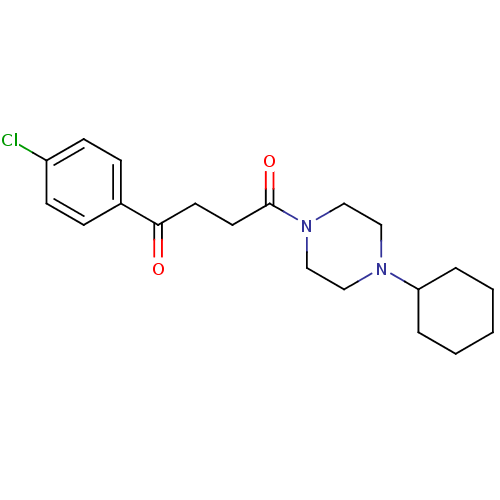 (1-(4-Chloro-phenyl)-4-(4-cyclohexyl-piperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146839 (1-(4-Chloro-phenyl)-4-(4-isopropyl-piperazin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146820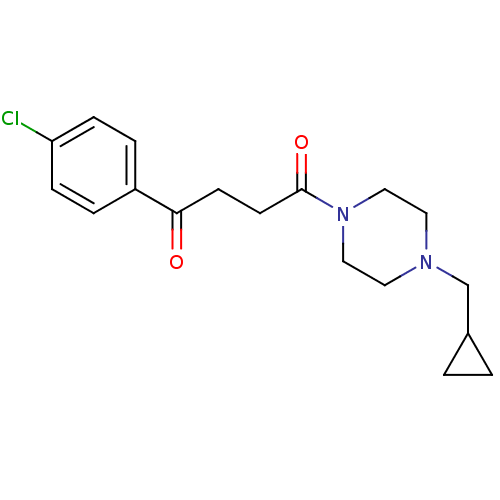 (1-(4-Chloro-phenyl)-4-(4-cyclopropylmethyl-piperaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370337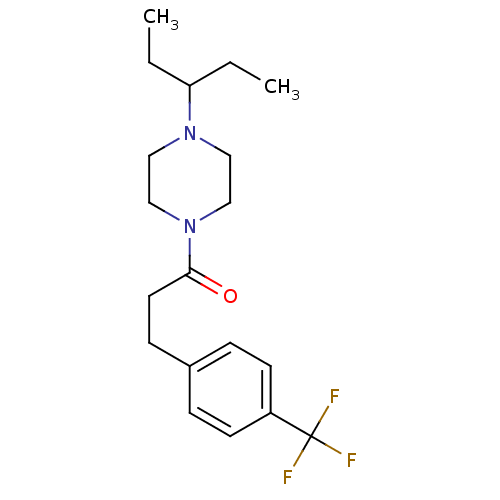 (CHEMBL1202902) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146818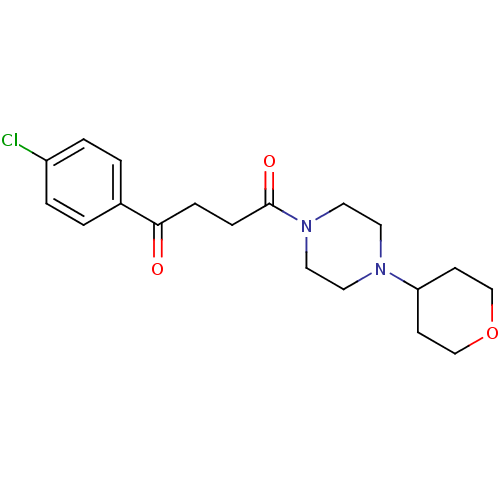 (1-(4-Chloro-phenyl)-4-[4-(tetrahydro-pyran-4-yl)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146829 (1-(4-Chloro-phenyl)-4-(4-cyclopropyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370334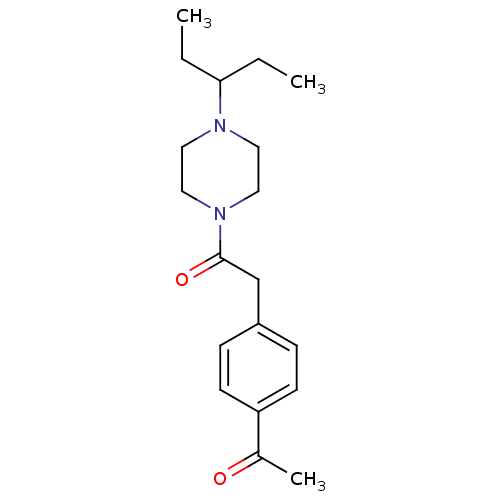 (CHEMBL1202897) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146842 (1-(4-Chloro-phenyl)-4-(4-cycloheptyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146837 (1-(4-Chloro-phenyl)-4-[4-(1,1-dimethyl-prop-2-ynyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370335 (CHEMBL1202895) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM27213 (4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370332 (CHEMBL1202898) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146819 (1-(4-Chloro-phenyl)-4-(4-cyclooctyl-piperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146841 (1-(4-Chloro-phenyl)-4-[4-(1-propyl-butyl)-piperazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50146844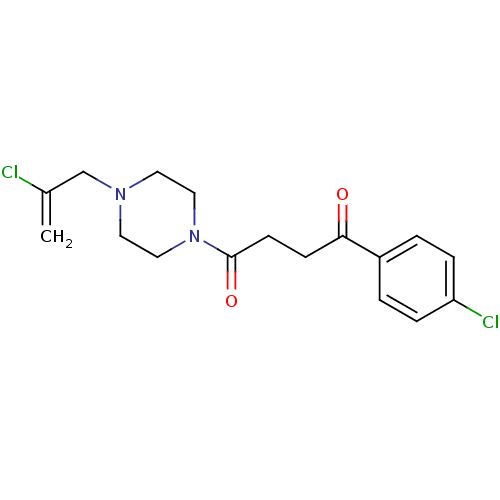 (1-[4-(2-Chloro-allyl)-piperazin-1-yl]-4-(4-chloro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay | J Med Chem 47: 2833-8 (2004) Article DOI: 10.1021/jm031028z BindingDB Entry DOI: 10.7270/Q2T154CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 8/ATP-sensitive inward rectifier potassium channel 11 (Homo sapiens (Human)) | BDBM50118351 ((2-Chloro-7,7-dioxo-4,7-dihydro-1,7lambda*6*-dithi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk Research and Development Curated by ChEMBL | Assay Description Inhibition of [3H]glibenclamide binding to HEK293 cells co-expressing human Sulfonylurea receptor SUR1 and Inward rectifier K+ channel Kir6.2 at high... | J Med Chem 45: 4171-87 (2002) BindingDB Entry DOI: 10.7270/Q2SF2VGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50202031 (3-(4-((1-(4-cyclohexenylphenyl)-3-(3,5-dichlorophe...