 Found 573 hits with Last Name = 'qin' and Initial = 'a'
Found 573 hits with Last Name = 'qin' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50587720
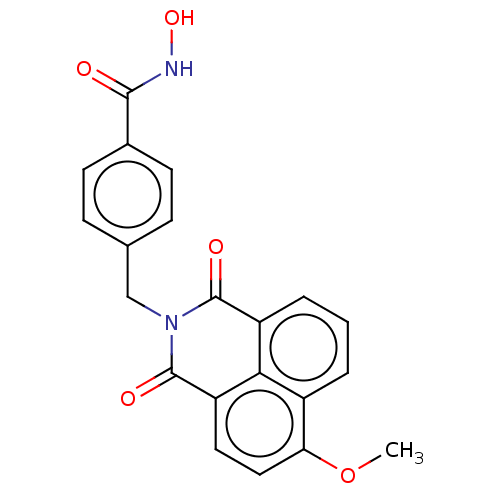
(CHEMBL5195946)Show SMILES COc1ccc2C(=O)N(Cc3ccc(cc3)C(=O)NO)C(=O)c3cccc1c23 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01782
BindingDB Entry DOI: 10.7270/Q2G73JP3 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50587716
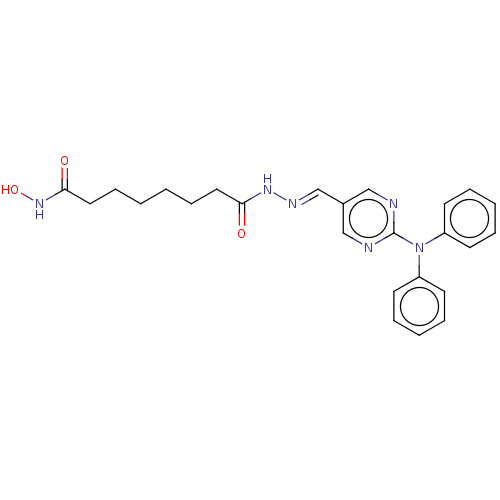
(CHEMBL5188297)Show SMILES ONC(=O)CCCCCCC(=O)N\N=C\c1cnc(nc1)N(c1ccccc1)c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01782
BindingDB Entry DOI: 10.7270/Q2G73JP3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50532351

(CHEMBL4565958)Show SMILES Cc1n[nH]cc1Nc1nccc(n1)-c1ccn([C@H](CO)c2ccc(Cl)c(F)c2)c(=O)c1 |r| Show InChI InChI=1S/C21H18ClFN6O2/c1-12-18(10-25-28-12)27-21-24-6-4-17(26-21)13-5-7-29(20(31)9-13)19(11-30)14-2-3-15(22)16(23)8-14/h2-10,19,30H,11H2,1H3,(H,25,28)(H,24,26,27)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50532351

(CHEMBL4565958)Show SMILES Cc1n[nH]cc1Nc1nccc(n1)-c1ccn([C@H](CO)c2ccc(Cl)c(F)c2)c(=O)c1 |r| Show InChI InChI=1S/C21H18ClFN6O2/c1-12-18(10-25-28-12)27-21-24-6-4-17(26-21)13-5-7-29(20(31)9-13)19(11-30)14-2-3-15(22)16(23)8-14/h2-10,19,30H,11H2,1H3,(H,25,28)(H,24,26,27)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50532350

(CHEMBL4571067)Show SMILES Cc1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)ccn1 |r| Show InChI InChI=1S/C23H19ClFN5O2/c1-14-10-17(4-7-26-14)28-23-27-8-5-20(29-23)15-6-9-30(22(32)12-15)21(13-31)16-2-3-18(24)19(25)11-16/h2-12,21,31H,13H2,1H3,(H,26,27,28,29)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50532350

(CHEMBL4571067)Show SMILES Cc1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)ccn1 |r| Show InChI InChI=1S/C23H19ClFN5O2/c1-14-10-17(4-7-26-14)28-23-27-8-5-20(29-23)15-6-9-30(22(32)12-15)21(13-31)16-2-3-18(24)19(25)11-16/h2-12,21,31H,13H2,1H3,(H,26,27,28,29)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM120086
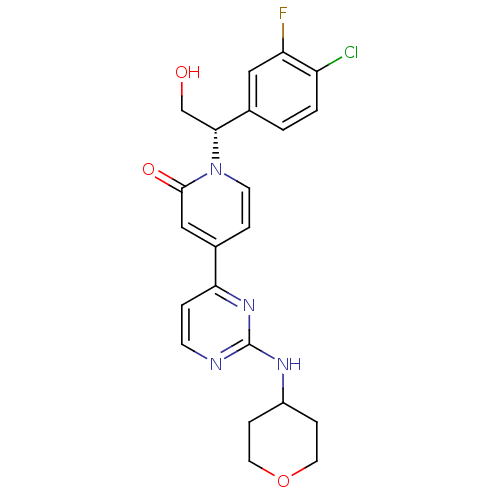
(US8697715, 1 | US9259470, 1)Show SMILES OC[C@H](c1ccc(Cl)c(F)c1)n1ccc(cc1=O)-c1ccnc(NC2CCOCC2)n1 |r| Show InChI InChI=1S/C22H22ClFN4O3/c23-17-2-1-15(11-18(17)24)20(13-29)28-8-4-14(12-21(28)30)19-3-7-25-22(27-19)26-16-5-9-31-10-6-16/h1-4,7-8,11-12,16,20,29H,5-6,9-10,13H2,(H,25,26,27)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM120086

