

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM203459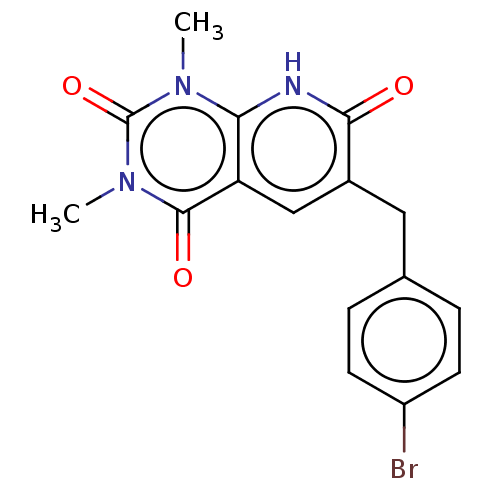 (US9242982, 1k) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | 22 |
COUNCIL OF SCIENTIFIC & INDUSTRIAL RESEARCH US Patent | Assay Description PDE3 inhibition assay was performed a BIOMOL GREEN Quantizyme Assay System (catalogue No. BML-AK800-0001). The Platelets isolated from human blood ... | US Patent US9242982 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM203457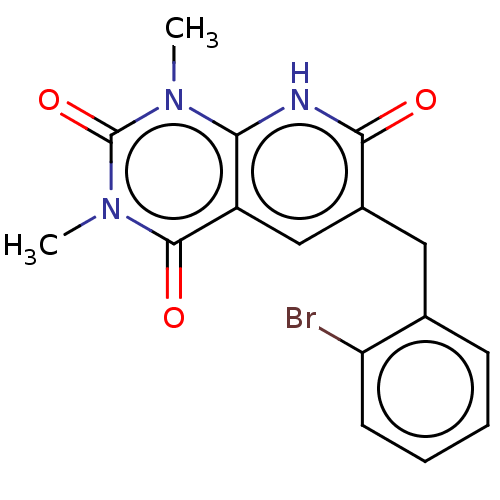 (US9242982, 1i) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | 22 |
COUNCIL OF SCIENTIFIC & INDUSTRIAL RESEARCH US Patent | Assay Description PDE3 inhibition assay was performed a BIOMOL GREEN Quantizyme Assay System (catalogue No. BML-AK800-0001). The Platelets isolated from human blood ... | US Patent US9242982 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM205353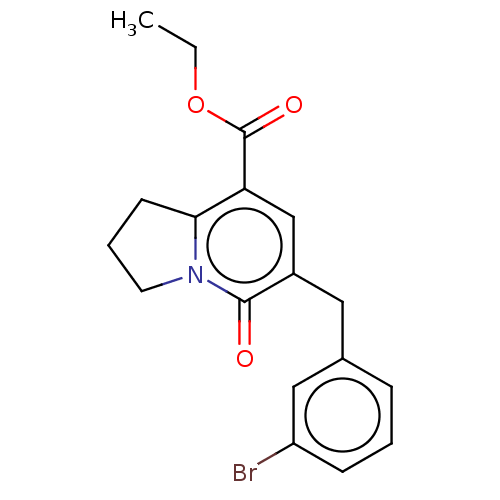 (US9249139, 1j) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11.3 | n/a | n/a | n/a | n/a | n/a | 22 |
Council of Scientific & Industrial Research US Patent | Assay Description PDE3 inhibition assay was performed a BIOMOL GREEN Quantizyme Assay System (catalogue No. BML-AK800-0001). The Platelets isolated from human blood ... | US Patent US9249139 (2016) BindingDB Entry DOI: 10.7270/Q27W6B1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM203455 (US9242982, 1g) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.5 | n/a | n/a | n/a | n/a | n/a | 22 |
COUNCIL OF SCIENTIFIC & INDUSTRIAL RESEARCH US Patent | Assay Description PDE3 inhibition assay was performed a BIOMOL GREEN Quantizyme Assay System (catalogue No. BML-AK800-0001). The Platelets isolated from human blood ... | US Patent US9242982 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM205346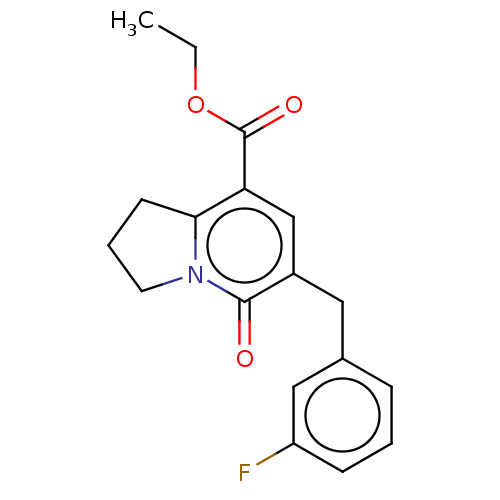 (US9249139, 1c) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.5 | n/a | n/a | n/a | n/a | n/a | 22 |
Council of Scientific & Industrial Research US Patent | Assay Description PDE3 inhibition assay was performed a BIOMOL GREEN Quantizyme Assay System (catalogue No. BML-AK800-0001). The Platelets isolated from human blood ... | US Patent US9249139 (2016) BindingDB Entry DOI: 10.7270/Q27W6B1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM203451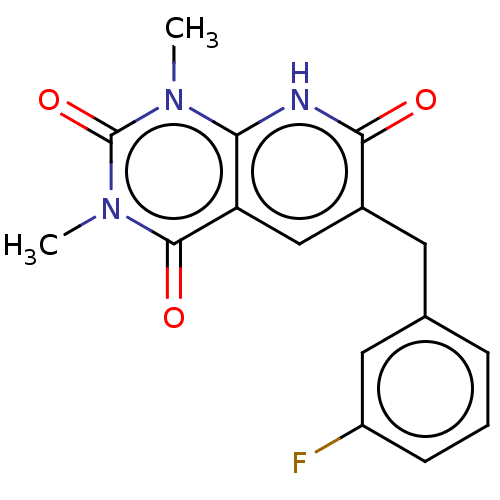 (US9242982, 1c) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32.3 | n/a | n/a | n/a | n/a | n/a | 22 |
