 Found 572 hits with Last Name = 'rawlins' and Initial = 'p'
Found 572 hits with Last Name = 'rawlins' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM203869
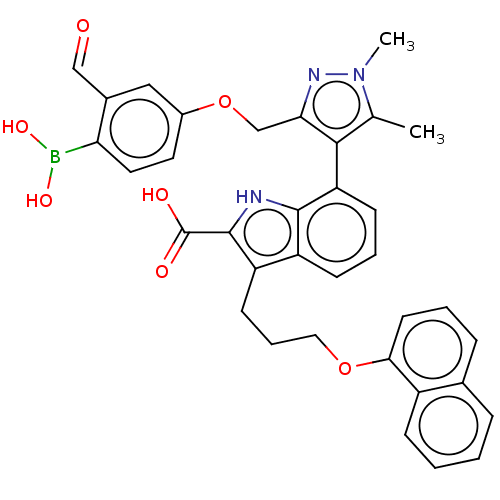
(7-(3-((4-Borono-3-formylphenoxy)methyl)-1,5-dimeth...)Show SMILES Cc1c(c(COc2ccc(B(O)O)c(C=O)c2)nn1C)-c1cccc2c(CCCOc3cccc4ccccc34)c([nH]c12)C(O)=O |(.05,-1.8,;-1.28,-2.57,;-2.53,-1.66,;-3.78,-2.57,;-5.11,-1.8,;-6.2,-2.88,;-7.53,-2.11,;-7.53,-.57,;-8.87,.2,;-10.2,-.57,;-11.29,.51,;-10.89,2,;-12.78,.91,;-10.2,-2.11,;-11.53,-2.88,;-10.76,-4.22,;-8.87,-2.88,;-3.3,-4.03,;-1.76,-4.03,;-.67,-5.12,;-2.48,-.6,;-3.81,.17,;-3.81,1.71,;-2.48,2.48,;-1.14,1.71,;.32,2.18,;-.08,3.67,;1.01,4.76,;.62,6.24,;1.7,7.33,;1.31,8.82,;-.03,9.59,;-.03,11.13,;1.31,11.9,;2.64,11.13,;3.97,11.9,;5.31,11.13,;5.31,9.59,;3.97,8.82,;2.64,9.59,;1.23,.94,;.32,-.31,;-1.14,.17,;2.77,.94,;3.86,-.15,;3.86,2.02,)| Show InChI InChI=1S/C35H32BN3O7/c1-21-32(30(38-39(21)2)20-46-24-15-16-29(36(43)44)23(18-24)19-40)28-12-6-11-26-27(34(35(41)42)37-33(26)28)13-7-17-45-31-14-5-9-22-8-3-4-10-25(22)31/h3-6,8-12,14-16,18-19,37,43-44H,7,13,17,20H2,1-2H3,(H,41,42) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | -51.5 | 3.40 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit
| Assay Description
TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... |
Nat Chem Biol 12: 931-936 (2016)
Article DOI: 10.1038/nchembio.2174
BindingDB Entry DOI: 10.7270/Q2FN1513 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50150074
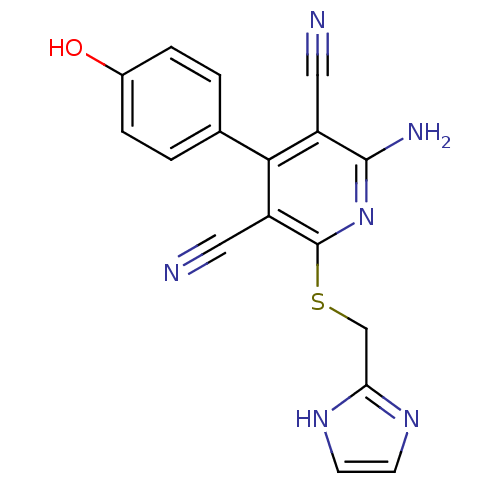
(2-Amino-4-(4-hydroxy-phenyl)-6-(1H-imidazol-2-ylme...)Show SMILES Nc1nc(SCc2ncc[nH]2)c(C#N)c(-c2ccc(O)cc2)c1C#N Show InChI InChI=1S/C17H12N6OS/c18-7-12-15(10-1-3-11(24)4-2-10)13(8-19)17(23-16(12)20)25-9-14-21-5-6-22-14/h1-6,24H,9H2,(H2,20,23)(H,21,22) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00099
BindingDB Entry DOI: 10.7270/Q23200WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM203875
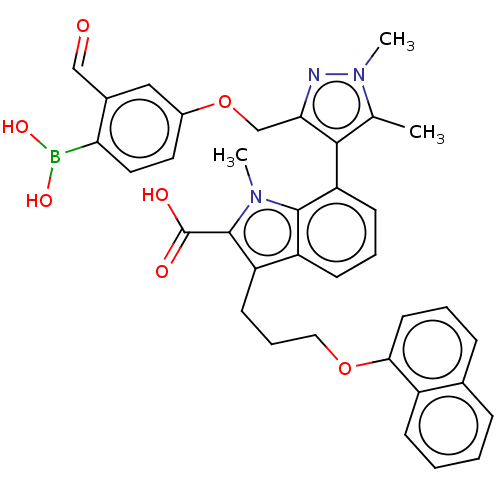