(US8697715, 1 | US9259470, 1)Show SMILES OC[C@H](c1ccc(Cl)c(F)c1)n1ccc(cc1=O)-c1ccnc(NC2CCOCC2)n1 |r| Show InChI InChI=1S/C22H22ClFN4O3/c23-17-2-1-15(11-18(17)24)20(13-29)28-8-4-14(12-21(28)30)19-3-7-25-22(27-19)26-16-5-9-31-10-6-16/h1-4,7-8,11-12,16,20,29H,5-6,9-10,13H2,(H,25,26,27)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM16016
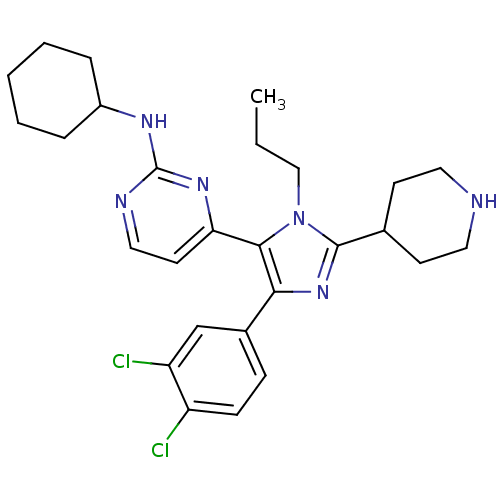
(CHEMBL437747 | N-cyclohexyl-4-[4-(3,4-dichlorophen...)Show SMILES CCCn1c(nc(c1-c1ccnc(NC2CCCCC2)n1)-c1ccc(Cl)c(Cl)c1)C1CCNCC1 Show InChI InChI=1S/C27H34Cl2N6/c1-2-16-35-25(23-12-15-31-27(33-23)32-20-6-4-3-5-7-20)24(19-8-9-21(28)22(29)17-19)34-26(35)18-10-13-30-14-11-18/h8-9,12,15,17-18,20,30H,2-7,10-11,13-14,16H2,1H3,(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK3 after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 315-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.010
BindingDB Entry DOI: 10.7270/Q24F1R0T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50532362

(CHEMBL4436718)Show SMILES CCn1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)cn1 |r| Show InChI InChI=1S/C22H20ClFN6O2/c1-2-29-12-16(11-26-29)27-22-25-7-5-19(28-22)14-6-8-30(21(32)10-14)20(13-31)15-3-4-17(23)18(24)9-15/h3-12,20,31H,2,13H2,1H3,(H,25,27,28)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50532362

(CHEMBL4436718)Show SMILES CCn1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)cn1 |r| Show InChI InChI=1S/C22H20ClFN6O2/c1-2-29-12-16(11-26-29)27-22-25-7-5-19(28-22)14-6-8-30(21(32)10-14)20(13-31)15-3-4-17(23)18(24)9-15/h3-12,20,31H,2,13H2,1H3,(H,25,27,28)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM417049
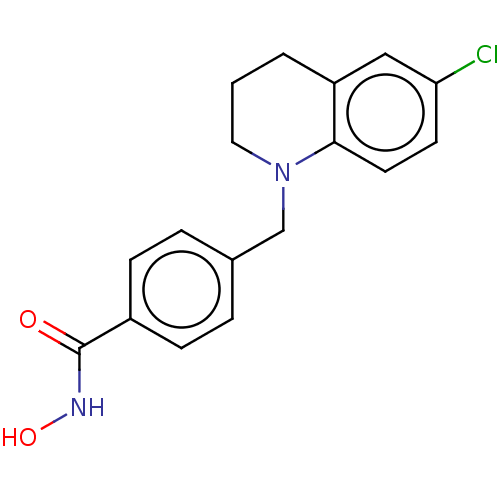
(4-((6-chloro-3,4-dihydroquinolin-1(2H)-yl)methyl)-...)Show InChI InChI=1S/C17H17ClN2O2/c18-15-7-8-16-14(10-15)2-1-9-20(16)11-12-3-5-13(6-4-12)17(21)19-22/h3-8,10,22H,1-2,9,11H2,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01782
BindingDB Entry DOI: 10.7270/Q2G73JP3 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM110036
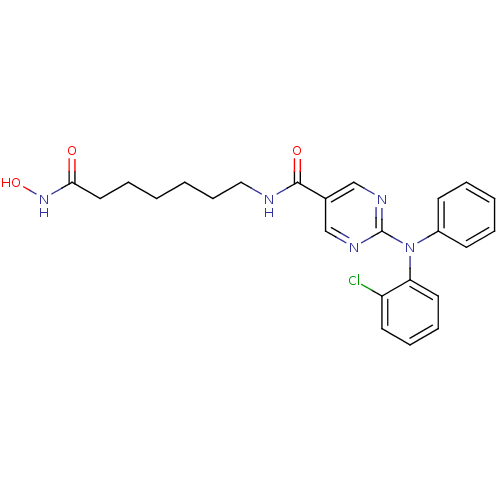
(US8609678, 2-((2-chlorophenyl)(phenyl)amino)-N-(7-...)Show SMILES ONC(=O)CCCCCCNC(=O)c1cnc(nc1)N(c1ccccc1)c1ccccc1Cl Show InChI InChI=1S/C24H26ClN5O3/c25-20-12-7-8-13-21(20)30(19-10-4-3-5-11-19)24-27-16-18(17-28-24)23(32)26-15-9-2-1-6-14-22(31)29-33/h3-5,7-8,10-13,16-17,33H,1-2,6,9,14-15H2,(H,26,32)(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01782
BindingDB Entry DOI: 10.7270/Q2G73JP3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM120086