COUNCIL OF SCIENTIFIC & INDUSTRIAL RESEARCH US Patent | Assay Description PDE3 inhibition assay was performed a BIOMOL GREEN Quantizyme Assay System (catalogue No. BML-AK800-0001). The Platelets isolated from human blood ... | US Patent US9242982 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM203450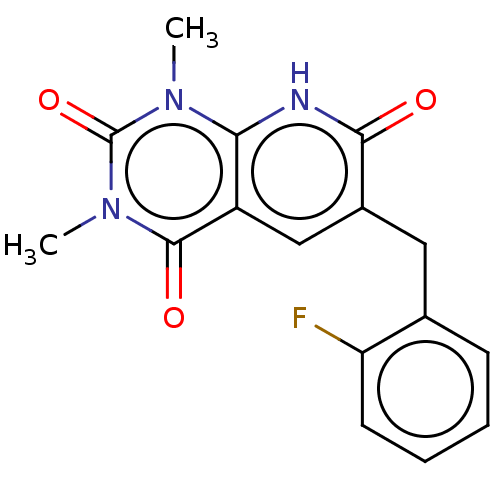 (US9242982, 1b) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 48.8 | n/a | n/a | n/a | n/a | n/a | 22 |
COUNCIL OF SCIENTIFIC & INDUSTRIAL RESEARCH US Patent | Assay Description PDE3 inhibition assay was performed a BIOMOL GREEN Quantizyme Assay System (catalogue No. BML-AK800-0001). The Platelets isolated from human blood ... | US Patent US9242982 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM205354 (US9249139, 1k) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 67.3 | n/a | n/a | n/a | n/a | n/a | 22 |
Council of Scientific & Industrial Research US Patent | Assay Description PDE3 inhibition assay was performed a BIOMOL GREEN Quantizyme Assay System (catalogue No. BML-AK800-0001). The Platelets isolated from human blood ... | US Patent US9249139 (2016) BindingDB Entry DOI: 10.7270/Q27W6B1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Rattus norvegicus) | BDBM50049730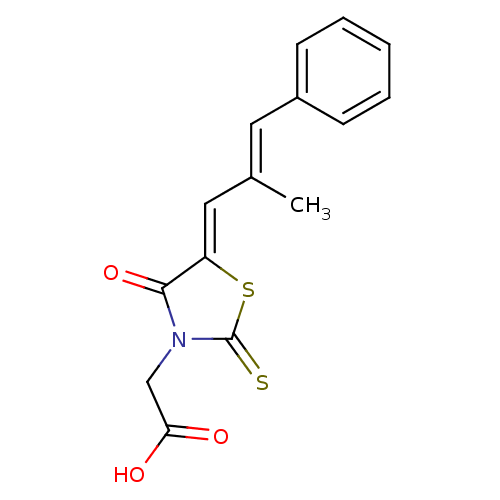 (2-(5-(2-methyl-3-phenylallylidene)-4-oxo-2-thioxot...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rat kidney NADPH-dependent aldose reductase assessed as DL-glyceraldehyde conversion to glycerol preincubated for 20 mins followed by N... | Eur J Med Chem 71: 53-66 (2014) Article DOI: 10.1016/j.ejmech.2013.10.043 BindingDB Entry DOI: 10.7270/Q2H996P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM205350 (US9249139, 1g) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | 22 |
Council of Scientific & Industrial Research US Patent | Assay Description PDE3 inhibition assay was performed a BIOMOL GREEN Quantizyme Assay System (catalogue No. BML-AK800-0001). The Platelets isolated from human blood ... | US Patent US9249139 (2016) BindingDB Entry DOI: 10.7270/Q27W6B1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM205348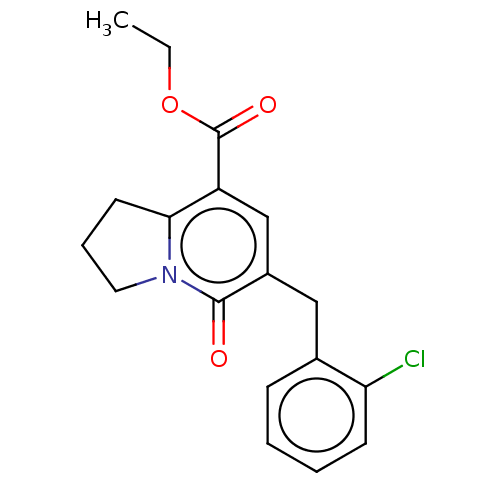 (US9249139, 1e) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | 22 |
Council of Scientific & Industrial Research US Patent | Assay Description PDE3 inhibition assay was performed a BIOMOL GREEN Quantizyme Assay System (catalogue No. BML-AK800-0001). The Platelets isolated from human blood ... | US Patent US9249139 (2016) BindingDB Entry DOI: 10.7270/Q27W6B1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Rattus norvegicus) | BDBM50444638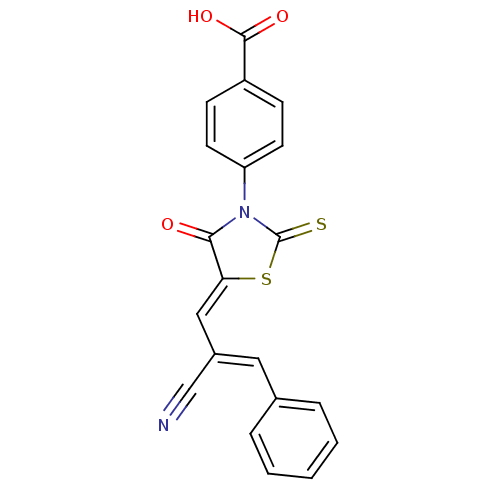 (CHEMBL3098361) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rat kidney NADPH-dependent aldose reductase assessed as DL-glyceraldehyde conversion to glycerol preincubated for 20 mins followed by N... | Eur J Med Chem 71: 53-66 (2014) Article DOI: 10.1016/j.ejmech.2013.10.043 BindingDB Entry DOI: 10.7270/Q2H996P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM205344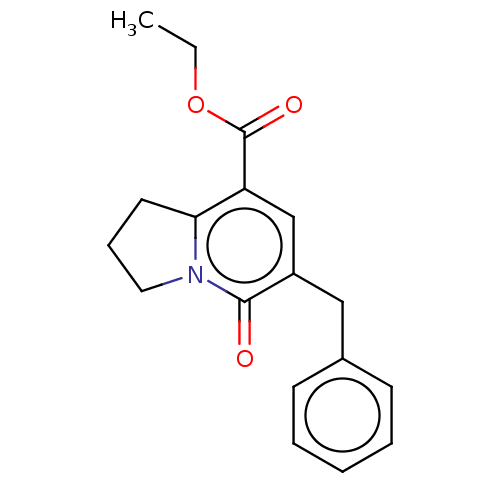 (US9249139, 1a) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 435 | n/a | n/a | n/a | n/a | n/a | 22 |
