

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM17945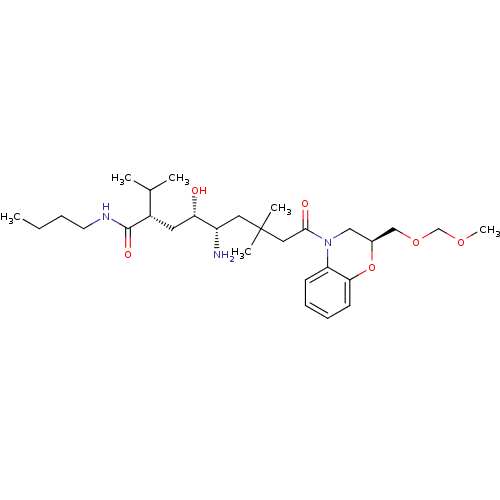 ((2S,4S,5S)-5-amino-N-butyl-4-hydroxy-9-[(2S)-2-[(m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description In vitro potencies of compounds against purified human recombinant renin were determined by its cleavage of substrate angiotensinogen. The angiotensi... | Chem Biol 7: 493-504 (2000) Article DOI: 10.1016/S1074-5521(00)00134-4 BindingDB Entry DOI: 10.7270/Q2V40SGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18313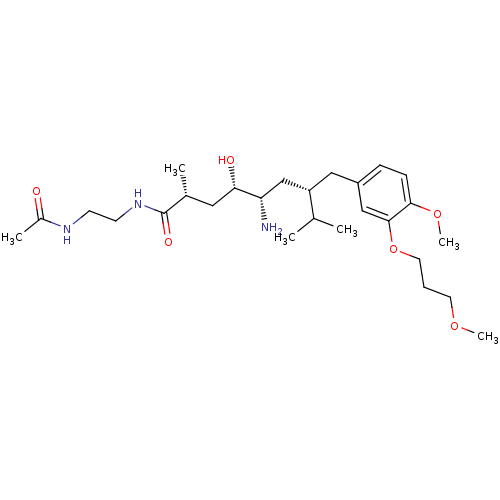 ((2R,4S,5S,7S)-5-amino-N-(2-acetamidoethyl)-4-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17949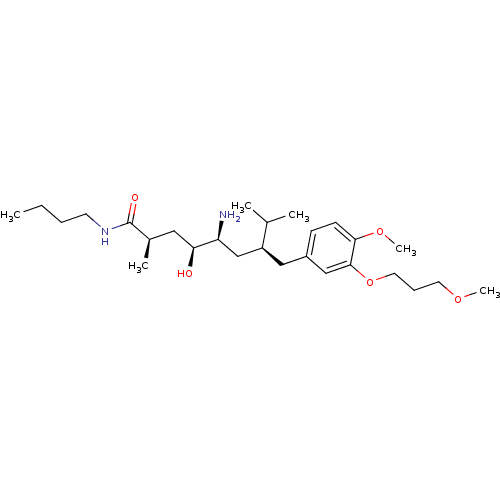 ((2R,4S,5S,7S)-5-amino-N-butyl-4-hydroxy-7-{[4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description In vitro potencies of compounds against purified human recombinant renin were determined by its cleavage of substrate angiotensinogen. The angiotensi... | Chem Biol 7: 493-504 (2000) Article DOI: 10.1016/S1074-5521(00)00134-4 BindingDB Entry DOI: 10.7270/Q2V40SGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18288 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022582 (CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of Human kideny renin | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17944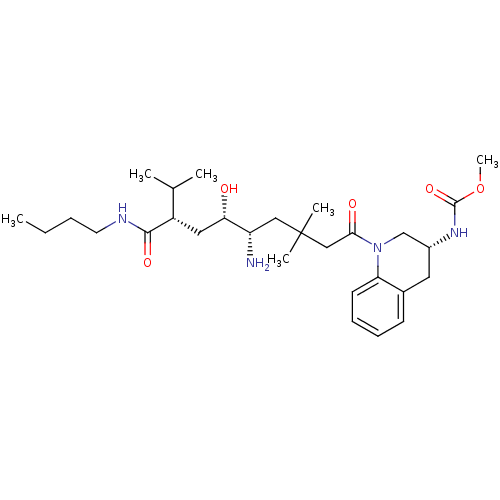 (Renin nonpeptide inhibitor, 4 | methyl N-[(3R)-1-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description In vitro potencies of compounds against purified human recombinant renin were determined by its cleavage of substrate angiotensinogen. The angiotensi... | Chem Biol 7: 493-504 (2000) Article DOI: 10.1016/S1074-5521(00)00134-4 BindingDB Entry DOI: 10.7270/Q2V40SGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18278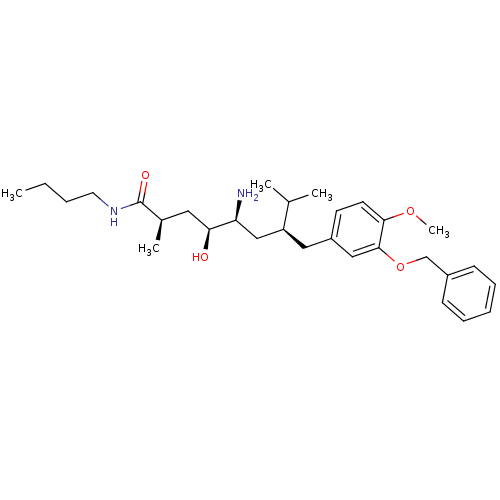 ((2R,4S,5S,7S)-5-amino-7-{[3-(benzyloxy)-4-methoxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17950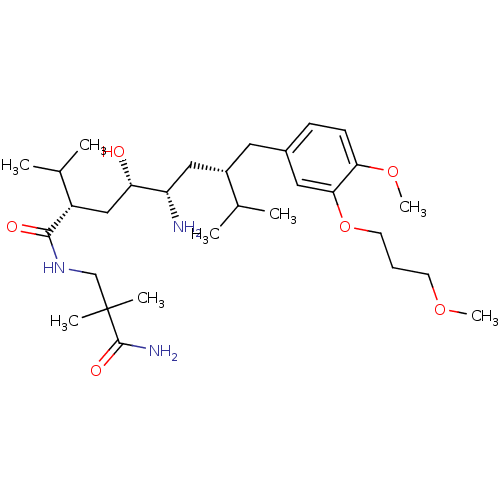 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description In vitro potencies of compounds against purified human recombinant renin were determined by its cleavage of substrate angiotensinogen. The angiotensi... | Chem Biol 7: 493-504 (2000) Article DOI: 10.1016/S1074-5521(00)00134-4 BindingDB Entry DOI: 10.7270/Q2V40SGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17943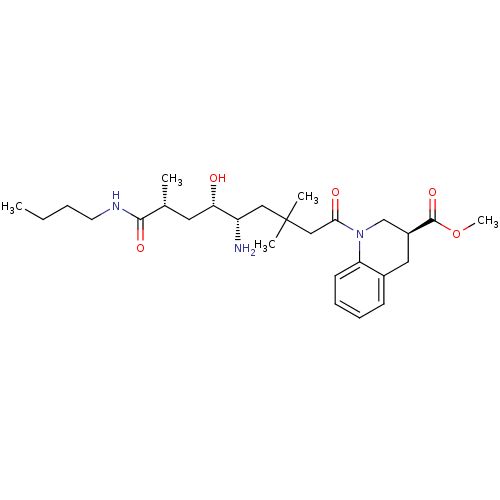 (Renin nonpeptide inhibitor, 3 | methyl (3S)-1-[(5S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description In vitro potencies of compounds against purified human recombinant renin were determined by its cleavage of substrate angiotensinogen. The angiotensi... | Chem Biol 7: 493-504 (2000) Article DOI: 10.1016/S1074-5521(00)00134-4 BindingDB Entry DOI: 10.7270/Q2V40SGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18289 ((2R,4S,5S,7S)-5-amino-4-hydroxy-N-(4-hydroxybutyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17948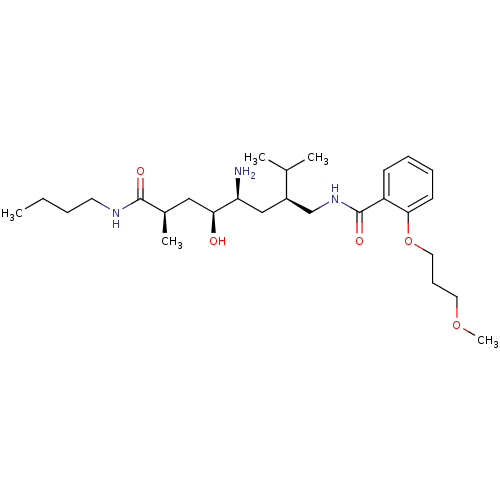 ((2R,4S,5S,7S)-5-amino-N-butyl-4-hydroxy-7-({[2-(3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description In vitro potencies of compounds against purified human recombinant renin were determined by its cleavage of substrate angiotensinogen. The angiotensi... | Chem Biol 7: 493-504 (2000) Article DOI: 10.1016/S1074-5521(00)00134-4 BindingDB Entry DOI: 10.7270/Q2V40SGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17941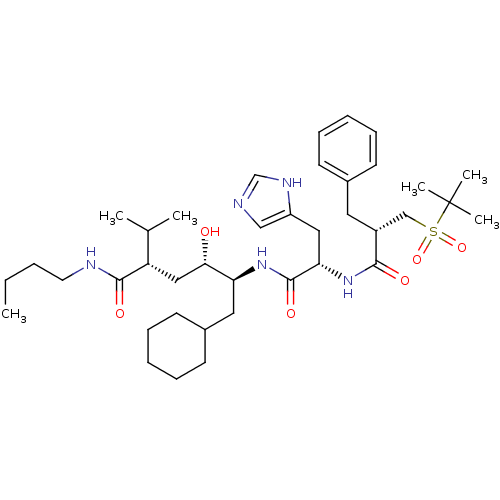 ((2S,4S,5S)-5-[(2S)-2-[(2S)-2-benzyl-3-[(2-methylpr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description In vitro potencies of compounds