(US8697715, 1 | US9259470, 1)Show SMILES OC[C@H](c1ccc(Cl)c(F)c1)n1ccc(cc1=O)-c1ccnc(NC2CCOCC2)n1 |r| Show InChI InChI=1S/C22H22ClFN4O3/c23-17-2-1-15(11-18(17)24)20(13-29)28-8-4-14(12-21(28)30)19-3-7-25-22(27-19)26-16-5-9-31-10-6-16/h1-4,7-8,11-12,16,20,29H,5-6,9-10,13H2,(H,25,26,27)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM120086

(US8697715, 1 | US9259470, 1)Show SMILES OC[C@H](c1ccc(Cl)c(F)c1)n1ccc(cc1=O)-c1ccnc(NC2CCOCC2)n1 |r| Show InChI InChI=1S/C22H22ClFN4O3/c23-17-2-1-15(11-18(17)24)20(13-29)28-8-4-14(12-21(28)30)19-3-7-25-22(27-19)26-16-5-9-31-10-6-16/h1-4,7-8,11-12,16,20,29H,5-6,9-10,13H2,(H,25,26,27)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50587718
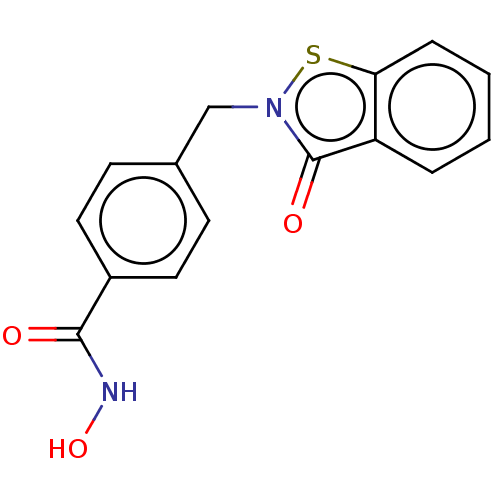
(CHEMBL5185889) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01782
BindingDB Entry DOI: 10.7270/Q2G73JP3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM120095
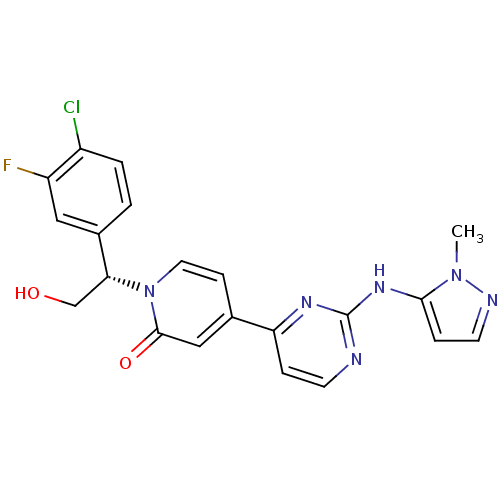
(US10525036, Example GDC-0994 | US10934304, Example...)Show SMILES Cn1nccc1Nc1nccc(n1)-c1ccn([C@H](CO)c2ccc(Cl)c(F)c2)c(=O)c1 |r| Show InChI InChI=1S/C21H18ClFN6O2/c1-28-19(5-8-25-28)27-21-24-7-4-17(26-21)13-6-9-29(20(31)11-13)18(12-30)14-2-3-15(22)16(23)10-14/h2-11,18,30H,12H2,1H3,(H,24,26,27)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM120095