Council of Scientific & Industrial Research US Patent | Assay Description PDE3 inhibition assay was performed a BIOMOL GREEN Quantizyme Assay System (catalogue No. BML-AK800-0001). The Platelets isolated from human blood ... | US Patent US9249139 (2016) BindingDB Entry DOI: 10.7270/Q27W6B1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM205351 (US9249139, 1h) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 577 | n/a | n/a | n/a | n/a | n/a | 22 |
Council of Scientific & Industrial Research US Patent | Assay Description PDE3 inhibition assay was performed a BIOMOL GREEN Quantizyme Assay System (catalogue No. BML-AK800-0001). The Platelets isolated from human blood ... | US Patent US9249139 (2016) BindingDB Entry DOI: 10.7270/Q27W6B1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Rattus norvegicus) | BDBM50444641 (CHEMBL3098447) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rat kidney NADPH-dependent aldose reductase assessed as DL-glyceraldehyde conversion to glycerol preincubated for 20 mins followed by N... | Eur J Med Chem 71: 53-66 (2014) Article DOI: 10.1016/j.ejmech.2013.10.043 BindingDB Entry DOI: 10.7270/Q2H996P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Rattus norvegicus) | BDBM50444640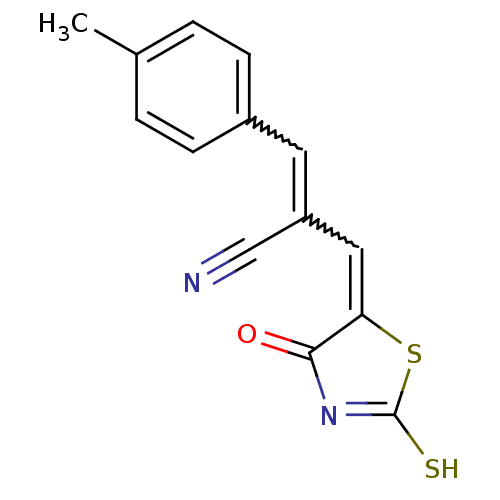 (CHEMBL3098450) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rat kidney NADPH-dependent aldose reductase assessed as DL-glyceraldehyde conversion to glycerol preincubated for 20 mins followed by N... | Eur J Med Chem 71: 53-66 (2014) Article DOI: 10.1016/j.ejmech.2013.10.043 BindingDB Entry DOI: 10.7270/Q2H996P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Rattus norvegicus) | BDBM50444642 (CHEMBL3098459) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rat kidney NADPH-dependent aldose reductase assessed as DL-glyceraldehyde conversion to glycerol preincubated for 20 mins followed by N... | Eur J Med Chem 71: 53-66 (2014) Article DOI: 10.1016/j.ejmech.2013.10.043 BindingDB Entry DOI: 10.7270/Q2H996P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Rattus norvegicus) | BDBM50444639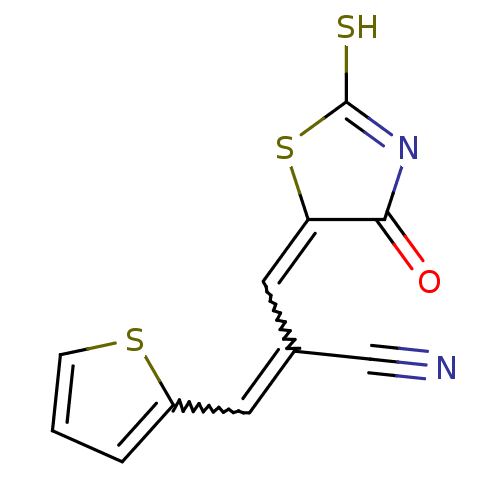 (CHEMBL3098360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rat kidney NADPH-dependent aldose reductase assessed as DL-glyceraldehyde conversion to glycerol preincubated for 20 mins followed by N... | Eur J Med Chem 71: 53-66 (2014) Article DOI: 10.1016/j.ejmech.2013.10.043 BindingDB Entry DOI: 10.7270/Q2H996P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM205347 (US9249139, 1d) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | 22 |