against purified human recombinant renin were determined by its cleavage of substrate angiotensinogen. The angiotensi... | Chem Biol 7: 493-504 (2000) Article DOI: 10.1016/S1074-5521(00)00134-4 BindingDB Entry DOI: 10.7270/Q2V40SGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022582 (CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of marmoset plasma renin | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17949 ((2R,4S,5S,7S)-5-amino-N-butyl-4-hydroxy-7-{[4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18306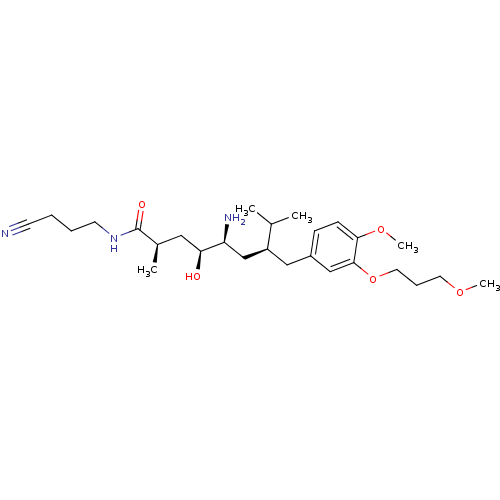 ((2R,4S,5S,7S)-5-amino-N-(3-cyanopropyl)-4-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18304 (8-phenyl-octanecarboxamide peptidomimetic, 61 | me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18308 ((2R,4S,5S,7S)-5-amino-N-(3-carbamoylpropyl)-4-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18298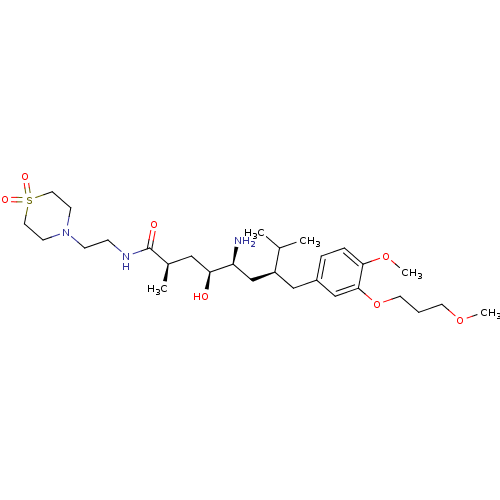 ((2R,4S,5S,7S)-5-amino-N-[2-(1,1-dioxo-1,4-thiomorp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18297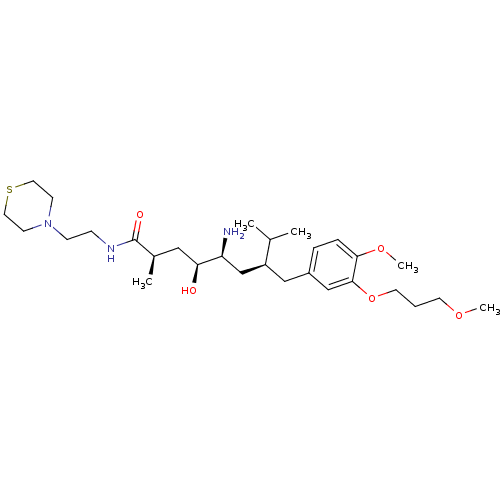 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18295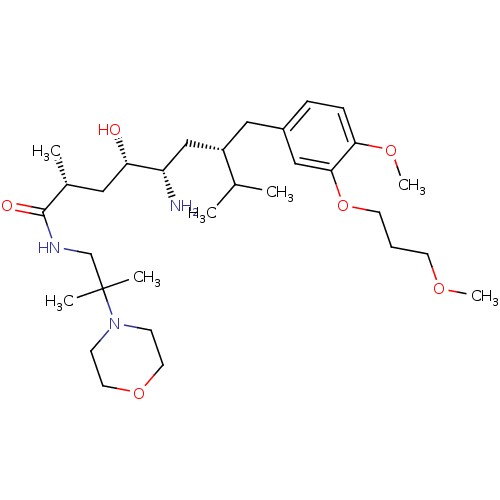 