(US10525036, Example GDC-0994 | US10934304, Example...)Show SMILES Cn1nccc1Nc1nccc(n1)-c1ccn([C@H](CO)c2ccc(Cl)c(F)c2)c(=O)c1 |r| Show InChI InChI=1S/C21H18ClFN6O2/c1-28-19(5-8-25-28)27-21-24-7-4-17(26-21)13-6-9-29(20(31)11-13)18(12-30)14-2-3-15(22)16(23)10-14/h2-11,18,30H,12H2,1H3,(H,24,26,27)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50532351

(CHEMBL4565958)Show SMILES Cc1n[nH]cc1Nc1nccc(n1)-c1ccn([C@H](CO)c2ccc(Cl)c(F)c2)c(=O)c1 |r| Show InChI InChI=1S/C21H18ClFN6O2/c1-12-18(10-25-28-12)27-21-24-6-4-17(26-21)13-5-7-29(20(31)9-13)19(11-30)14-2-3-15(22)16(23)8-14/h2-10,19,30H,11H2,1H3,(H,25,28)(H,24,26,27)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50532351

(CHEMBL4565958)Show SMILES Cc1n[nH]cc1Nc1nccc(n1)-c1ccn([C@H](CO)c2ccc(Cl)c(F)c2)c(=O)c1 |r| Show InChI InChI=1S/C21H18ClFN6O2/c1-12-18(10-25-28-12)27-21-24-6-4-17(26-21)13-5-7-29(20(31)9-13)19(11-30)14-2-3-15(22)16(23)8-14/h2-10,19,30H,11H2,1H3,(H,25,28)(H,24,26,27)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50532350

(CHEMBL4571067)Show SMILES Cc1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)ccn1 |r| Show InChI InChI=1S/C23H19ClFN5O2/c1-14-10-17(4-7-26-14)28-23-27-8-5-20(29-23)15-6-9-30(22(32)12-15)21(13-31)16-2-3-18(24)19(25)11-16/h2-12,21,31H,13H2,1H3,(H,26,27,28,29)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50532350

(CHEMBL4571067)Show SMILES Cc1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)ccn1 |r| Show InChI InChI=1S/C23H19ClFN5O2/c1-14-10-17(4-7-26-14)28-23-27-8-5-20(29-23)15-6-9-30(22(32)12-15)21(13-31)16-2-3-18(24)19(25)11-16/h2-12,21,31H,13H2,1H3,(H,26,27,28,29)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16016

(CHEMBL437747 | N-cyclohexyl-4-[4-(3,4-dichlorophen...)Show SMILES CCCn1c(nc(c1-c1ccnc(NC2CCCCC2)n1)-c1ccc(Cl)c(Cl)c1)C1CCNCC1 Show InChI InChI=1S/C27H34Cl2N6/c1-2-16-35-25(23-12-15-31-27(33-23)32-20-6-4-3-5-7-20)24(19-8-9-21(28)22(29)17-19)34-26(35)18-10-13-30-14-11-18/h8-9,12,15,17-18,20,30H,2-7,10-11,13-14,16H2,1H3,(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant p38alpha after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 315-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.010
BindingDB Entry DOI: 10.7270/Q24F1R0T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50532361

(CHEMBL4435650)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)cn1 |r| Show InChI InChI=1S/C21H18ClFN6O2/c1-28-11-15(10-25-28)26-21-24-6-4-18(27-21)13-5-7-29(20(31)9-13)19(12-30)14-2-3-16(22)17(23)8-14/h2-11,19,30H,12H2,1H3,(H,24,26,27)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50532361

(CHEMBL4435650)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)cn1 |r| Show InChI InChI=1S/C21H18ClFN6O2/c1-28-11-15(10-25-28)26-21-24-6-4-18(27-21)13-5-7-29(20(31)9-13)19(12-30)14-2-3-16(22)17(23)8-14/h2-11,19,30H,12H2,1H3,(H,24,26,27)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50439674
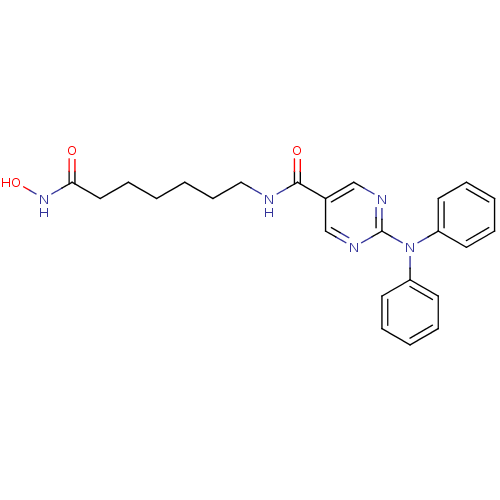
(RICOLINOSTAT | US10858323, Compound 2 | US11207431...)Show SMILES ONC(=O)CCCCCCNC(=O)c1cnc(nc1)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H27N5O3/c30-22(28-32)15-9-1-2-10-16-25-23(31)19-17-26-24(27-18-19)29(20-11-5-3-6-12-20)21-13-7-4-8-14-21/h3-8,11-14,17-18,32H,1-2,9-10,15-16H2,(H,25,31)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01782
BindingDB Entry DOI: 10.7270/Q2G73JP3 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-2
(Human) | BDBM50598619