(7-(3-((4-Borono-3-formylphenoxy)methyl)-1,5-dimeth...)Show SMILES Cc1c(c(COc2ccc(B(O)O)c(C=O)c2)nn1C)-c1cccc2c(CCCOc3cccc4ccccc34)c(C(O)=O)n(C)c12 |(.34,-2.6,;-1.28,-2.57,;-2.53,-1.66,;-3.78,-2.57,;-5.11,-1.8,;-6.2,-2.88,;-7.53,-2.11,;-7.53,-.57,;-8.87,.2,;-10.2,-.57,;-11.29,.51,;-10.89,2,;-12.78,.91,;-10.2,-2.11,;-11.53,-2.88,;-10.76,-4.22,;-8.87,-2.88,;-3.3,-4.03,;-1.76,-4.03,;-.67,-5.12,;-2.48,-.6,;-3.81,.17,;-3.81,1.71,;-2.48,2.48,;-1.14,1.71,;.32,2.18,;-.08,3.67,;1.01,4.76,;.62,6.24,;1.7,7.33,;1.31,8.82,;-.03,9.59,;-.03,11.13,;1.31,11.9,;2.64,11.13,;3.97,11.9,;5.31,11.13,;5.31,9.59,;3.97,8.82,;2.64,9.59,;1.23,.94,;2.77,.94,;3.86,-.15,;3.86,2.02,;.32,-.31,;1.22,-1.52,;-1.14,.17,)| Show InChI InChI=1S/C36H34BN3O7/c1-22-33(31(38-40(22)3)21-47-25-16-17-30(37(44)45)24(19-25)20-41)29-13-7-12-27-28(35(36(42)43)39(2)34(27)29)14-8-18-46-32-15-6-10-23-9-4-5-11-26(23)32/h4-7,9-13,15-17,19-20,44-45H,8,14,18,21H2,1-3H3,(H,42,43) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | -50.7 | 4.20 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit
| Assay Description
TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... |
Nat Chem Biol 12: 931-936 (2016)
Article DOI: 10.1038/nchembio.2174
BindingDB Entry DOI: 10.7270/Q2FN1513 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM203870
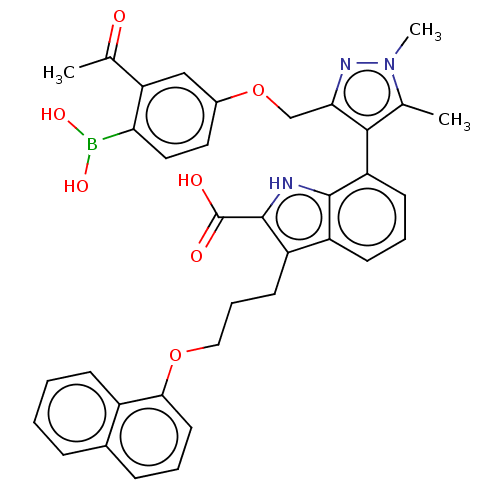
(7-(3-((3-Acetyl-4-boronophenoxy)methyl)-1,5-dimeth...)Show SMILES CC(=O)c1cc(OCc2nn(C)c(C)c2-c2cccc3c(CCCOc4cccc5ccccc45)c([nH]c23)C(O)=O)ccc1B(O)O |(-13.02,-2.49,;-11.53,-2.88,;-12.16,-4.44,;-10.2,-2.11,;-8.87,-2.88,;-7.53,-2.11,;-6.2,-2.88,;-5.11,-1.8,;-3.78,-2.57,;-3.3,-4.03,;-1.76,-4.03,;-.67,-5.12,;-1.28,-2.57,;.05,-1.8,;-2.53,-1.66,;-2.48,-.6,;-3.81,.17,;-3.81,1.71,;-2.48,2.48,;-1.14,1.71,;.32,2.18,;-.08,3.67,;1.01,4.76,;.62,6.24,;1.7,7.33,;1.31,8.82,;-.03,9.59,;-.03,11.13,;1.31,11.9,;2.64,11.13,;3.97,11.9,;5.31,11.13,;5.31,9.59,;3.97,8.82,;2.64,9.59,;1.23,.94,;.32,-.31,;-1.14,.17,;2.77,.94,;3.86,-.15,;3.86,2.02,;-7.53,-.57,;-8.87,.2,;-10.2,-.57,;-11.29,.51,;-10.89,2,;-12.78,.91,)| Show InChI