

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Toxin B (Peptoclostridium difficile) | BDBM50454459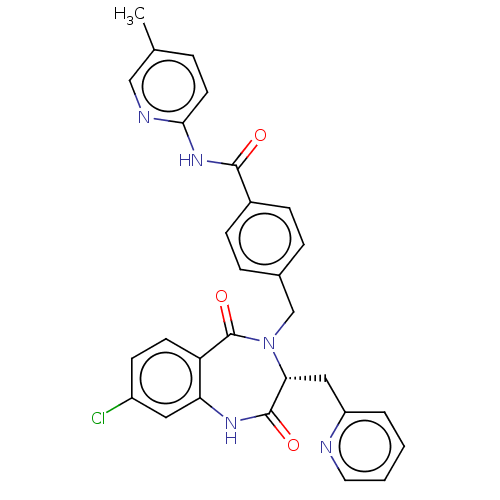 (CHEMBL4215036) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of Clostridium difficile toxin B transfected in CHO cells assessed as reduction in caspase 3/7 activation pre-incubated for 1 hr before Tc... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311657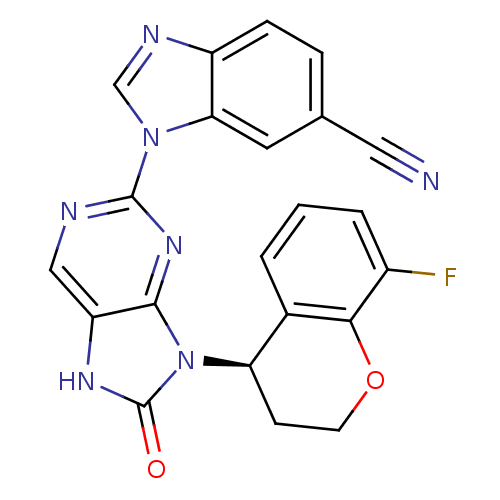 (1-(9-(8-fluorochroman-4-yl)-8-oxo-8,9-dihydro-7H-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454459 (CHEMBL4215036) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507130 (CHEMBL4452983) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454498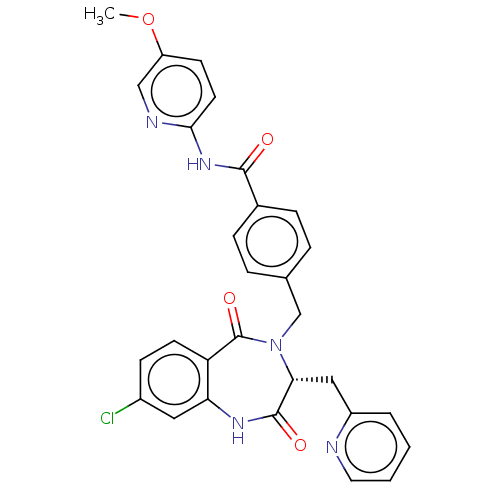 (CHEMBL4215657) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454497 (CHEMBL4214079) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of Clostridium difficile toxin B transfected in CHO cells assessed as reduction in caspase 3/7 activation pre-incubated for 1 hr before Tc... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311656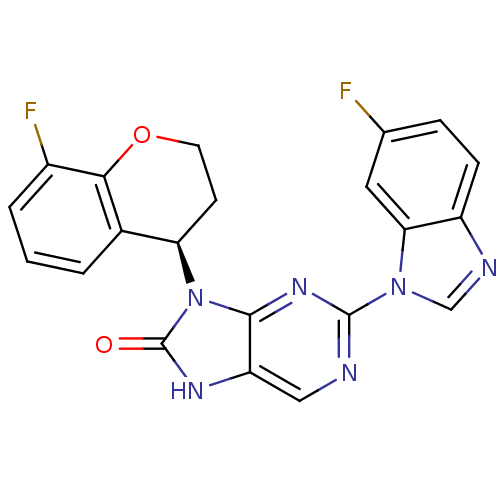 (2-(6-fluoro-1H-benzo[d]imidazol-1-yl)-9-(8-fluoroc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507108 (CHEMBL4529863) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454500 (CHEMBL4212258) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507107 (CHEMBL4473557) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507115 (CHEMBL4520413) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507128 (CHEMBL4451752) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507135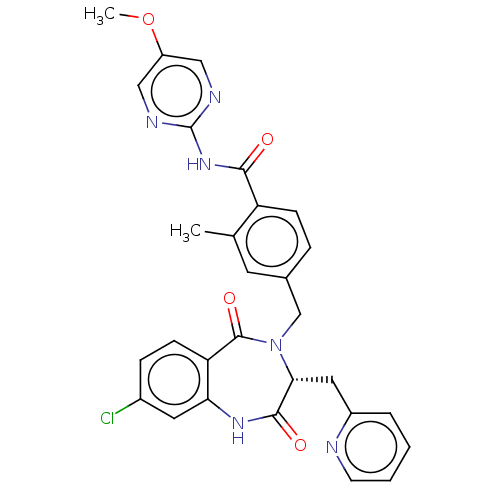 (CHEMBL4470638) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507134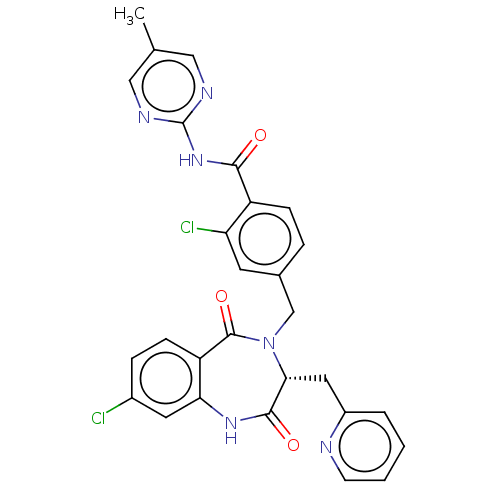 (CHEMBL4444965) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50311657 (1-(9-(8-fluorochroman-4-yl)-8-oxo-8,9-dihydro-7H-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK2 (532-1132) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507127 (CHEMBL4476228) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507120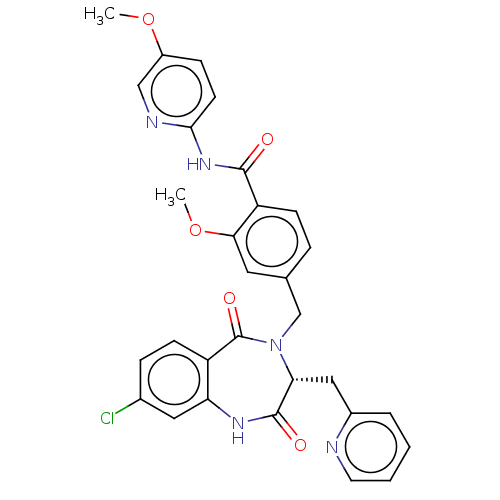 (CHEMBL4473443) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507105 (CHEMBL4557223) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311644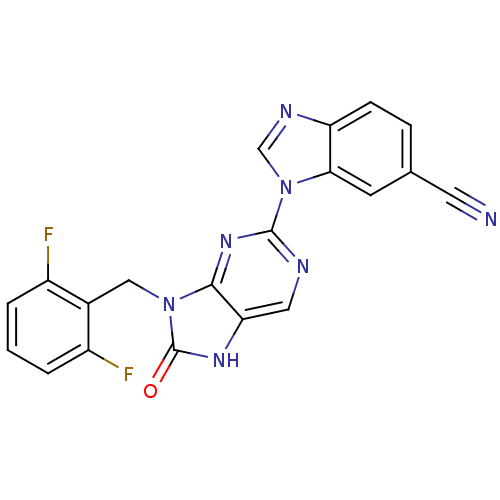 (1-(9-(2,6-difluorobenzyl)-8-oxo-8,9-dihydro-7H-pur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507133 (CHEMBL4461315) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311640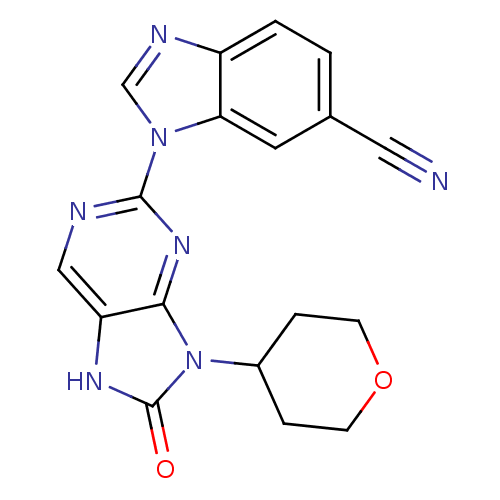 (1-(8-oxo-9-(tetrahydro-2H-pyran-4-yl)-8,9-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507136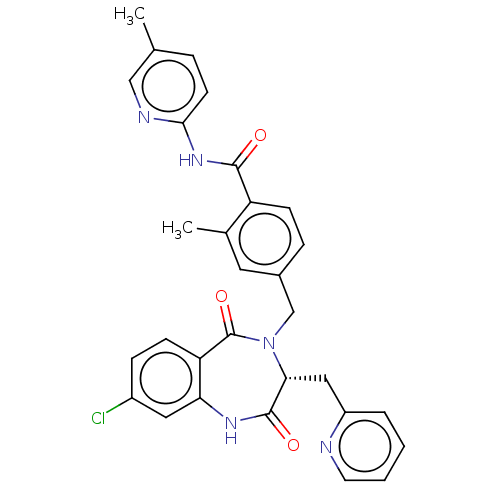 (CHEMBL4454294) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454497 (CHEMBL4214079) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507126 (CHEMBL4483028) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507113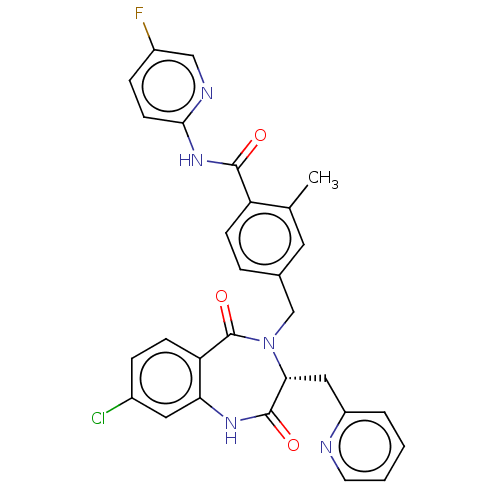 (CHEMBL4436890) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507114 (CHEMBL4553525) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507116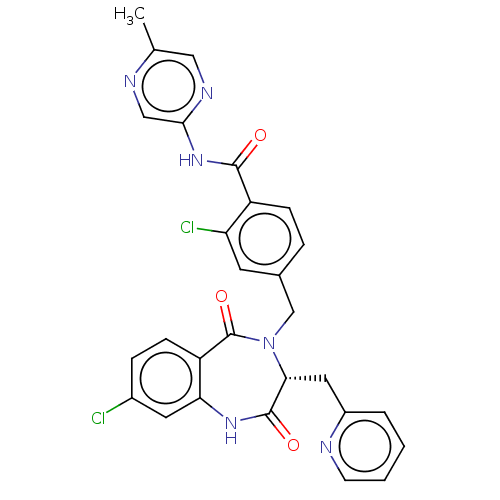 (CHEMBL4452876) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454486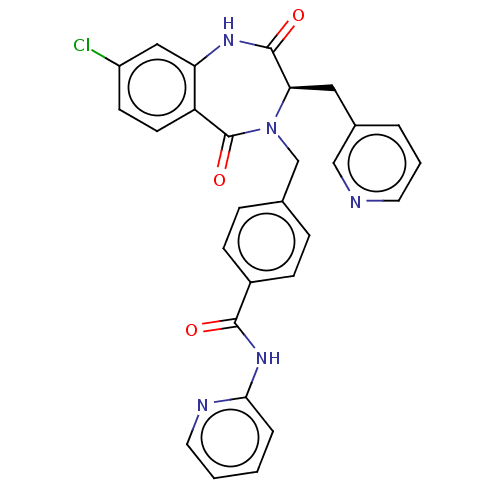 (CHEMBL4203819) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507119 (CHEMBL4441033) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507106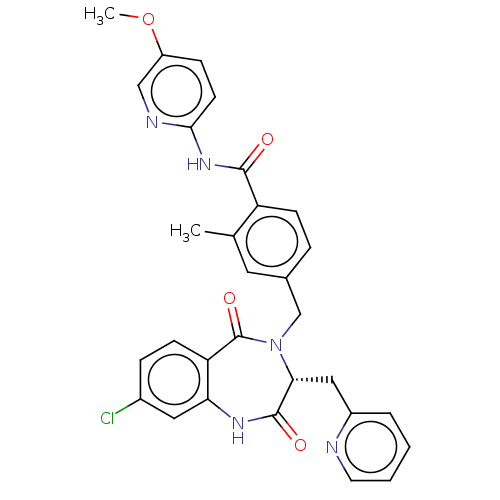 (CHEMBL4472977) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311639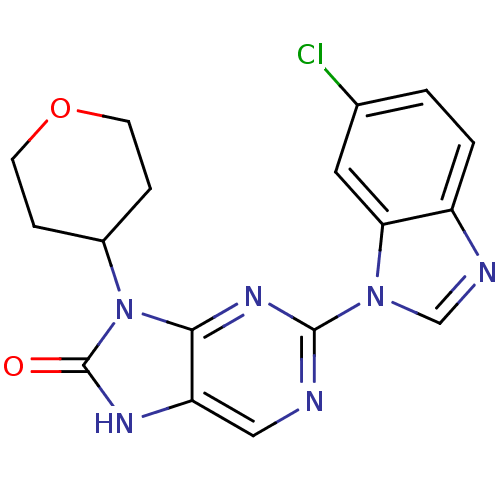 (2-(6-chloro-1H-benzo[d]imidazol-1-yl)-9-(tetrahydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507129 (CHEMBL4456814) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311655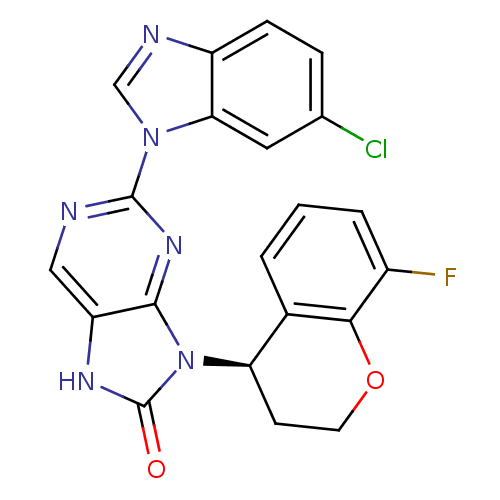 (2-(6-chloro-1H-benzo[d]imidazol-1-yl)-9-(8-fluoroc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311642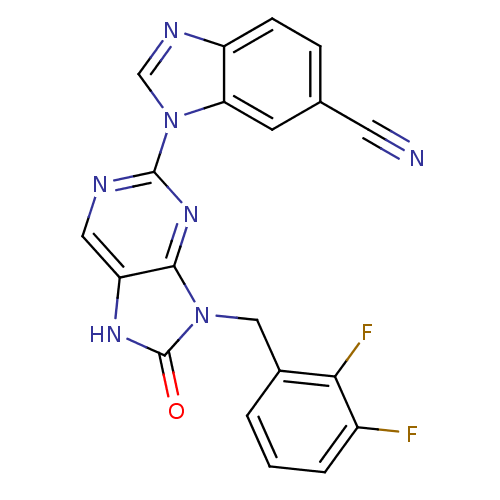 (1-(9-(2,3-difluorobenzyl)-8-oxo-8,9-dihydro-7H-pur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454504 (CHEMBL4210388) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507112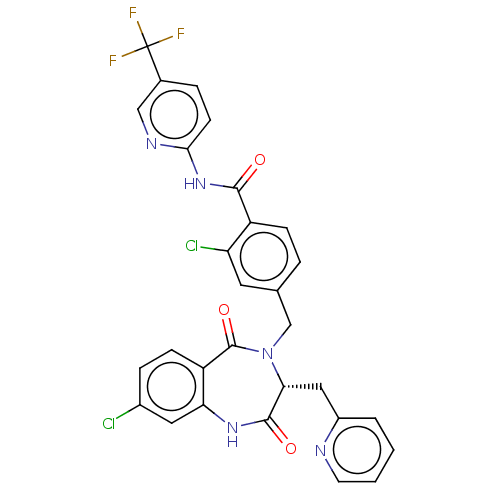 (CHEMBL4547929) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507118 (CHEMBL4456808) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507117 (CHEMBL4591470) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507105 (CHEMBL4557223) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of Clostridium difficile toxin B transfected in CHO cells assessed as reduction in caspase 3/7 activation pre-incubated for 1 hr before Tc... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50311656 (2-(6-fluoro-1H-benzo[d]imidazol-1-yl)-9-(8-fluoroc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK2 (532-1132) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50311644 (1-(9-(2,6-difluorobenzyl)-8-oxo-8,9-dihydro-7H-pur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK2 (532-1132) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311653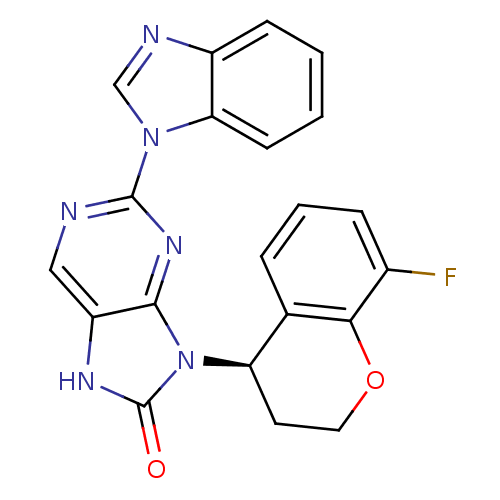 ((R)-2-(1H-benzo[d]imidazol-1-yl)-9-(8-fluorochroma...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311648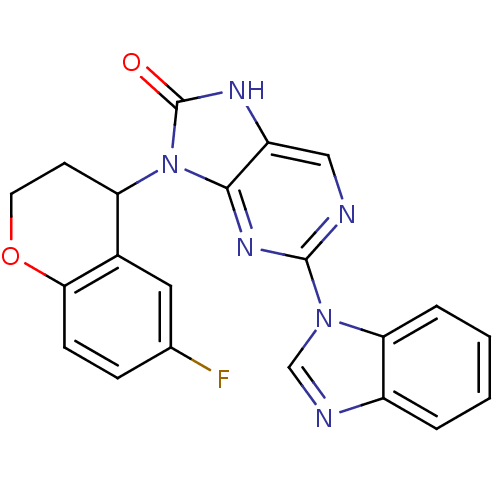 ((+/-)-2-(1H-benzo[d]imidazol-1-yl)-9-(6-fluorochro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507135 (CHEMBL4470638) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of Clostridium difficile toxin B transfected in CHO cells assessed as reduction in caspase 3/7 activation pre-incubated for 1 hr before Tc... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311651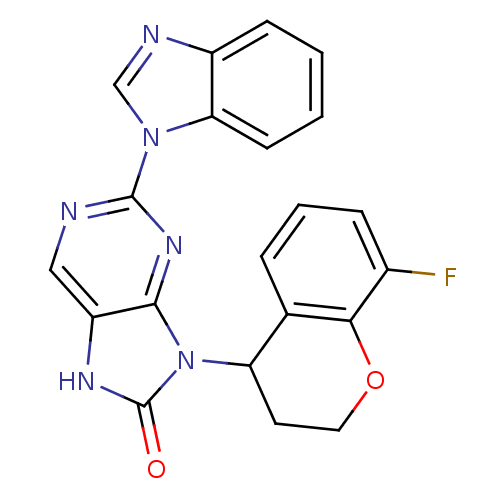 ((+/-)-2-(1H-benzo[d]imidazol-1-yl)-9-(8-fluorochro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50311638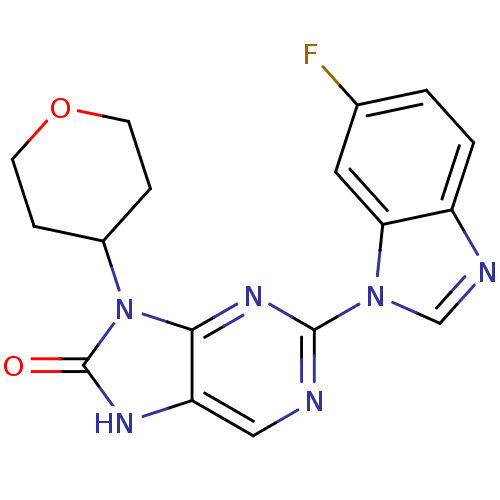 (2-(6-fluoro-1H-benzo[d]imidazol-1-yl)-9-(tetrahydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay | Bioorg Med Chem Lett 19: 6788-92 (2009) Article DOI: 10.1016/j.bmcl.2009.09.080 BindingDB Entry DOI: 10.7270/Q24B31G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507109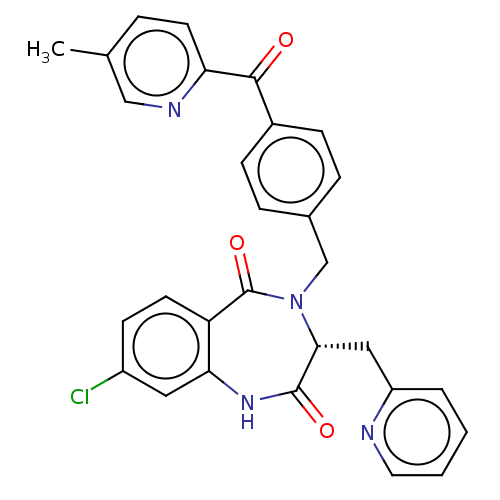 (CHEMBL4530206) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507121 (CHEMBL4589634) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454503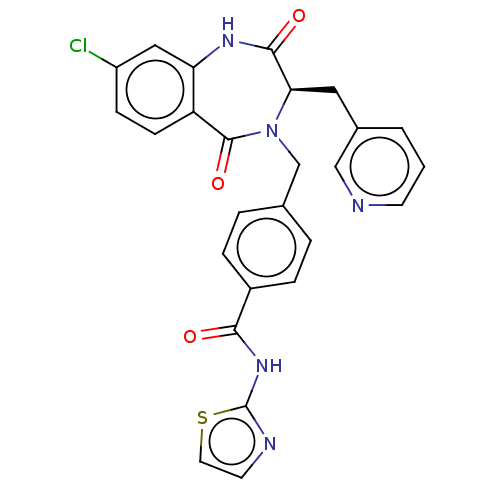 (CHEMBL4211420) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454498 (CHEMBL4215657) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of Clostridium difficile toxin B transfected in CHO cells assessed as reduction in caspase 3/7 activation pre-incubated for 1 hr before Tc... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 190 total ) | Next | Last >> |