(CHEMBL5199008)Show SMILES CCOc1ncc2cc(C3=CC4N=C(CCN5CCC(O)C5)N(C)C4C=C3)c(=O)n(-c3ccc(OC(F)F)cc3)c2n1 |c:27,t:9,12| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00395
BindingDB Entry DOI: 10.7270/Q28G8QRH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50532362

(CHEMBL4436718)Show SMILES CCn1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)cn1 |r| Show InChI InChI=1S/C22H20ClFN6O2/c1-2-29-12-16(11-26-29)27-22-25-7-5-19(28-22)14-6-8-30(21(32)10-14)20(13-31)15-3-4-17(23)18(24)9-15/h3-12,20,31H,2,13H2,1H3,(H,25,27,28)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50532362

(CHEMBL4436718)Show SMILES CCn1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)cn1 |r| Show InChI InChI=1S/C22H20ClFN6O2/c1-2-29-12-16(11-26-29)27-22-25-7-5-19(28-22)14-6-8-30(21(32)10-14)20(13-31)15-3-4-17(23)18(24)9-15/h3-12,20,31H,2,13H2,1H3,(H,25,27,28)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM206343
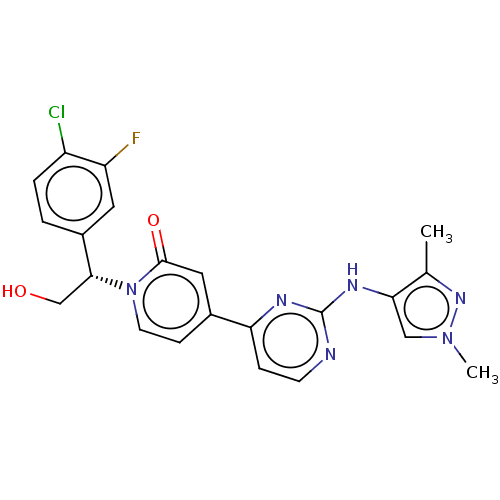
(US9259470, 167)Show SMILES Cc1nn(C)cc1Nc1nccc(n1)-c1ccn([C@H](CO)c2ccc(Cl)c(F)c2)c(=O)c1 |r| Show InChI InChI=1S/C22H20ClFN6O2/c1-13-19(11-29(2)28-13)27-22-25-7-5-18(26-22)14-6-8-30(21(32)10-14)20(12-31)15-3-4-16(23)17(24)9-15/h3-11,20,31H,12H2,1-2H3,(H,25,26,27)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM206343

(US9259470, 167)Show SMILES Cc1nn(C)cc1Nc1nccc(n1)-c1ccn([C@H](CO)c2ccc(Cl)c(F)c2)c(=O)c1 |r| Show InChI InChI=1S/C22H20ClFN6O2/c1-13-19(11-29(2)28-13)27-22-25-7-5-18(26-22)14-6-8-30(21(32)10-14)20(12-31)15-3-4-16(23)17(24)9-15/h3-11,20,31H,12H2,1-2H3,(H,25,26,27)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50035907
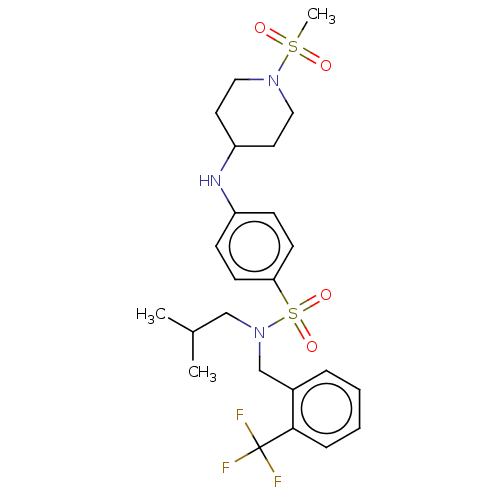
(CHEMBL3361050)Show SMILES CC(C)CN(Cc1ccccc1C(F)(F)F)S(=O)(=O)c1ccc(NC2CCN(CC2)S(C)(=O)=O)cc1 Show InChI InChI=1S/C24H32F3N3O4S2/c1-18(2)16-30(17-19-6-4-5-7-23(19)24(25,26)27)36(33,34)22-10-8-20(9-11-22)28-21-12-14-29(15-13-21)35(3,31)32/h4-11,18,21,28H,12-17H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]25-hydroxycholesterol from human RORc-LBD expressed in bacterial expression system after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 5769-76 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.037
BindingDB Entry DOI: 10.7270/Q2125V8F |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50035909
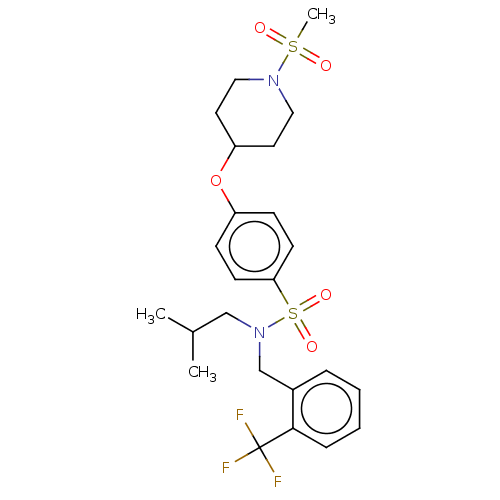
(CHEMBL3361052)Show SMILES CC(C)CN(Cc1ccccc1C(F)(F)F)S(=O)(=O)c1ccc(OC2CCN(CC2)S(C)(=O)=O)cc1 Show InChI InChI=1S/C24H31F3N2O5S2/c1-18(2)16-29(17-19-6-4-5-7-23(19)24(25,26)27)36(32,33)22-10-8-20(9-11-22)34-21-12-14-28(15-13-21)35(3,30)31/h4-11,18,21H,12-17H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]25-hydroxycholesterol from human RORc-LBD expressed in bacterial expression system after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 5769-76 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.037
BindingDB Entry DOI: 10.7270/Q2125V8F |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM120095