InChI=1S/C36H34BN3O7/c1-21-33(31(39-40(21)3)20-47-24-16-17-30(37(44)45)29(19-24)22(2)41)28-13-7-12-26-27(35(36(42)43)38-34(26)28)14-8-18-46-32-15-6-10-23-9-4-5-11-25(23)32/h4-7,9-13,15-17,19,38,44-45H,8,14,18,20H2,1-3H3,(H,42,43) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | -50.7 | 4.70 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit
| Assay Description
TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... |
Nat Chem Biol 12: 931-936 (2016)
Article DOI: 10.1038/nchembio.2174
BindingDB Entry DOI: 10.7270/Q2FN1513 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM203876
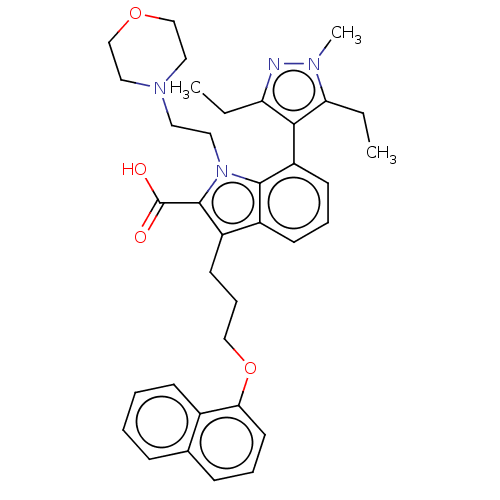
(Mcl-1 inhibitor 12)Show SMILES CCc1nn(C)c(CC)c1-c1cccc2c(CCCOc3cccc4ccccc34)c(C(O)=O)n(CCN3CCOCC3)c12 |(-6.2,-2.88,;-5.11,-1.8,;-3.78,-2.57,;-3.3,-4.03,;-1.76,-4.03,;-.67,-5.12,;-1.28,-2.57,;-.02,-2.6,;.3,-4.24,;-2.53,-1.66,;-2.48,-.6,;-3.81,.17,;-3.81,1.71,;-2.48,2.48,;-1.14,1.71,;.32,2.18,;-.08,3.67,;1.01,4.76,;.62,6.24,;1.7,7.33,;1.31,8.82,;-.03,9.59,;-.03,11.13,;1.31,11.9,;2.64,11.13,;3.97,11.9,;5.31,11.13,;5.31,9.59,;3.97,8.82,;2.64,9.59,;1.23,.94,;2.77,.94,;3.86,-.15,;3.86,2.02,;.32,-.31,;1.22,-1.52,;2.76,-1.52,;3.16,-3.01,;1.82,-3.78,;1.82,-5.32,;3.16,-6.09,;4.49,-5.32,;4.49,-3.78,;-1.14,.17,)| Show InChI InChI=1S/C36H42N4O4/c1-4-30-33(31(5-2)38(3)37-30)29-15-9-14-27-28(16-10-22-44-32-17-8-12-25-11-6-7-13-26(25)32)35(36(41)42)40(34(27)29)19-18-39-20-23-43-24-21-39/h6-9,11-15,17H,4-5,10,16,18-24H2,1-3H3,(H,41,42) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | -50.0 | 5.96 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit
| Assay Description
TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... |
Nat Chem Biol 12: 931-936 (2016)
Article DOI: 10.1038/nchembio.2174
BindingDB Entry DOI: 10.7270/Q2FN1513 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM203871
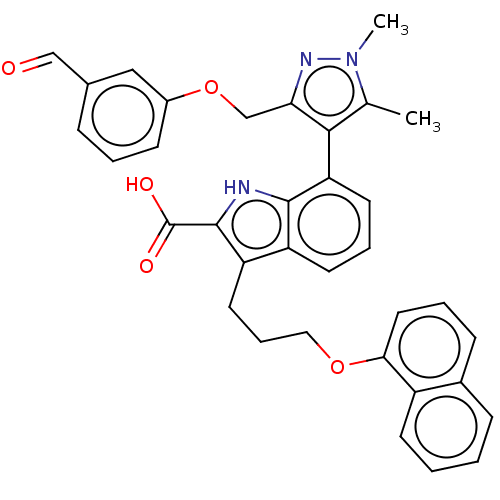
