

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50108119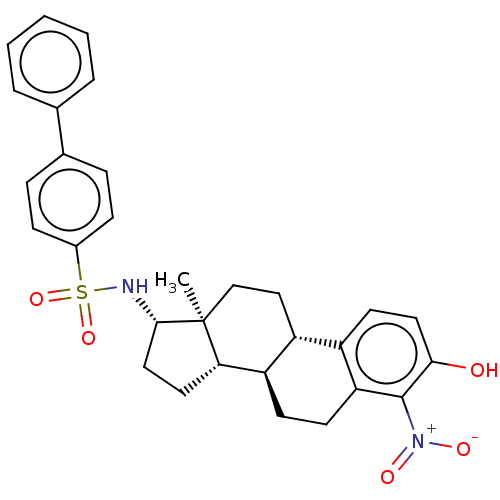 (CHEMBL3600587) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Reversible inhibition of STS (unknown origin) using 4-MUS as substrate measured over 10 mins by fluorescence assay | Bioorg Med Chem 23: 5681-92 (2015) Article DOI: 10.1016/j.bmc.2015.07.019 BindingDB Entry DOI: 10.7270/Q2R78H03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50108118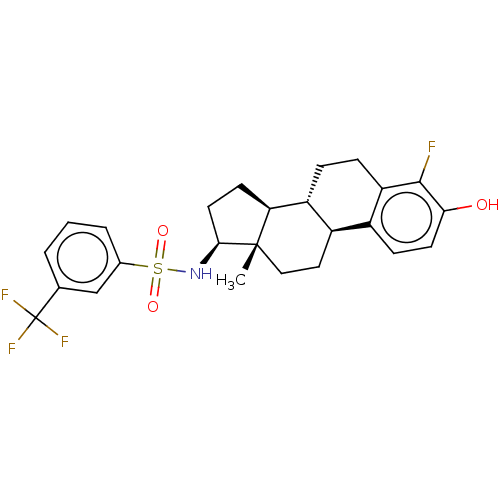 (CHEMBL3600594) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Reversible inhibition of STS (unknown origin) using 4-MUS as substrate measured over 10 mins by fluorescence assay | Bioorg Med Chem 23: 5681-92 (2015) Article DOI: 10.1016/j.bmc.2015.07.019 BindingDB Entry DOI: 10.7270/Q2R78H03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50534432 (CHEMBL4463709) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of MB-COMT in Wistar rat brain assessed as metanephrine formation preincubated for 20 mins followed by addition of adrenaline as substrate... | J Med Chem 59: 7584-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00666 BindingDB Entry DOI: 10.7270/Q2HQ43DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50108877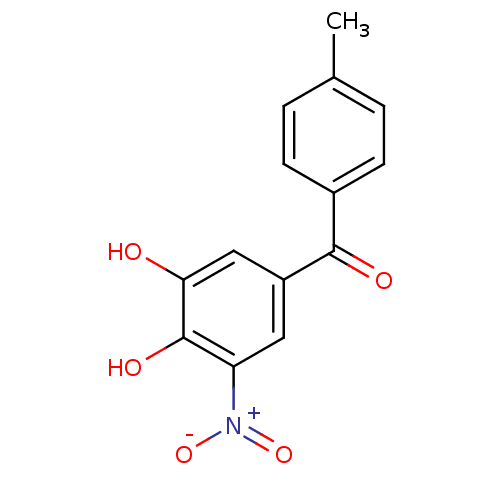 ((3,4-Dihydroxy-5-nitro-phenyl)-p-tolyl-methanone |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of MB-COMT in Wistar rat brain assessed as metanephrine formation preincubated for 20 mins followed by addition of adrenaline as substrate... | J Med Chem 59: 7584-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00666 BindingDB Entry DOI: 10.7270/Q2HQ43DN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50108877 ((3,4-Dihydroxy-5-nitro-phenyl)-p-tolyl-methanone |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.912 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of MB-COMT in Wistar rat brain assessed as metanephrine formation preincubated for 20 mins followed by addition of adrenaline as substrate... | J Med Chem 59: 7584-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00666 BindingDB Entry DOI: 10.7270/Q2HQ43DN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50534437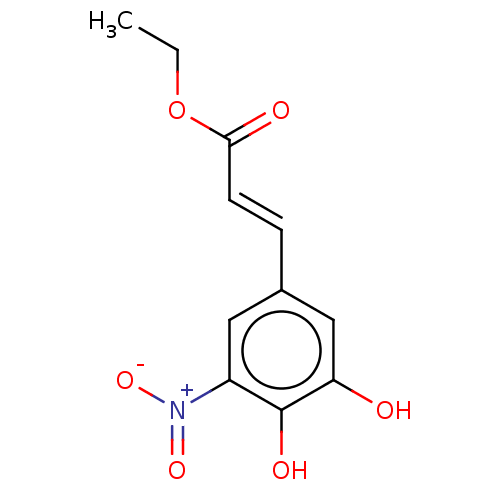 (CHEMBL4437043) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of MB-COMT in Wistar rat brain assessed as metanephrine formation preincubated for 20 mins followed by addition of adrenaline as substrate... | J Med Chem 59: 7584-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00666 BindingDB Entry DOI: 10.7270/Q2HQ43DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Bioorg Med Chem Lett 20: 3606-9 (2010) Article DOI: 10.1016/j.bmcl.2010.04.108 BindingDB Entry DOI: 10.7270/Q2GM87GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50534438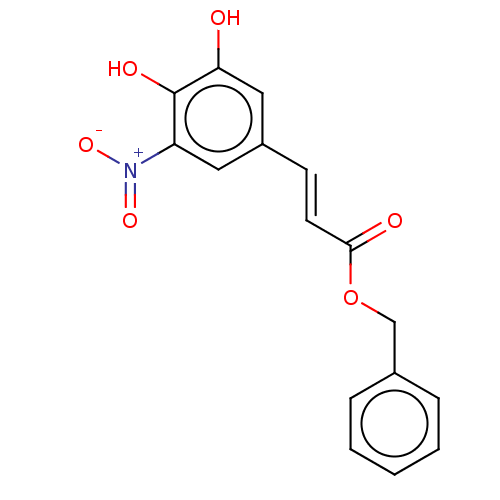 (CHEMBL4447349) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of MB-COMT in Wistar rat brain assessed as metanephrine formation preincubated for 20 mins followed by addition of adrenaline as substrate... | J Med Chem 59: 7584-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00666 BindingDB Entry DOI: 10.7270/Q2HQ43DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50534438 (CHEMBL4447349) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of MB-COMT in Wistar rat brain assessed as metanephrine formation preincubated for 20 mins followed by addition of adrenaline as substrate... | J Med Chem 59: 7584-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00666 BindingDB Entry DOI: 10.7270/Q2HQ43DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50534433 (CHEMBL4448310) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of MB-COMT in Wistar rat brain assessed as metanephrine formation preincubated for 20 mins followed by addition of adrenaline as substrate... | J Med Chem 59: 7584-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00666 BindingDB Entry DOI: 10.7270/Q2HQ43DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50108879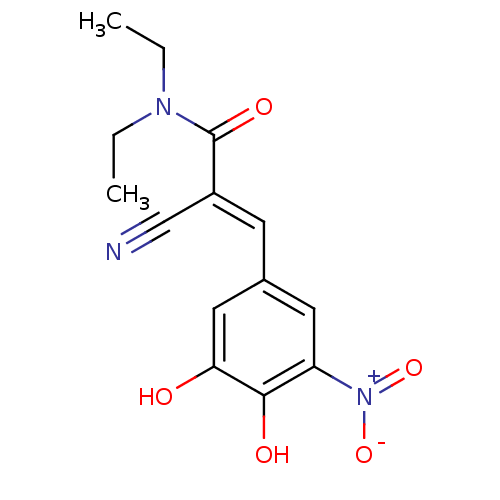 ((2E)-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.47 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of MB-COMT in Wistar rat brain assessed as metanephrine formation preincubated for 20 mins followed by addition of adrenaline as substrate... | J Med Chem 59: 7584-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00666 BindingDB Entry DOI: 10.7270/Q2HQ43DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50108879 ((2E)-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of MB-COMT in Wistar rat brain assessed as metanephrine formation preincubated for 20 mins followed by addition of adrenaline as substrate... | J Med Chem 59: 7584-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00666 BindingDB Entry DOI: 10.7270/Q2HQ43DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50534435 (CHEMBL4543735) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of MB-COMT in Wistar rat brain assessed as metanephrine formation preincubated for 20 mins followed by addition of adrenaline as substrate... | J Med Chem 59: 7584-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00666 BindingDB Entry DOI: 10.7270/Q2HQ43DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50534435 (CHEMBL4543735) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of MB-COMT in Wistar rat brain assessed as metanephrine formation preincubated for 20 mins followed by addition of adrenaline as substrate... | J Med Chem 59: 7584-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00666 BindingDB Entry DOI: 