(US10525036, Example GDC-0994 | US10934304, Example...)Show SMILES Cn1nccc1Nc1nccc(n1)-c1ccn([C@H](CO)c2ccc(Cl)c(F)c2)c(=O)c1 |r| Show InChI InChI=1S/C21H18ClFN6O2/c1-28-19(5-8-25-28)27-21-24-7-4-17(26-21)13-6-9-29(20(31)11-13)18(12-30)14-2-3-15(22)16(23)10-14/h2-11,18,30H,12H2,1H3,(H,24,26,27)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM120095

(US10525036, Example GDC-0994 | US10934304, Example...)Show SMILES Cn1nccc1Nc1nccc(n1)-c1ccn([C@H](CO)c2ccc(Cl)c(F)c2)c(=O)c1 |r| Show InChI InChI=1S/C21H18ClFN6O2/c1-28-19(5-8-25-28)27-21-24-7-4-17(26-21)13-6-9-29(20(31)11-13)18(12-30)14-2-3-15(22)16(23)10-14/h2-11,18,30H,12H2,1H3,(H,24,26,27)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50562487
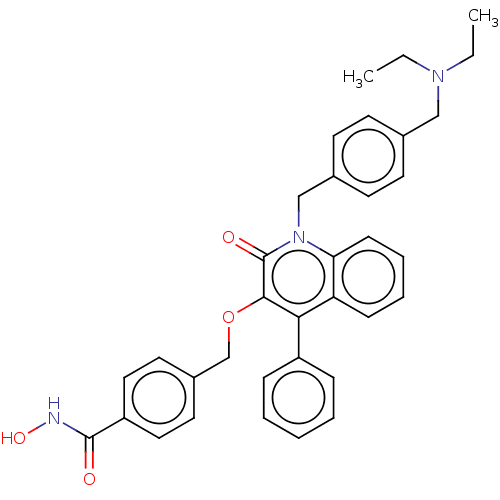
(CHEMBL4757504)Show SMILES CCN(CC)Cc1ccc(Cn2c3ccccc3c(-c3ccccc3)c(OCc3ccc(cc3)C(=O)NO)c2=O)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01782
BindingDB Entry DOI: 10.7270/Q2G73JP3 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50035910