(7-(3-((3-formylphenoxy)methyl)-1,5-dimethyl-1H-pyr...)Show SMILES Cc1c(c(COc2cccc(C=O)c2)nn1C)-c1cccc2c(CCCOc3cccc4ccccc34)c([nH]c12)C(O)=O |(.05,-1.8,;-1.28,-2.57,;-2.53,-1.66,;-3.78,-2.57,;-5.11,-1.8,;-6.2,-2.88,;-7.53,-2.11,;-7.53,-.57,;-8.87,.2,;-10.2,-.57,;-10.2,-2.11,;-11.53,-2.88,;-10.76,-4.22,;-8.87,-2.88,;-3.3,-4.03,;-1.76,-4.03,;-.67,-5.12,;-2.48,-.6,;-3.81,.17,;-3.81,1.71,;-2.48,2.48,;-1.14,1.71,;.32,2.18,;-.08,3.67,;1.01,4.76,;.62,6.24,;1.7,7.33,;1.31,8.82,;-.03,9.59,;-.03,11.13,;1.31,11.9,;2.64,11.13,;3.97,11.9,;5.31,11.13,;5.31,9.59,;3.97,8.82,;2.64,9.59,;1.23,.94,;.32,-.31,;-1.14,.17,;2.77,.94,;3.86,-.15,;3.86,2.02,)| Show InChI InChI=1S/C35H31N3O5/c1-22-32(30(37-38(22)2)21-43-25-12-5-9-23(19-25)20-39)29-15-7-14-27-28(34(35(40)41)36-33(27)29)16-8-18-42-31-17-6-11-24-10-3-4-13-26(24)31/h3-7,9-15,17,19-20,36H,8,16,18,21H2,1-2H3,(H,40,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | -44.3 | 59.5 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit
| Assay Description
TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... |
Nat Chem Biol 12: 931-936 (2016)
Article DOI: 10.1038/nchembio.2174
BindingDB Entry DOI: 10.7270/Q2FN1513 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM203873
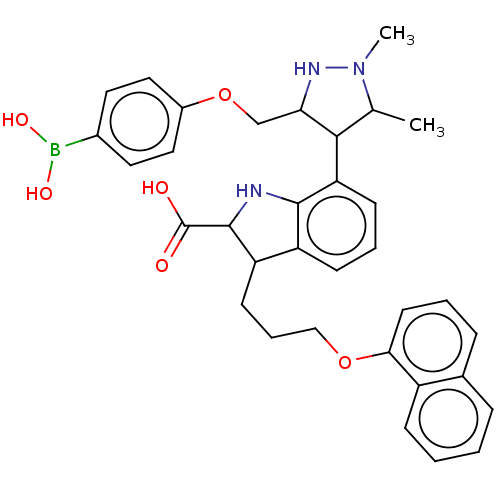
(7-(3-((4-Boronophenoxy)methyl)-1,5-dimethyl-1H-pyr...)Show SMILES CC1C(C(COc2ccc(cc2)B(O)O)NN1C)c1cccc2C(CCCOc3cccc4ccccc34)C(Nc12)C(O)=O Show InChI InChI=1S/C34H38BN3O6/c1-21-31(29(37-38(21)2)20-44-24-17-15-23(16-18-24)35(41)42)28-12-6-11-26-27(33(34(39)40)36-32(26)28)13-7-19-43-30-14-5-9-22-8-3-4-10-25(22)30/h3-6,8-12,14-18,21,27,29,31,33,36-37,41-42H,7,13,19-20H2,1-2H3,(H,39,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 44 | -41.8 | 162 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit
| Assay Description
TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... |
Nat Chem Biol 12: 931-936 (2016)
Article DOI: 10.1038/nchembio.2174
BindingDB Entry DOI: 10.7270/Q2FN1513 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM203872
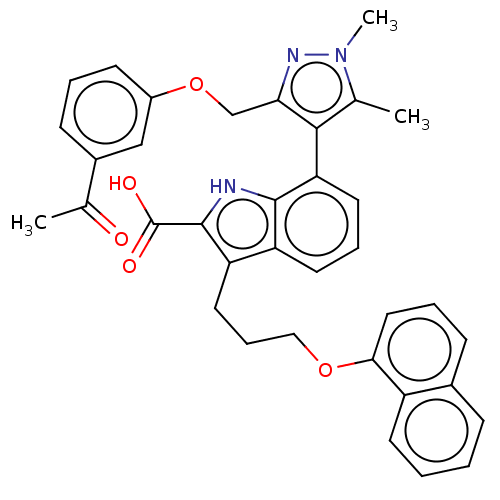