Council of Scientific & Industrial Research US Patent | Assay Description PDE3 inhibition assay was performed a BIOMOL GREEN Quantizyme Assay System (catalogue No. BML-AK800-0001). The Platelets isolated from human blood ... | US Patent US9249139 (2016) BindingDB Entry DOI: 10.7270/Q27W6B1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM50485540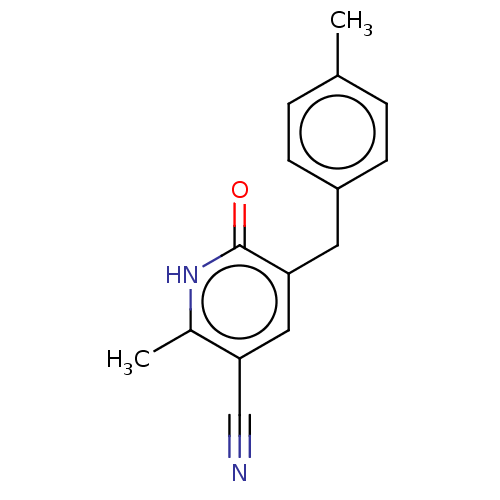 (CHEMBL2069990) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of PDE3 by Biomol Green quantizyme assay system | Bioorg Med Chem Lett 22: 6010-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.019 BindingDB Entry DOI: 10.7270/Q2P84FRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM50485536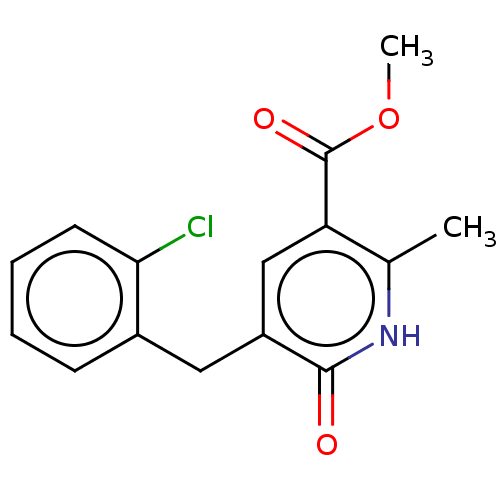 (CHEMBL2069919) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of PDE3 by Biomol Green quantizyme assay system | Bioorg Med Chem Lett 22: 6010-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.019 BindingDB Entry DOI: 10.7270/Q2P84FRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM15296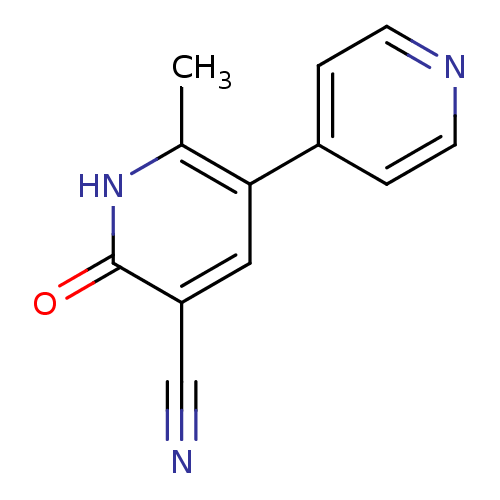 (6-methyl-2-oxo-5-(pyridin-4-yl)-1,2-dihydropyridin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | 22 |
COUNCIL OF SCIENTIFIC & INDUSTRIAL RESEARCH US Patent | Assay Description PDE3 inhibition assay was performed a BIOMOL GREEN Quantizyme Assay System (catalogue No. BML-AK800-0001). The Platelets isolated from human blood ... | US Patent US9242982 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM15296 (6-methyl-2-oxo-5-(pyridin-4-yl)-1,2-dihydropyridin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of PDE3 by Biomol Green quantizyme assay system | Bioorg Med Chem Lett 22: 6010-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.019 BindingDB Entry DOI: 10.7270/Q2P84FRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM15296 (6-methyl-2-oxo-5-(pyridin-4-yl)-1,2-dihydropyridin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | 22 |