(CHEMBL3361053)Show SMILES CC(C)CN(Cc1ccccc1C(F)(F)F)S(=O)(=O)c1ccc(cc1)C(=O)C1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C25H31F3N2O5S2/c1-18(2)16-30(17-21-6-4-5-7-23(21)25(26,27)28)37(34,35)22-10-8-19(9-11-22)24(31)20-12-14-29(15-13-20)36(3,32)33/h4-11,18,20H,12-17H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]25-hydroxycholesterol from human RORc-LBD expressed in bacterial expression system after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 5769-76 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.037
BindingDB Entry DOI: 10.7270/Q2125V8F |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM120114
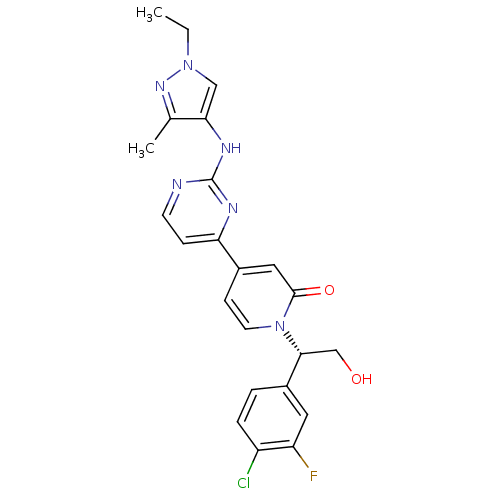
(US8697715, 173 | US9259470, 173)Show SMILES CCn1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)c(C)n1 |r| Show InChI InChI=1S/C23H22ClFN6O2/c1-3-30-12-20(14(2)29-30)28-23-26-8-6-19(27-23)15-7-9-31(22(33)11-15)21(13-32)16-4-5-17(24)18(25)10-16/h4-12,21,32H,3,13H2,1-2H3,(H,26,27,28)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50587722
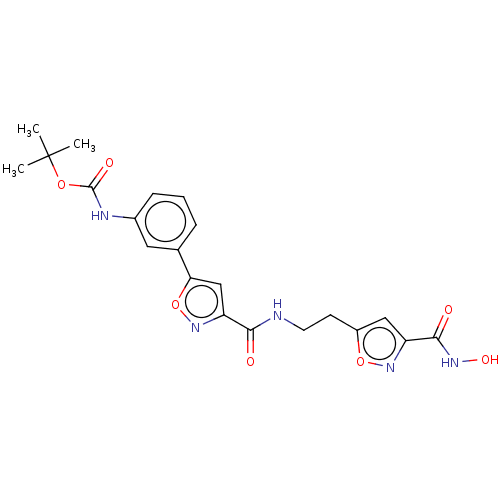
(CHEMBL5173000)Show SMILES CC(C)(C)OC(=O)Nc1cccc(c1)-c1cc(no1)C(=O)NCCc1cc(no1)C(=O)NO | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01782
BindingDB Entry DOI: 10.7270/Q2G73JP3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM120114

(US8697715, 173 | US9259470, 173)Show SMILES CCn1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)c(C)n1 |r| Show InChI InChI=1S/C23H22ClFN6O2/c1-3-30-12-20(14(2)29-30)28-23-26-8-6-19(27-23)15-7-9-31(22(33)11-15)21(13-32)16-4-5-17(24)18(25)10-16/h4-12,21,32H,3,13H2,1-2H3,(H,26,27,28)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50532361

(CHEMBL4435650)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)cn1 |r| Show InChI InChI=1S/C21H18ClFN6O2/c1-28-11-15(10-25-28)26-21-24-6-4-18(27-21)13-5-7-29(20(31)9-13)19(12-30)14-2-3-16(22)17(23)8-14/h2-11,19,30H,12H2,1H3,(H,24,26,27)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50532361

(CHEMBL4435650)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)cn1 |r| Show InChI InChI=1S/C21H18ClFN6O2/c1-28-11-15(10-25-28)26-21-24-6-4-18(27-21)13-5-7-29(20(31)9-13)19(12-30)14-2-3-16(22)17(23)8-14/h2-11,19,30H,12H2,1H3,(H,24,26,27)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
S-adenosylmethionine synthase isoform type-2
(Human) | BDBM50575978
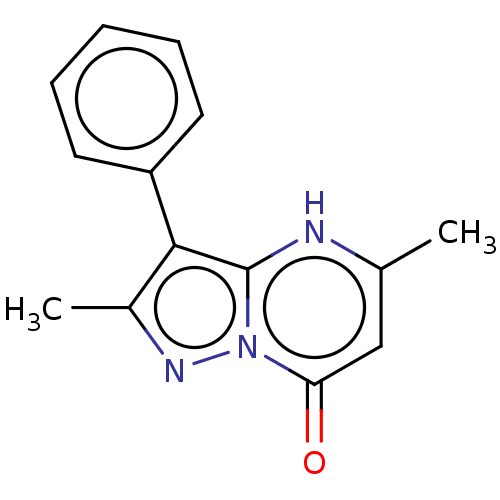
(CHEMBL4860456) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00395
BindingDB Entry DOI: 10.7270/Q28G8QRH |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50035914
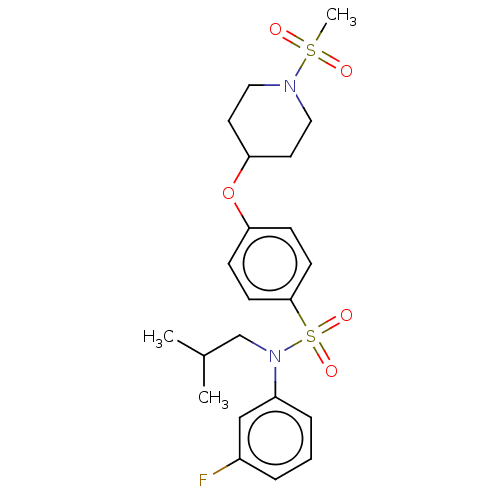
(CHEMBL3361057)Show SMILES CC(C)CN(c1cccc(F)c1)S(=O)(=O)c1ccc(OC2CCN(CC2)S(C)(=O)=O)cc1 Show InChI InChI=1S/C22H29FN2O5S2/c1-17(2)16-25(19-6-4-5-18(23)15-19)32(28,29)22-9-7-20(8-10-22)30-21-11-13-24(14-12-21)31(3,26)27/h4-10,15,17,21H,11-14,16H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]25-hydroxycholesterol from human RORc-LBD expressed in bacterial expression system after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 5769-76 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.037
BindingDB Entry DOI: 10.7270/Q2125V8F |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50587716