(7-(3-((3-Acetylphenoxy)methyl)-1,5-dimethyl-1H-pyr...)Show SMILES CC(=O)c1cccc(OCc2nn(C)c(C)c2-c2cccc3c(CCCOc4cccc5ccccc45)c([nH]c23)C(O)=O)c1 |(-12.87,-2.11,;-11.53,-2.88,;-12.01,-4.36,;-10.2,-2.11,;-10.2,-.57,;-8.87,.2,;-7.53,-.57,;-7.53,-2.11,;-6.2,-2.88,;-5.11,-1.8,;-3.78,-2.57,;-3.3,-4.03,;-1.76,-4.03,;-.67,-5.12,;-1.28,-2.57,;.05,-1.8,;-2.53,-1.66,;-2.48,-.6,;-3.81,.17,;-3.81,1.71,;-2.48,2.48,;-1.14,1.71,;.32,2.18,;-.08,3.67,;1.01,4.76,;.62,6.24,;1.7,7.33,;1.31,8.82,;-.03,9.59,;-.03,11.13,;1.31,11.9,;2.64,11.13,;3.97,11.9,;5.31,11.13,;5.31,9.59,;3.97,8.82,;2.64,9.59,;1.23,.94,;.32,-.31,;-1.14,.17,;2.77,.94,;3.86,-.15,;3.86,2.02,;-8.87,-2.88,)| Show InChI InChI=1S/C36H33N3O5/c1-22-33(31(38-39(22)3)21-44-26-13-6-12-25(20-26)23(2)40)30-16-8-15-28-29(35(36(41)42)37-34(28)30)17-9-19-43-32-18-7-11-24-10-4-5-14-27(24)32/h4-8,10-16,18,20,37H,9,17,19,21H2,1-3H3,(H,41,42) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 64 | -40.9 | 237 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit
| Assay Description
TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... |
Nat Chem Biol 12: 931-936 (2016)
Article DOI: 10.1038/nchembio.2174
BindingDB Entry DOI: 10.7270/Q2FN1513 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM82022
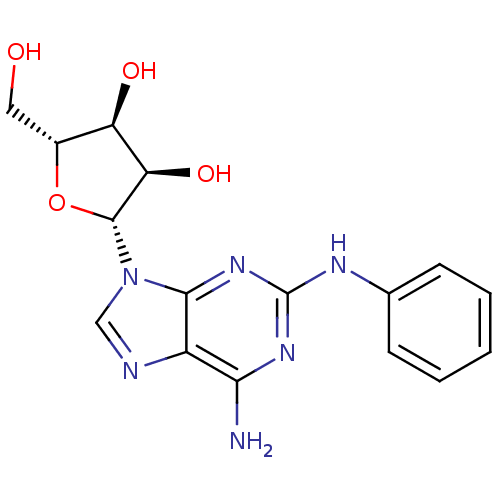
(2-(Phenylamino)ado (CV-1808) | 2-[6-Amino-2-(3-cyc...)Show SMILES Nc1nc(Nc2ccccc2)nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C16H18N6O4/c17-13-10-14(21-16(20-13)19-8-4-2-1-3-5-8)22(7-18-10)15-12(25)11(24)9(6-23)26-15/h1-5,7,9,11-12,15,23-25H,6H2,(H3,17,19,20,21)/t9-,11-,12-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00099
BindingDB Entry DOI: 10.7270/Q23200WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM203874
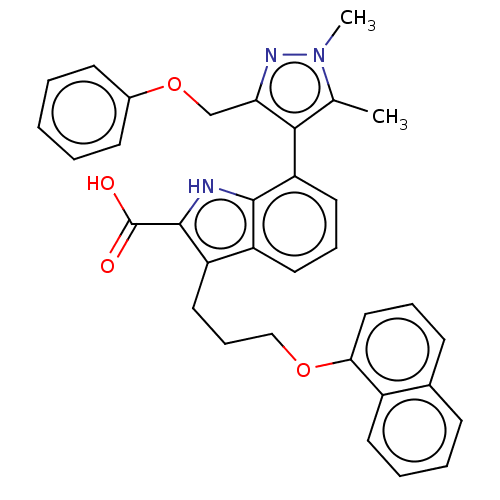
(Mcl-1 inhibitor 10)Show SMILES Cc1c(c(COc2ccccc2)nn1C)-c1cccc2c(CCCOc3cccc4ccccc34)c([nH]c12)C(O)=O |(.05,-1.8,;-1.28,-2.57,;-2.53,-1.66,;-3.78,-2.57,;-5.11,-1.8,;-6.2,-2.88,;-7.53,-2.11,;-7.53,-.57,;-8.87,.2,;-10.2,-.57,;-10.2,-2.11,;-8.87,-2.88,;-3.3,-4.03,;-1.76,-4.03,;-.67,-5.12,;-2.48,-.6,;-3.81,.17,;-3.81,1.71,;-2.48,2.48,;-1.14,1.71,;.32,2.18,;-.08,3.67,;1.01,4.76,;.62,6.24,;1.7,7.33,;1.31,8.82,;-.03,9.59,;-.03,11.13,;1.31,11.9,;2.64,11.13,;3.97,11.9,;5.31,11.13,;5.31,9.59,;3.97,8.82,;2.64,9.59,;1.23,.94,;.32,-.31,;-1.14,.17,;2.77,.94,;3.86,-.15,;3.86,2.02,)| Show