10.7270/Q2HQ43DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50534434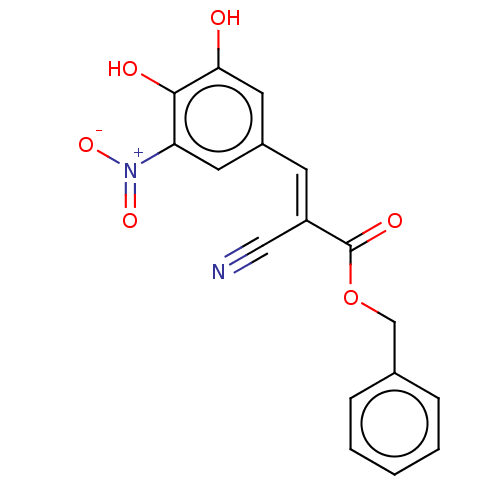 (CHEMBL4444830) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of MB-COMT in Wistar rat brain assessed as metanephrine formation preincubated for 20 mins followed by addition of adrenaline as substrate... | J Med Chem 59: 7584-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00666 BindingDB Entry DOI: 10.7270/Q2HQ43DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50534434 (CHEMBL4444830) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of MB-COMT in Wistar rat brain assessed as metanephrine formation preincubated for 20 mins followed by addition of adrenaline as substrate... | J Med Chem 59: 7584-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00666 BindingDB Entry DOI: 10.7270/Q2HQ43DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50479610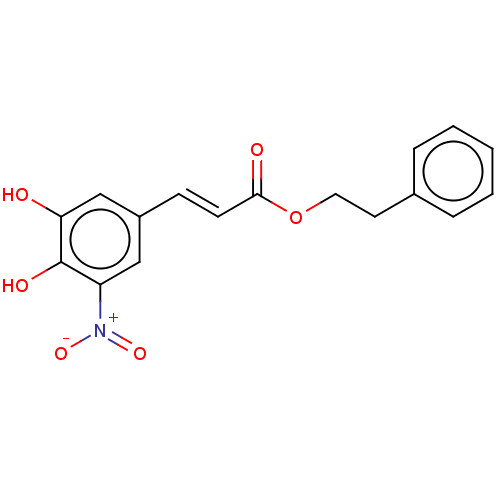 (CHEMBL481644) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of MB-COMT in Wistar rat brain assessed as metanephrine formation preincubated for 20 mins followed by addition of adrenaline as substrate... | J Med Chem 59: 7584-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00666 BindingDB Entry DOI: 10.7270/Q2HQ43DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50479610 (CHEMBL481644) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of MB-COMT in Wistar rat brain assessed as metanephrine formation preincubated for 20 mins followed by addition of adrenaline as substrate... | J Med Chem 59: 7584-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00666 BindingDB Entry DOI: 10.7270/Q2HQ43DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50534436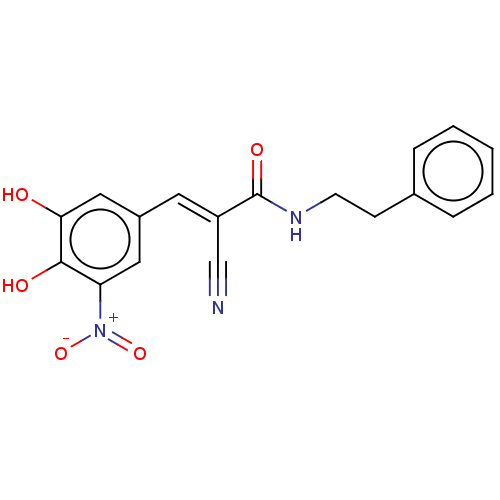 (CHEMBL4437795) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of MB-COMT in Wistar rat brain assessed as metanephrine formation preincubated for 20 mins followed by addition of adrenaline as substrate... | J Med Chem 59: 7584-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00666 BindingDB Entry DOI: 10.7270/Q2HQ43DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50479631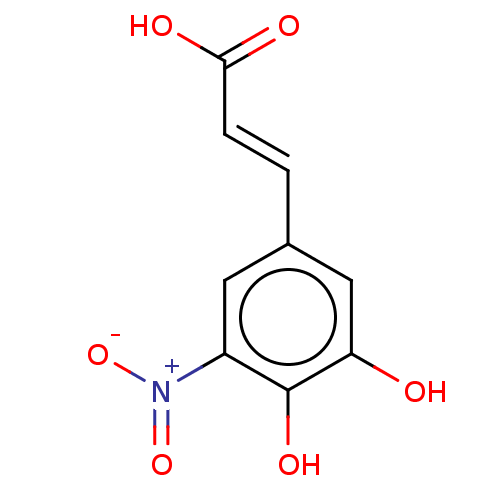 (CHEMBL137555) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of MB-COMT in Wistar rat brain assessed as metanephrine formation preincubated for 20 mins followed by addition of adrenaline as substrate... | J Med Chem 59: 7584-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00666 BindingDB Entry DOI: 10.7270/Q2HQ43DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50108126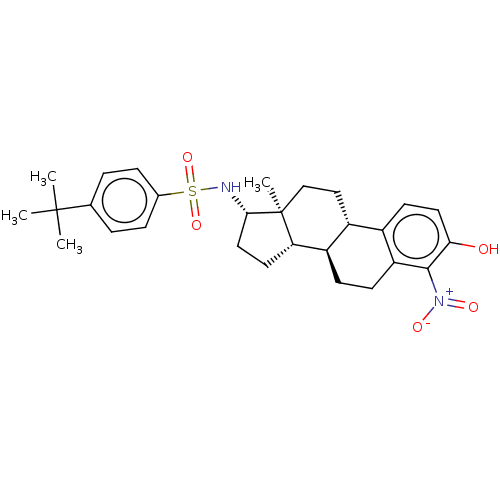 (CHEMBL3600586) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of STS (unknown origin) using 4-MUS as substrate measured over 10 mins by fluorescence assay | Bioorg Med Chem 23: 5681-92 (2015) Article DOI: 10.1016/j.bmc.2015.07.019 BindingDB Entry DOI: 10.7270/Q2R78H03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM13065 (5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluor...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hacettepe Curated by ChEMBL | Assay Description Inhibition of ovine COX1 by fluorescence assay | Bioorg Med Chem 20: 2912-22 (2012) Article DOI: 10.1016/j.bmc.2012.03.021 BindingDB Entry DOI: 10.7270/Q2F76DKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50363576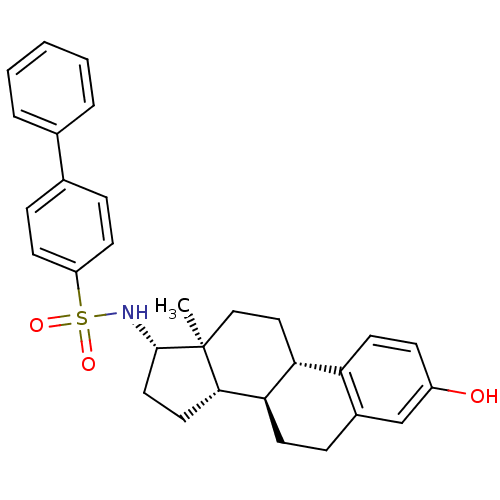 (CHEMBL1945904) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of STS (unknown origin) using 4-MUS as substrate measured over 10 mins by fluorescence assay | Bioorg Med Chem 23: 5681-92 (2015) Article DOI: 10.1016/j.bmc.2015.07.019 BindingDB Entry DOI: 10.7270/Q2R78H03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 10.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE | Bioorg Med Chem Lett 20: 3606-9 (2010) Article DOI: 10.1016/j.bmcl.2010.04.108 BindingDB Entry DOI: 10.7270/Q2GM87GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50534437 (CHEMBL4437043) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of S-COMT in Wistar rat liver assessed as metanephrine formation preincubated for 20 mins followed by addition of adrenaline as substrate ... | J Med Chem 59: 7584-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00666 BindingDB Entry DOI: 10.7270/Q2HQ43DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50108127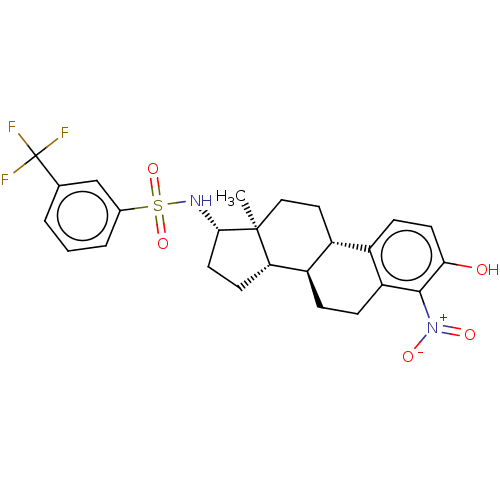 (CHEMBL3600585) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of STS (unknown origin) using 4-MUS as substrate measured over 10 mins by fluorescence assay | Bioorg Med Chem 23: 5681-92 (2015) Article DOI: 10.1016/j.bmc.2015.07.019 BindingDB Entry DOI: 10.7270/Q2R78H03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50108128 (CHEMBL3600584) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of STS (unknown origin) using 4-MUS as substrate measured over 10 mins by fluorescence assay | Bioorg Med Chem 23: 5681-92 (2015) Article DOI: 10.1016/j.bmc.2015.07.019 BindingDB Entry DOI: 10.7270/Q2R78H03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50479631 (CHEMBL137555) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of S-COMT in Wistar rat liver assessed as metanephrine formation preincubated for 20 mins followed by addition of adrenaline as substrate ... | J Med Chem 59: 7584-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00666 BindingDB Entry DOI: 10.7270/Q2HQ43DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50534432 (CHEMBL4463709) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of S-COMT in Wistar rat liver assessed as metanephrine formation preincubated for 20 mins followed by addition of adrenaline as substrate ... | J Med Chem 59: 