Council of Scientific & Industrial Research US Patent | Assay Description PDE3 inhibition assay was performed a BIOMOL GREEN Quantizyme Assay System (catalogue No. BML-AK800-0001). The Platelets isolated from human blood ... | US Patent US9249139 (2016) BindingDB Entry DOI: 10.7270/Q27W6B1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM50485544 (CHEMBL2069920) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of PDE3 by Biomol Green quantizyme assay system | Bioorg Med Chem Lett 22: 6010-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.019 BindingDB Entry DOI: 10.7270/Q2P84FRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM50485546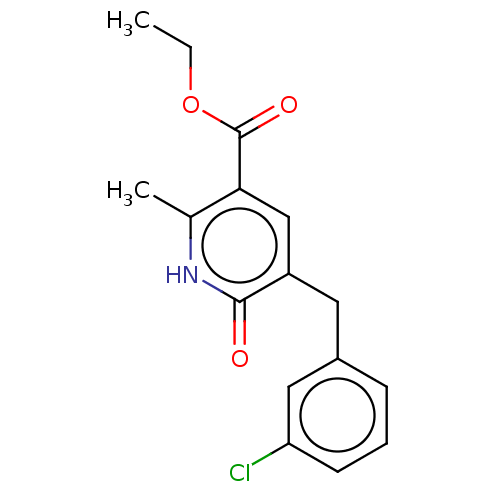 (CHEMBL2069912) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of PDE3 by Biomol Green quantizyme assay system | Bioorg Med Chem Lett 22: 6010-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.019 BindingDB Entry DOI: 10.7270/Q2P84FRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM50485538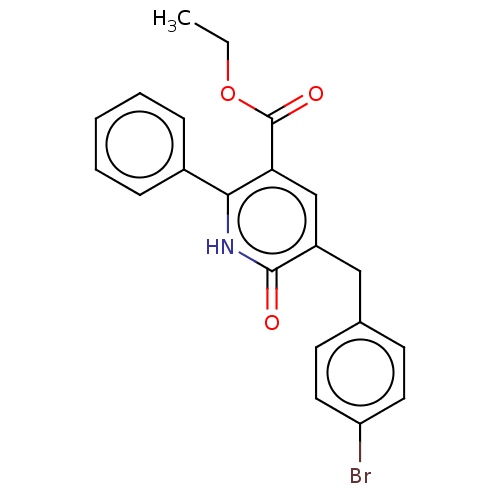 (CHEMBL2069983) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of PDE3 by Biomol Green quantizyme assay system | Bioorg Med Chem Lett 22: 6010-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.019 BindingDB Entry DOI: 10.7270/Q2P84FRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM203449 (US9242982, 1a) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | 22 |
COUNCIL OF SCIENTIFIC & INDUSTRIAL RESEARCH US Patent | Assay Description PDE3 inhibition assay was performed a BIOMOL GREEN Quantizyme Assay System (catalogue No. BML-AK800-0001). The Platelets isolated from human blood ... | US Patent US9242982 (2016) BindingDB Entry DOI: 10.7270/Q2BV7FF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50401967 (CHEMBL2207959) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North Maharashtra University Curated by ChEMBL | Assay Description Inhibition of liver glucosidase in Swiss mouse liver extracts | Bioorg Med Chem Lett 22: 7011-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.025 BindingDB Entry DOI: 10.7270/Q2FN17CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Sus scrofa (Pig)) | BDBM50401976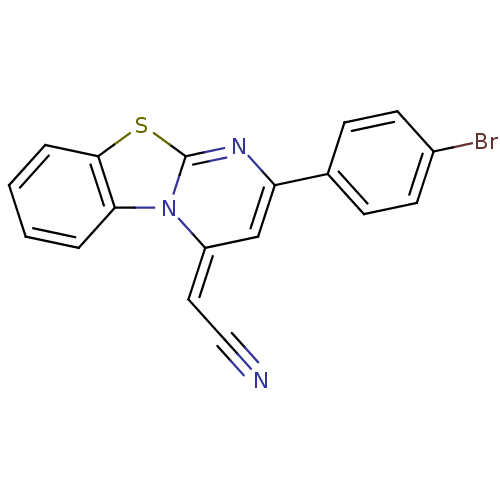 (CHEMBL2203334) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North Maharashtra University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic alpha amylase using starch as substrate by Bernfeld method | Bioorg Med Chem Lett 22: 7011-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.025 BindingDB Entry DOI: 10.7270/Q2FN17CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Sus scrofa (Pig)) | BDBM50425183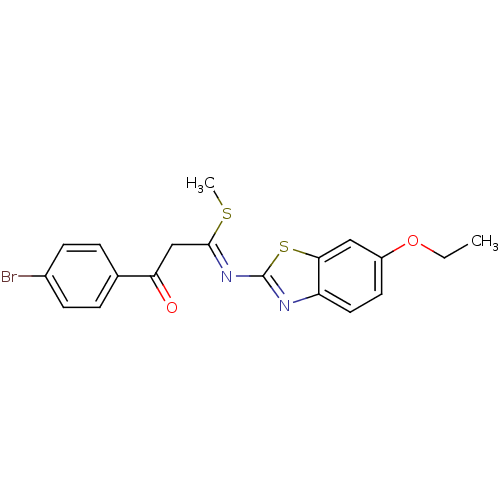 (CHEMBL2313583) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North Maharashtra University Curated by ChEMBL | Assay Description Inhibition of pig pancreatic alpha-amylase by Bernfeld method | Eur J Med Chem 59: 304-9 (2013) Article DOI: 10.1016/j.ejmech.2012.11.020 BindingDB Entry DOI: 