InChI InChI=1S/C34H31N3O4/c1-22-31(29(36-37(22)2)21-41-24-13-4-3-5-14-24)28-17-9-16-26-27(33(34(38)39)35-32(26)28)18-10-20-40-30-19-8-12-23-11-6-7-15-25(23)30/h3-9,11-17,19,35H,10,18,20-21H2,1-2H3,(H,38,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 104 | -39.7 | 383 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit
| Assay Description
TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... |
Nat Chem Biol 12: 931-936 (2016)
Article DOI: 10.1038/nchembio.2174
BindingDB Entry DOI: 10.7270/Q2FN1513 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM14487

((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H13N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h2-4,6-7,10,16-18H,1H2,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00099
BindingDB Entry DOI: 10.7270/Q23200WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM14487

((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H13N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h2-4,6-7,10,16-18H,1H2,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00099
BindingDB Entry DOI: 10.7270/Q23200WW |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21190
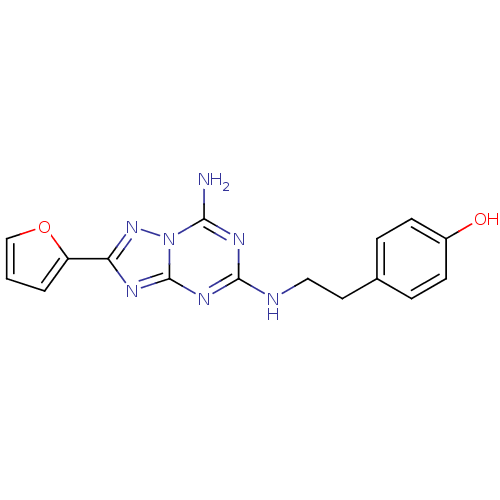
(4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...)Show InChI InChI=1S/C16H15N7O2/c17-14-20-15(18-8-7-10-3-5-11(24)6-4-10)21-16-19-13(22-23(14)16)12-2-1-9-25-12/h1-6,9,24H,7-8H2,(H3,17,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 395 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00099
BindingDB Entry DOI: 10.7270/Q23200WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505991

(CHEMBL4470113)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cc2C(=O)N(Cc3cccc(Cl)c3)CCn2c1 Show InChI InChI=1S/C23H22ClN7O/c1-15-12-25-23(27-20-6-7-26-29(20)2)28-21(15)17-11-19-22(32)31(9-8-30(19)14-17)13-16-4-3-5-18(24)10-16/h3-7,10-12,14H,8-9,13H2,1-2H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265959