7584-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00666 BindingDB Entry DOI: 10.7270/Q2HQ43DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50363575 (CHEMBL1945903) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of STS (unknown origin) using 4-MUS as substrate measured over 10 mins by fluorescence assay | Bioorg Med Chem 23: 5681-92 (2015) Article DOI: 10.1016/j.bmc.2015.07.019 BindingDB Entry DOI: 10.7270/Q2R78H03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BChE incubated with compounf for 5 mins before addition of substrate S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE | Bioorg Med Chem Lett 20: 3606-9 (2010) Article DOI: 10.1016/j.bmcl.2010.04.108 BindingDB Entry DOI: 10.7270/Q2GM87GC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM199186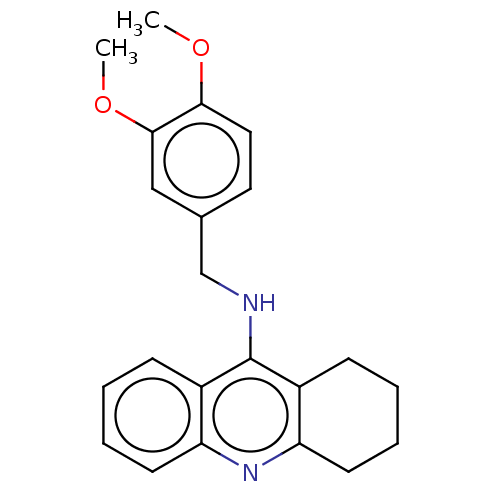 (N-(3,4-Dimethoxybenzyl)-1,2,3,4-tetrahydroacridin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM199186 (N-(3,4-Dimethoxybenzyl)-1,2,3,4-tetrahydroacridin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human BuChE using S-butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition by Ellman's method | Bioorg Med Chem Lett 27: 2443-2449 (2017) Article DOI: 10.1016/j.bmcl.2017.04.006 BindingDB Entry DOI: 10.7270/Q2WQ05X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of equine BuChE by Ellman's method | Bioorg Med Chem Lett 21: 5881-7 (2011) Article DOI: 10.1016/j.bmcl.2011.07.091 BindingDB Entry DOI: 10.7270/Q2C829P7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50363588 (CHEMBL1947221) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of STS (unknown origin) using 4-MUS as substrate measured over 10 mins by fluorescence assay | Bioorg Med Chem 23: 5681-92 (2015) Article DOI: 10.1016/j.bmc.2015.07.019 BindingDB Entry DOI: 10.7270/Q2R78H03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50363580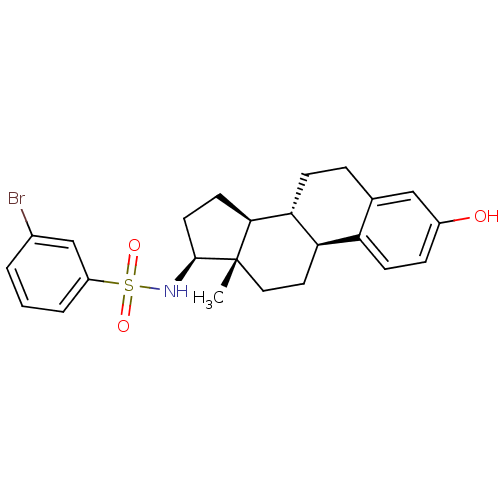 (CHEMBL1945908) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of STS (unknown origin) using 4-MUS as substrate measured over 10 mins by fluorescence assay | Bioorg Med Chem 23: 5681-92 (2015) Article DOI: 10.1016/j.bmc.2015.07.019 BindingDB Entry DOI: 10.7270/Q2R78H03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50534433 (CHEMBL4448310) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of S-COMT in Wistar rat liver assessed as metanephrine formation preincubated for 20 mins followed by addition of adrenaline as substrate ... | J Med Chem 59: 7584-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00666 BindingDB Entry DOI: 10.7270/Q2HQ43DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 60 to 300 se... | Eur J Med Chem 126: 823-843 (2017) Article DOI: 10.1016/j.ejmech.2016.12.005 BindingDB Entry DOI: 10.7270/Q2D220WF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50108877 ((3,4-Dihydroxy-5-nitro-phenyl)-p-tolyl-methanone |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of S-COMT in Wistar rat liver assessed as metanephrine formation preincubated for 20 mins followed by addition of adrenaline as substrate ... | J Med Chem 59: 7584-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00666 BindingDB Entry DOI: 10.7270/Q2HQ43DN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE by Ellman's assay | Bioorg Med Chem Lett 21: 5881-7 (2011) Article DOI: 10.1016/j.bmcl.2011.07.091 BindingDB Entry DOI: 10.7270/Q2C829P7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate after 5 mins by DTNB method | Bioorg Med Chem Lett 22: 4707-12 (2012) Article DOI: 10.1016/j.bmcl.2012.05.077 BindingDB Entry DOI: 10.7270/Q27S7PSZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE incubated with compounf for 5 mins before addition of substrate acetylthiocholine iodide by Ellman's method | Bioorg Med Chem 19: 2269-81 (2011) Article DOI: 10.1016/j.bmc.2011.02.030 BindingDB Entry DOI: 10.7270/Q2Q240JH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50108122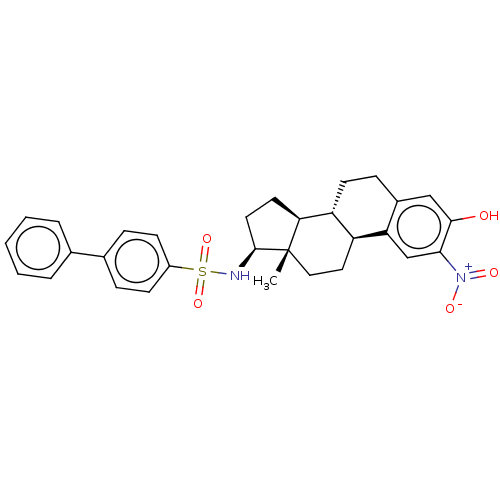 (CHEMBL3600591) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of STS (unknown origin) using 4-MUS as substrate measured over 10 mins by fluorescence assay | Bioorg Med Chem 23: 5681-92 (2015) Article DOI: 10.1016/j.bmc.2015.07.019 BindingDB Entry DOI: 10.7270/Q2R78H03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50108879 ((2E)-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of S-COMT in Wistar rat liver assessed as metanephrine formation preincubated for 20 mins followed by addition of adrenaline as substrate ... | J Med Chem 59: 7584-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00666 BindingDB Entry DOI: 10.7270/Q2HQ43DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Waterloo | Assay Description Test compounds were prepared in DMSO (maximum concentration used 1% v/v), and 10 μL of each (0.001-25 μm final concentration range) was inc... | Chem Biol Drug Des 88: 710-723 (2016) Article DOI: 10.1111/cbdd.12800 BindingDB Entry DOI: 10.7270/Q26D5RSX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human BuChE using S-butyrylthiocholine iodide as substrate treated 5 mins before substrate addition measured up to 4 mins by Ellman's m... | Bioorg Med Chem Lett 23: 4336-41 (2013) Article DOI: 10.1016/j.bmcl.2013.05.092 BindingDB Entry DOI: 10.7270/Q2JD50Q1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate treated 5 mins before substrate addition measured up to 4 mins by Ellman's metho... | Bioorg Med Chem Lett 23: 4336-41 (2013) Article DOI: 10.1016/j.bmcl.2013.05.092 BindingDB Entry DOI: 10.7270/Q2JD50Q1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 518 total ) | Next | Last >> |