10.7270/Q2028SV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50401973 (CHEMBL2207953) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North Maharashtra University Curated by ChEMBL | Assay Description Inhibition of liver glucosidase in Swiss mouse liver extracts | Bioorg Med Chem Lett 22: 7011-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.025 BindingDB Entry DOI: 10.7270/Q2FN17CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Sus scrofa (Pig)) | BDBM50425181 (CHEMBL2313585) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North Maharashtra University Curated by ChEMBL | Assay Description Inhibition of pig pancreatic alpha-amylase by Bernfeld method | Eur J Med Chem 59: 304-9 (2013) Article DOI: 10.1016/j.ejmech.2012.11.020 BindingDB Entry DOI: 10.7270/Q2028SV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Sus scrofa (Pig)) | BDBM50425180 (CHEMBL2313586) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North Maharashtra University Curated by ChEMBL | Assay Description Inhibition of pig pancreatic alpha-amylase by Bernfeld method | Eur J Med Chem 59: 304-9 (2013) Article DOI: 10.1016/j.ejmech.2012.11.020 BindingDB Entry DOI: 10.7270/Q2028SV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Sus scrofa (Pig)) | BDBM50401967 (CHEMBL2207959) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North Maharashtra University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic alpha amylase using starch as substrate by Bernfeld method | Bioorg Med Chem Lett 22: 7011-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.025 BindingDB Entry DOI: 10.7270/Q2FN17CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Sus scrofa (Pig)) | BDBM50425182 (CHEMBL2313584) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North Maharashtra University Curated by ChEMBL | Assay Description Inhibition of pig pancreatic alpha-amylase by Bernfeld method | Eur J Med Chem 59: 304-9 (2013) Article DOI: 10.1016/j.ejmech.2012.11.020 BindingDB Entry DOI: 10.7270/Q2028SV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Sus scrofa (Pig)) | BDBM50425178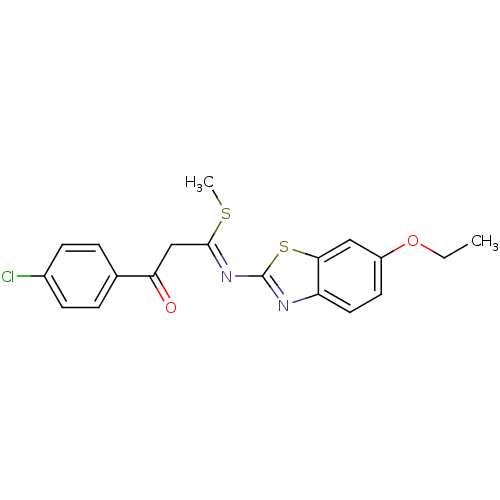 (CHEMBL2313588) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North Maharashtra University Curated by ChEMBL | Assay Description Inhibition of pig pancreatic alpha-amylase by Bernfeld method | Eur J Med Chem 59: 304-9 (2013) Article DOI: 10.1016/j.ejmech.2012.11.020 BindingDB Entry DOI: 10.7270/Q2028SV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50401970 (CHEMBL2207956) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North Maharashtra University Curated by ChEMBL | Assay Description Inhibition of