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibition of human cloned glucagon receptor expressed in BHK cells | J Med Chem 50: 113-28 (2007) Article DOI: 10.1021/jm058026u BindingDB Entry DOI: 10.7270/Q26M36GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM29124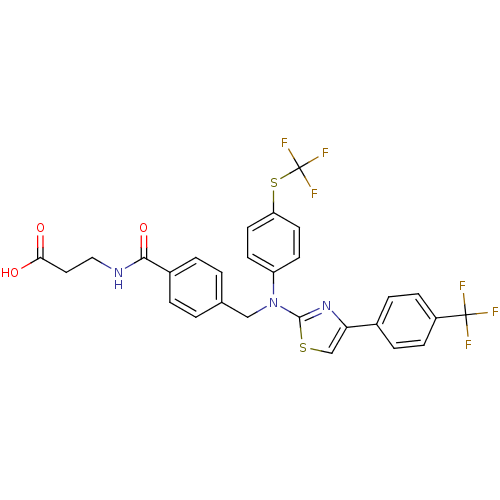 (aminothiazole, 21) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novo Nordisk A/S | Assay Description Wheat Germ Agglutinin derivatized SPA beads containing a scintillant (WGA beads) (Amersham) bound the membranes of BHK cells transfected with the hum... | J Med Chem 52: 2989-3000 (2009) Article DOI: 10.1021/jm8016249 BindingDB Entry DOI: 10.7270/Q21Z42RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50202047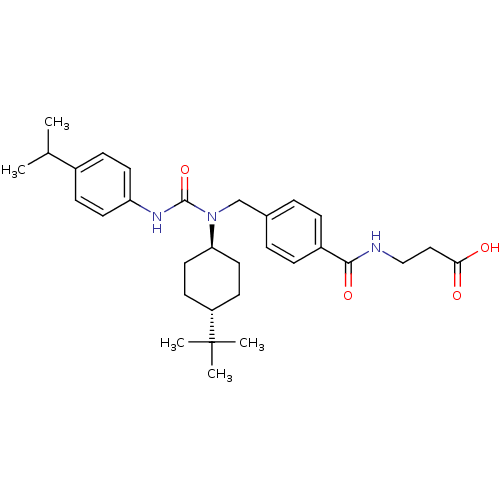 (3-(4-((1-(4-tert-butylcyclohexyl)-3-(4-isopropylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibition of human cloned glucagon receptor expressed in BHK cells | J Med Chem 50: 113-28 (2007) Article DOI: 10.1021/jm058026u BindingDB Entry DOI: 10.7270/Q26M36GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM29106 (aminothiazole, 3) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novo Nordisk A/S | Assay Description Wheat Germ Agglutinin derivatized SPA beads containing a scintillant (WGA beads) (Amersham) bound the membranes of BHK cells transfected with the hum... | J Med Chem 52: 2989-3000 (2009) Article DOI: 10.1021/jm8016249 BindingDB Entry DOI: 10.7270/Q21Z42RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50202056 (CHEMBL219244 | cis-3-{4-[1-(4-tert-butylcyclohexyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibition of human cloned glucagon receptor expressed in BHK cells | J Med Chem 50: 113-28 (2007) Article DOI: 10.1021/jm058026u BindingDB Entry DOI: 10.7270/Q26M36GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM29107 (aminothiazole, 4) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novo Nordisk A/S | Assay Description Wheat Germ Agglutinin derivatized SPA beads containing a scintillant (WGA beads) (Amersham) bound the membranes of BHK cells transfected with the hum... | J Med Chem 52: 2989-3000 (2009) Article DOI: 10.1021/jm8016249 BindingDB Entry DOI: 10.7270/Q21Z42RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM29109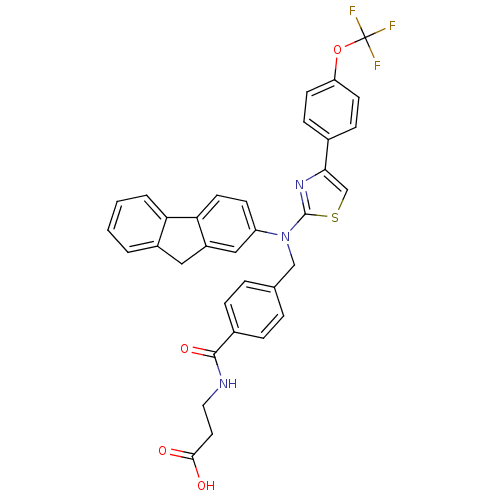 (aminothiazole, 6) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novo Nordisk A/S | Assay Description Wheat Germ Agglutinin derivatized SPA beads containing a scintillant (WGA beads) (Amersham) bound the membranes of BHK cells transfected with the hum... | J Med Chem 52: 2989-3000 (2009) Article DOI: 10.1021/jm8016249 BindingDB Entry DOI: 10.7270/Q21Z42RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50144009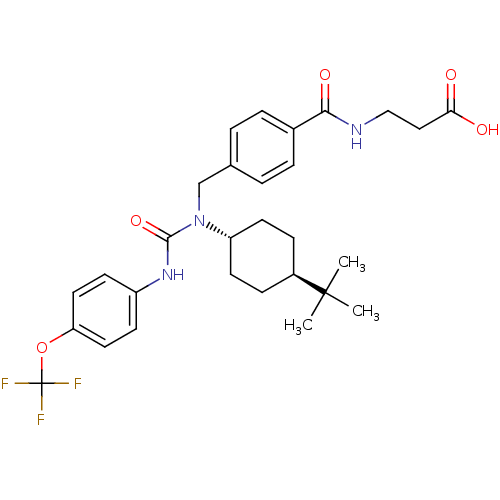 (3-{4-[1-(4-tert-Butyl-cyclohexyl)-3-(4-trifluorome...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibition of human cloned glucagon receptor expressed in BHK cells | J Med Chem 50: 113-28 (2007) Article DOI: 10.1021/jm058026u BindingDB Entry DOI: 10.7270/Q26M36GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50202041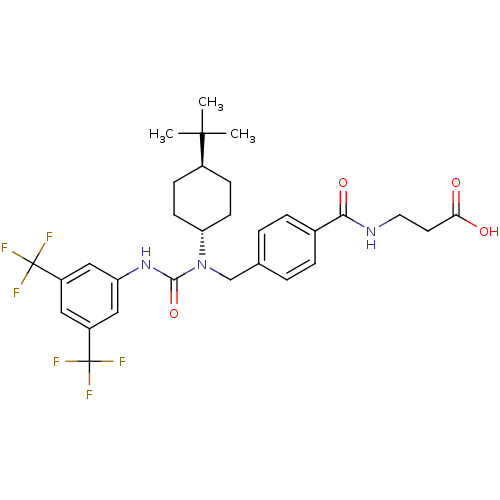 (CHEMBL375167 | trans-3-{4-[3-(3,5-Bis(trifluoromet...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibition of human cloned glucagon receptor expressed in BHK cells | J Med Chem 50: 113-28 (2007) Article DOI: 10.1021/jm058026u BindingDB Entry DOI: 10.7270/Q26M36GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50202051 (CHEMBL373824 | trans-3-(4-((1-(4-tert-butylcyclohe...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibition of human cloned glucagon receptor expressed in BHK cells | J Med Chem 50: 113-28 (2007) Article DOI: 10.1021/jm058026u BindingDB Entry DOI: 10.7270/Q26M36GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50144009 (3-{4-[1-(4-tert-Butyl-cyclohexyl)-3-(4-trifluorome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibition of rat glucagon receptor | J Med Chem 50: 113-28 (2007) Article DOI: 10.1021/jm058026u BindingDB Entry DOI: 10.7270/Q26M36GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50202029 (3-(4-((1-(4-isopropylcyclohexyl)-3-(4-(trifluorome...