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18294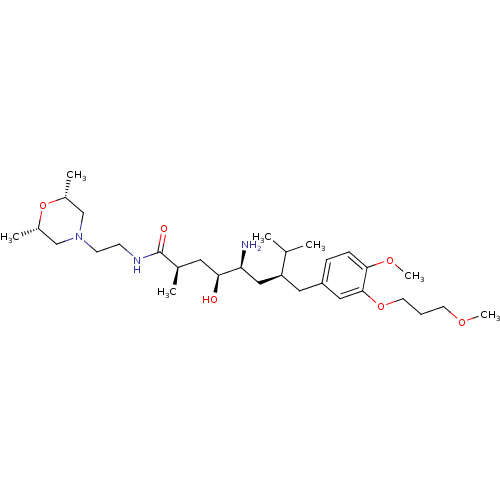 ((2R,4S,5S,7S)-5-amino-N-{2-[(2R,6S)-2,6-dimethylmo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18293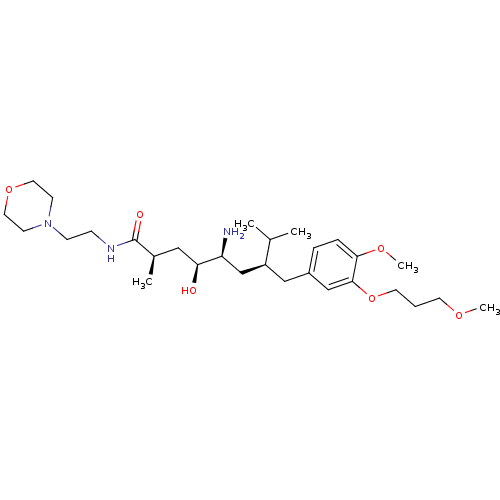 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18287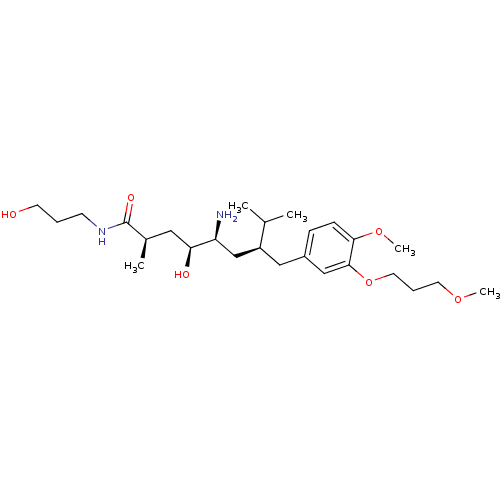 ((2R,4S,5S,7S)-5-amino-4-hydroxy-N-(3-hydroxypropyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18284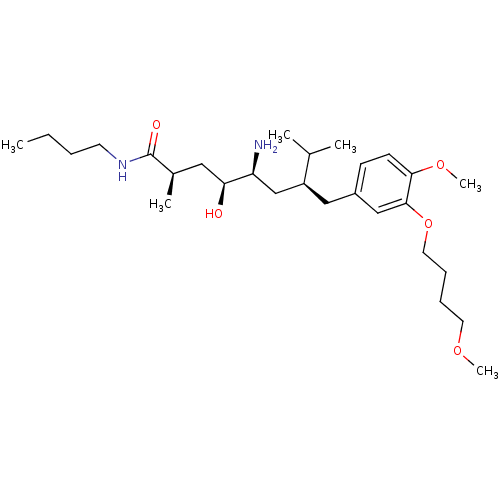 ((2R,4S,5S,7S)-5-amino-N-butyl-4-hydroxy-7-{[4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18320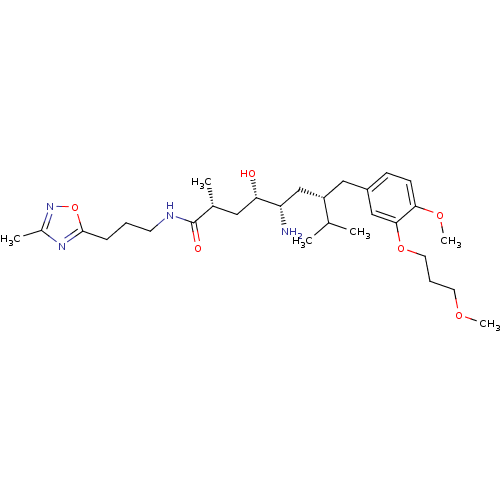 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022582 (CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of porcine plasma renin | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022582 (CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of marmoset plasma renin | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022582 (CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of Human plasma renin | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022586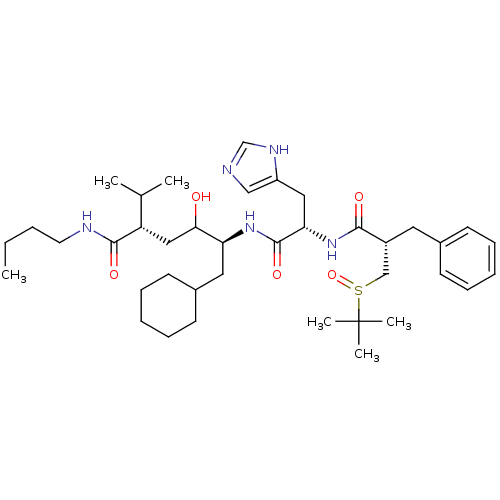 (5-[2-[2-Benzyl-3-(2-methyl-propane-2-sulfinyl)-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18316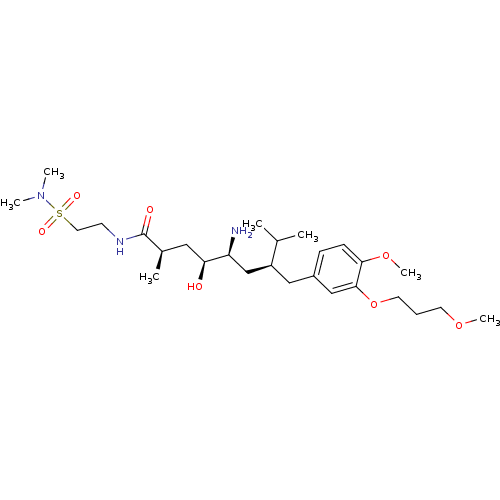 ((2R,4S,5S,7S)-5-amino-N-[2-(dimethylsulfamoyl)ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18310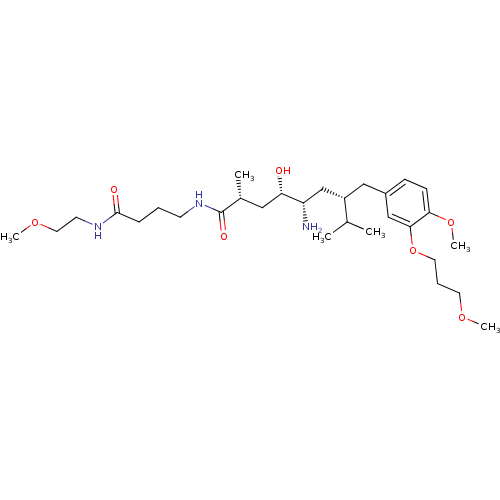 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18311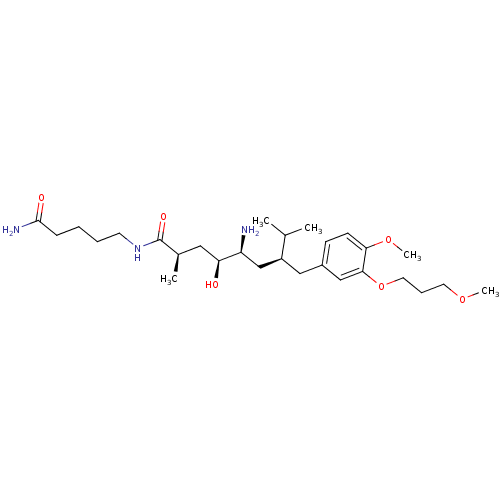 ((2R,4S,5S,7S)-5-amino-N-(4-carbamoylbutyl)-4-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18312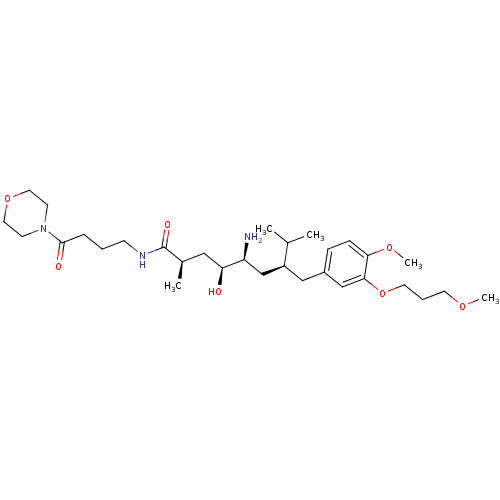 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022582 (CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18319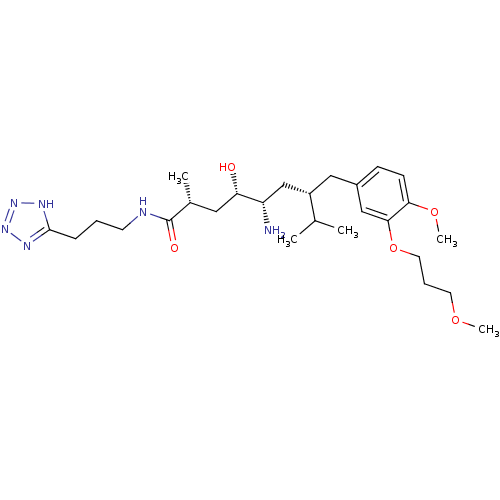 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17946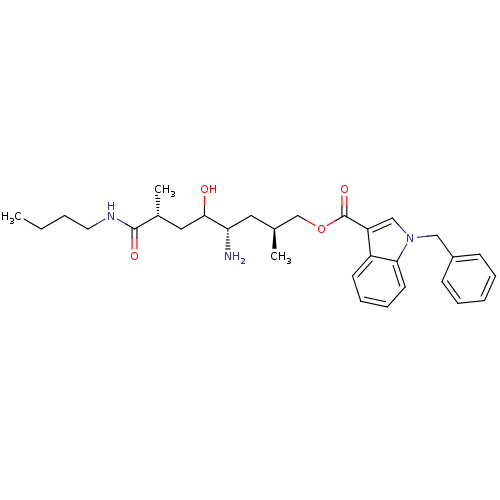 ((2S,4S,7R)-4-amino-7-(butylcarbamoyl)-5-hydroxy-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description In vitro potencies of compounds against purified human recombinant renin were determined by its cleavage of substrate angiotensinogen. The angiotensi... | Chem Biol 7: 493-504 (2000) Article DOI: 10.1016/S1074-5521(00)00134-4 BindingDB Entry DOI: 10.7270/Q2V40SGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18309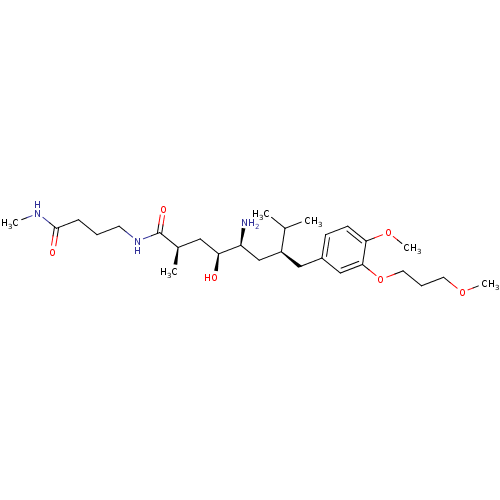 