liver glucosidase in Swiss mouse liver extracts | Bioorg Med Chem Lett 22: 7011-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.025 BindingDB Entry DOI: 10.7270/Q2FN17CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Sus scrofa (Pig)) | BDBM50425176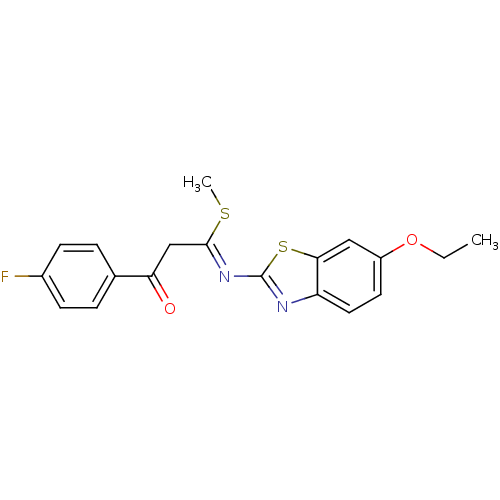 (CHEMBL2313590) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North Maharashtra University Curated by ChEMBL | Assay Description Inhibition of pig pancreatic alpha-amylase by Bernfeld method | Eur J Med Chem 59: 304-9 (2013) Article DOI: 10.1016/j.ejmech.2012.11.020 BindingDB Entry DOI: 10.7270/Q2028SV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Sus scrofa (Pig)) | BDBM50425175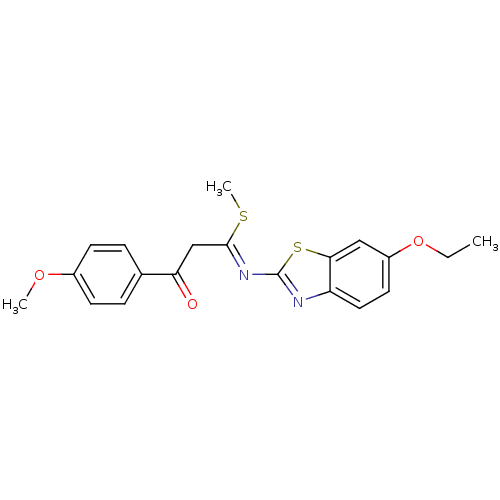 (CHEMBL2313591) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North Maharashtra University Curated by ChEMBL | Assay Description Inhibition of pig pancreatic alpha-amylase by Bernfeld method | Eur J Med Chem 59: 304-9 (2013) Article DOI: 10.1016/j.ejmech.2012.11.020 BindingDB Entry DOI: 10.7270/Q2028SV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50401968 (CHEMBL2207958) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North Maharashtra University Curated by ChEMBL | Assay Description Inhibition of liver glucosidase in Swiss mouse liver extracts | Bioorg Med Chem Lett 22: 7011-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.025 BindingDB Entry DOI: 10.7270/Q2FN17CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Sus scrofa (Pig)) | BDBM50401973 (CHEMBL2207953) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North Maharashtra University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic alpha amylase using starch as substrate by Bernfeld method | Bioorg Med Chem Lett 22: 7011-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.025 BindingDB Entry DOI: 10.7270/Q2FN17CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Sus scrofa (Pig)) | BDBM50425184 (CHEMBL2313582) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North Maharashtra University Curated by ChEMBL | Assay Description Inhibition of pig pancreatic alpha-amylase by Bernfeld method | Eur J Med Chem 59: 304-9 (2013) Article DOI: 10.1016/j.ejmech.2012.11.020 BindingDB Entry DOI: 10.7270/Q2028SV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Sus scrofa (Pig)) | BDBM50401970 (CHEMBL2207956) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North Maharashtra University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic alpha amylase using starch as substrate by Bernfeld method | Bioorg Med Chem Lett 22: 7011-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.025 BindingDB