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibition of human cloned glucagon receptor expressed in BHK cells | J Med Chem 50: 113-28 (2007) Article DOI: 10.1021/jm058026u BindingDB Entry DOI: 10.7270/Q26M36GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50202039 (3-(4-((1-(4-cyclohexylphenyl)-3-(4-(trifluorometho...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibition of human cloned glucagon receptor expressed in BHK cells | J Med Chem 50: 113-28 (2007) Article DOI: 10.1021/jm058026u BindingDB Entry DOI: 10.7270/Q26M36GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM29119 (aminothiazole, 16) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novo Nordisk A/S | Assay Description Wheat Germ Agglutinin derivatized SPA beads containing a scintillant (WGA beads) (Amersham) bound the membranes of BHK cells transfected with the hum... | J Med Chem 52: 2989-3000 (2009) Article DOI: 10.1021/jm8016249 BindingDB Entry DOI: 10.7270/Q21Z42RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM29114 (aminothiazole, 11) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novo Nordisk A/S | Assay Description Wheat Germ Agglutinin derivatized SPA beads containing a scintillant (WGA beads) (Amersham) bound the membranes of BHK cells transfected with the hum... | J Med Chem 52: 2989-3000 (2009) Article DOI: 10.1021/jm8016249 BindingDB Entry DOI: 10.7270/Q21Z42RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM29128 (aminothiazole, 25) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novo Nordisk A/S | Assay Description Wheat Germ Agglutinin derivatized SPA beads containing a scintillant (WGA beads) (Amersham) bound the membranes of BHK cells transfected with the hum... | J Med Chem 52: 2989-3000 (2009) Article DOI: 10.1021/jm8016249 BindingDB Entry DOI: 10.7270/Q21Z42RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 184 total ) | Next | Last >> |