(CHEMBL5188297)Show SMILES ONC(=O)CCCCCCC(=O)N\N=C\c1cnc(nc1)N(c1ccccc1)c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01782
BindingDB Entry DOI: 10.7270/Q2G73JP3 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50035906

(CHEMBL3361049)Show SMILES CC(C)CN(Cc1ccccc1C(F)(F)F)S(=O)(=O)c1ccc(cc1)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C24H30F3N3O3S/c1-18(2)16-30(17-20-6-4-5-7-23(20)24(25,26)27)34(32,33)22-10-8-21(9-11-22)29-14-12-28(13-15-29)19(3)31/h4-11,18H,12-17H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]25-hydroxycholesterol from human RORc-LBD expressed in bacterial expression system after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 5769-76 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.037
BindingDB Entry DOI: 10.7270/Q2125V8F |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50532349
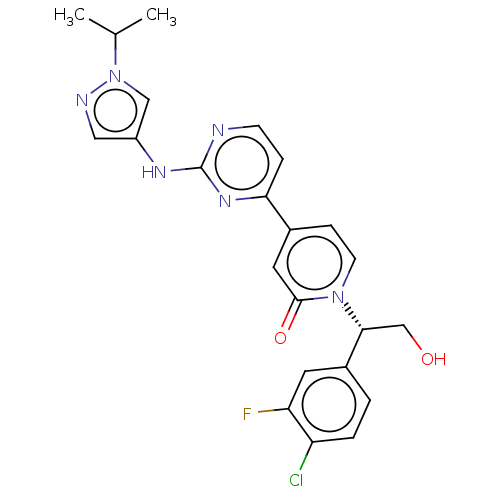
(CHEMBL4474917)Show SMILES CC(C)n1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)cn1 |r| Show InChI InChI=1S/C23H22ClFN6O2/c1-14(2)31-12-17(11-27-31)28-23-26-7-5-20(29-23)15-6-8-30(22(33)10-15)21(13-32)16-3-4-18(24)19(25)9-16/h3-12,14,21,32H,13H2,1-2H3,(H,26,28,29)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50532349

(CHEMBL4474917)Show SMILES CC(C)n1cc(Nc2nccc(n2)-c2ccn([C@H](CO)c3ccc(Cl)c(F)c3)c(=O)c2)cn1 |r| Show InChI InChI=1S/C23H22ClFN6O2/c1-14(2)31-12-17(11-27-31)28-23-26-7-5-20(29-23)15-6-8-30(22(33)10-15)21(13-32)16-3-4-18(24)19(25)9-16/h3-12,14,21,32H,13H2,1-2H3,(H,26,28,29)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
J Med Chem 59: 5650-60 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00389
BindingDB Entry DOI: 10.7270/Q2M90D42 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50035911

(CHEMBL3361054)Show SMILES CC(C)CN(Cc1ccccc1C(F)(F)F)S(=O)(=O)c1ccc(cc1)C(O)C1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C25H33F3N2O5S2/c1-18(2)16-30(17-21-6-4-5-7-23(21)25(26,27)28)37(34,35)22-10-8-19(9-11-22)24(31)20-12-14-29(15-13-20)36(3,32)33/h4-11,18,20,24,31H,12-17H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]25-hydroxycholesterol from human RORc-LBD expressed in bacterial expression system after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 5769-76 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.037
BindingDB Entry DOI: 10.7270/Q2125V8F |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50035885

(CHEMBL3361064)Show SMILES CC(C)CN(Cc1ccccc1C(F)(F)F)S(=O)(=O)c1ccc(OC2CCN(CC2)S(C)(=O)=O)cn1 Show InChI InChI=1S/C23H30F3N3O5S2/c1-17(2)15-29(16-18-6-4-5-7-21(18)23(24,25)26)36(32,33)22-9-8-20(14-27-22)34-19-10-12-28(13-11-19)35(3,30)31/h4-9,14,17,19H,10-13,15-16H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]25-hydroxycholesterol from human RORc-LBD expressed in bacterial expression system after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 5769-76 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.037
BindingDB Entry DOI: 10.7270/Q2125V8F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data