Entry DOI: 10.7270/Q2FN17CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50401972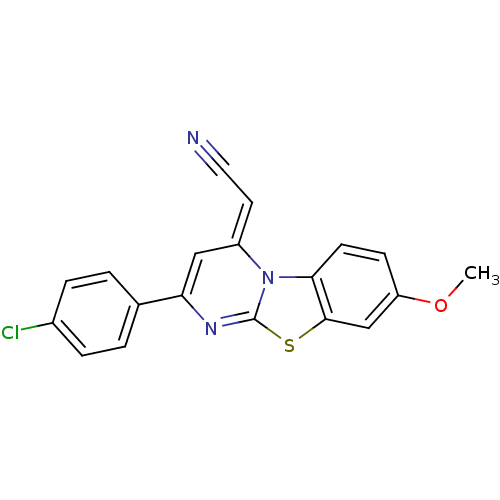 (CHEMBL2207954) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North Maharashtra University Curated by ChEMBL | Assay Description Inhibition of liver glucosidase in Swiss mouse liver extracts | Bioorg Med Chem Lett 22: 7011-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.025 BindingDB Entry DOI: 10.7270/Q2FN17CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Sus scrofa (Pig)) | BDBM50425177 (CHEMBL2313589) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North Maharashtra University Curated by ChEMBL | Assay Description Inhibition of pig pancreatic alpha-amylase by Bernfeld method | Eur J Med Chem 59: 304-9 (2013) Article DOI: 10.1016/j.ejmech.2012.11.020 BindingDB Entry DOI: 10.7270/Q2028SV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Sus scrofa (Pig)) | BDBM50401974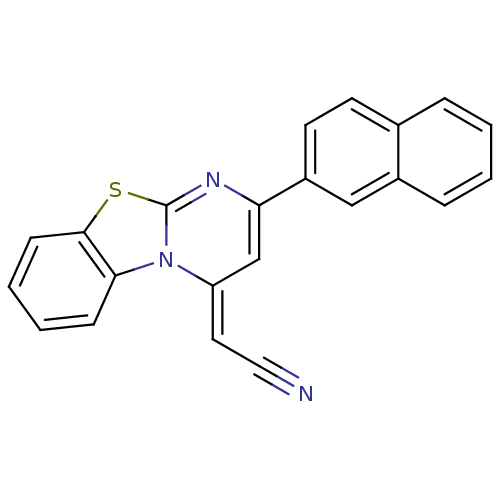 (CHEMBL2207952) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North Maharashtra University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic alpha amylase using starch as substrate by Bernfeld method | Bioorg Med Chem Lett 22: 7011-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.025 BindingDB Entry DOI: 10.7270/Q2FN17CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Sus scrofa (Pig)) | BDBM50401972 (CHEMBL2207954) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North Maharashtra University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic alpha amylase using starch as substrate by Bernfeld method | Bioorg Med Chem Lett 22: 7011-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.025 BindingDB Entry DOI: 10.7270/Q2FN17CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50401971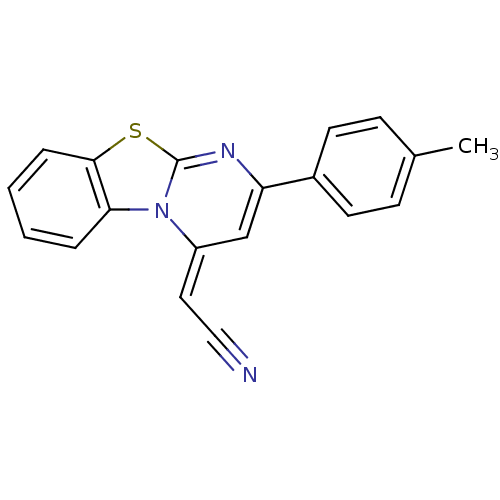 (CHEMBL2207955) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North Maharashtra University Curated by ChEMBL | Assay Description Inhibition of liver glucosidase in Swiss mouse liver extracts | Bioorg Med Chem Lett 22: 7011-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.025 BindingDB Entry DOI: 10.7270/Q2FN17CD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Sus scrofa (Pig)) | BDBM50425179 (CHEMBL2313587) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
North Maharashtra University Curated by ChEMBL | Assay Description Inhibition of pig pancreatic alpha-amylase by Bernfeld method | Eur J Med Chem 59: 304-9 (2013) Article DOI: 10.1016/j.ejmech.2012.11.020 BindingDB Entry DOI: 10.7270/Q2028SV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 79 total ) | Next | Last >> |