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18282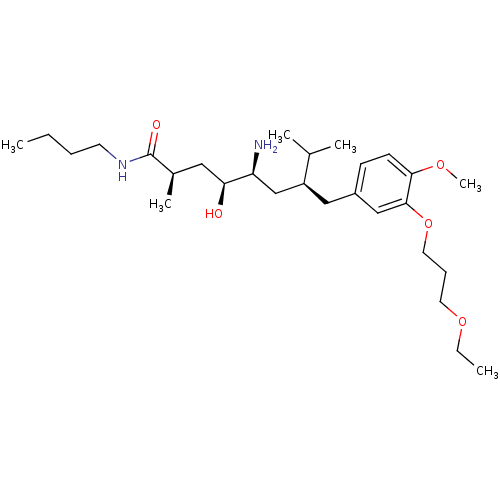 ((2R,4S,5S,7S)-5-amino-N-butyl-7-{[3-(3-ethoxypropo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18302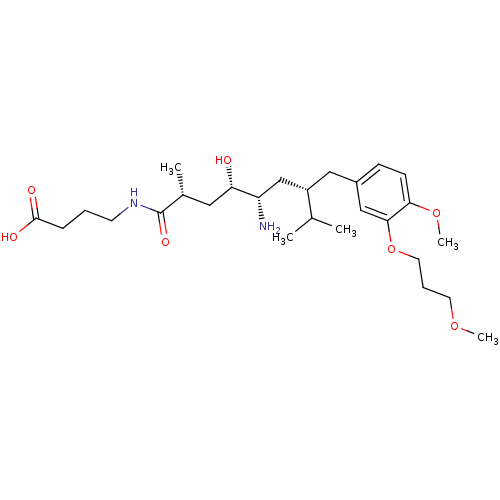 (4-[(2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18318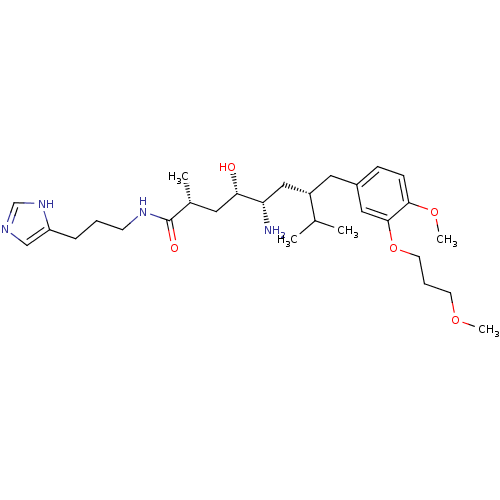 ((2R,4S,5S,7S)-5-amino-4-hydroxy-N-[3-(1H-imidazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18270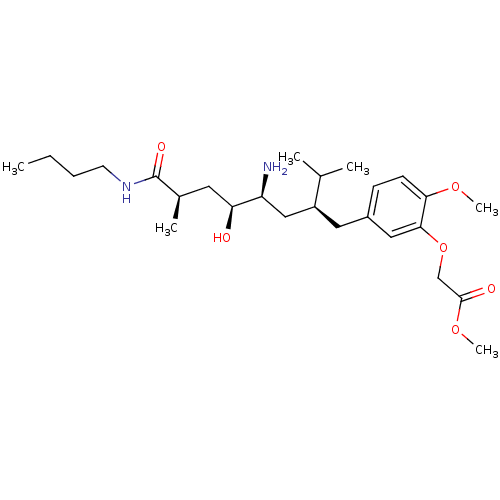 (8-phenyl-octanecarboxamide peptidomimetic, 28 | me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18280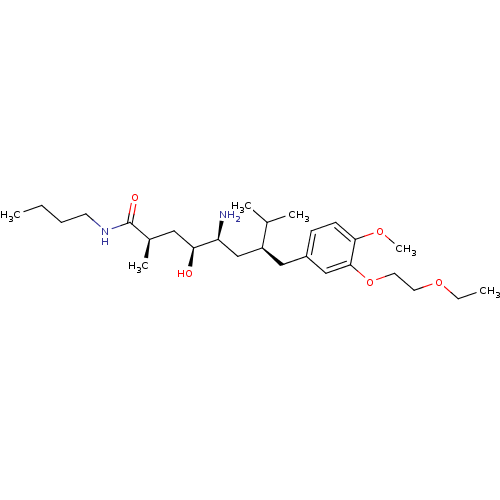 ((2R,4S,5S,7S)-5-amino-N-butyl-7-{[3-(2-ethoxyethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18286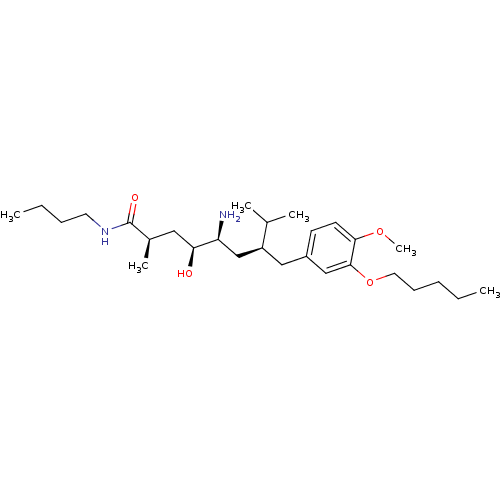 ((2R,4S,5S,7S)-5-amino-N-butyl-4-hydroxy-7-{[4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18303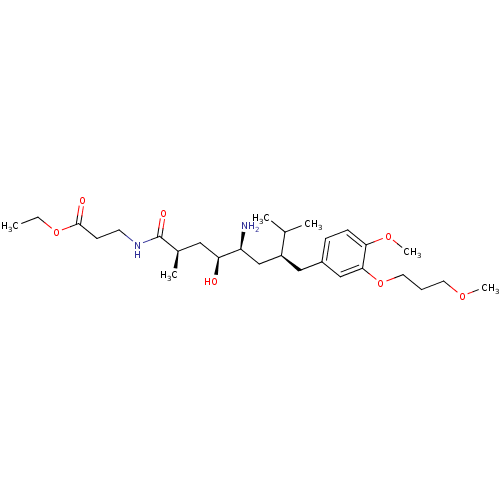 (8-phenyl-octanecarboxamide peptidomimetic, 60 | et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18314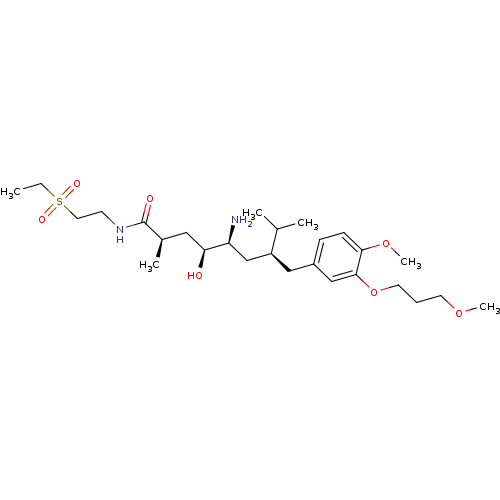 ((2R,4S,5S,7S)-5-amino-N-[2-(ethanesulfonyl)ethyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022588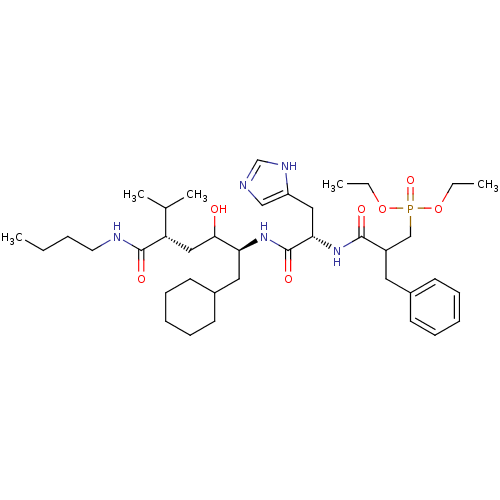 (CHEMBL291787 | {2-[1-(4-Butylcarbamoyl-1-cyclohexy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022587 (5-[2-(2-tert-Butylsulfanylmethyl-3-phenyl-propiony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18300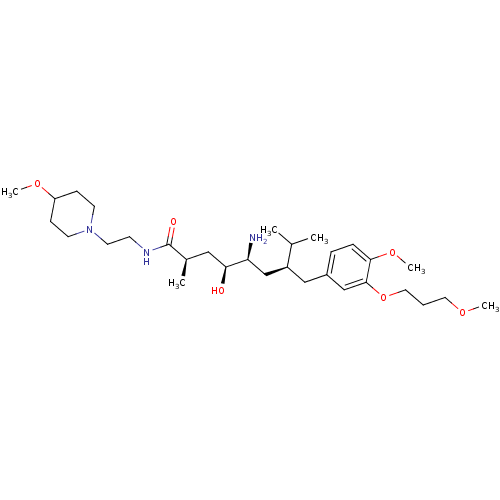 ((2R,4S,5S,7S)-5-amino-4-hydroxy-7-{[4-methoxy-3-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18277 ((2R,4S,5S,7S)-5-amino-N-butyl-4-hydroxy-7-{[4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Novartis Pharmaceuticals | Assay Description Enzyme inhibition by test compounds was determined using human recombinant renin, incubating with a synthetic tetradecapeptide substrate. The Ang I g... | J Med Chem 50: 4818-31 (2007) Article DOI: 10.1021/jm070314y BindingDB Entry DOI: 10.7270/Q2MG7MSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022584 (2-Benzyl-5,5-dimethyl-4-oxo-hexanoic acid [1-(4-bu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited Curated by ChEMBL | Assay Description In vitro inhibition of purified Human kidney renin (250 pg/mL) incubated with angiotensinogen at pH 7.2 | J Med Chem 31: 1839-46 (1988) BindingDB Entry DOI: 10.7270/Q2Z03744 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 108 total ) | Next | Last >> |