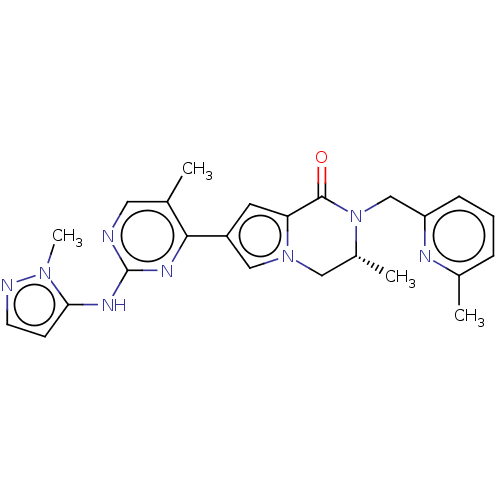
(CHEMBL4071576)Show SMILES C[C@@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H26N8O/c1-15-11-25-24(28-21-8-9-26-30(21)4)29-22(15)18-10-20-23(33)32(17(3)12-31(20)13-18)14-19-7-5-6-16(2)27-19/h5-11,13,17H,12,14H2,1-4H3,(H,25,28,29)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505993

(CHEMBL4587118)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cc2C(=O)N(Cc3ccc(F)c(F)c3)CCn2c1 Show InChI InChI=1S/C23H21F2N7O/c1-14-11-26-23(28-20-5-6-27-30(20)2)29-21(14)16-10-19-22(33)32(8-7-31(19)13-16)12-15-3-4-17(24)18(25)9-15/h3-6,9-11,13H,7-8,12H2,1-2H3,(H,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505988
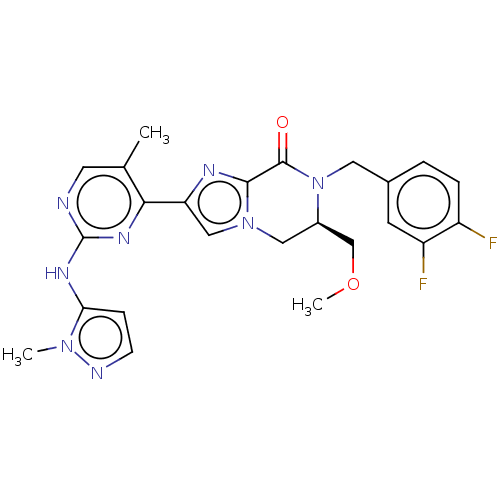
(CHEMBL4482864)Show SMILES COC[C@H]1Cn2cc(nc2C(=O)N1Cc1ccc(F)c(F)c1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H24F2N8O2/c1-14-9-27-24(30-20-6-7-28-32(20)2)31-21(14)19-12-33-11-16(13-36-3)34(23(35)22(33)29-19)10-15-4-5-17(25)18(26)8-15/h4-9,12,16H,10-11,13H2,1-3H3,(H,27,30,31)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505994

(CHEMBL4440109)Show SMILES C[C@H]1Cn2cc(nc2C(=O)N1Cc1ccc(F)c(F)c1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C23H22F2N8O/c1-13-9-26-23(29-19-6-7-27-31(19)3)30-20(13)18-12-32-10-14(2)33(22(34)21(32)28-18)11-15-4-5-16(24)17(25)8-15/h4-9,12,14H,10-11H2,1-3H3,(H,26,29,30)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505989

(CHEMBL4551714)Show SMILES COC[C@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C25H28N8O2/c1-16-11-26-25(29-22-8-9-27-31(22)3)30-23(16)18-10-21-24(34)33(13-19-7-5-6-17(2)28-19)20(15-35-4)14-32(21)12-18/h5-12,20H,13-15H2,1-4H3,(H,26,29,30)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505992

(CHEMBL4452853)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cn2CCN(Cc3cccc(Cl)c3)C(=O)c2n1 Show InChI InChI=1S/C22H21ClN8O/c1-14-11-24-22(27-18-6-7-25-29(18)2)28-19(14)17-13-30-8-9-31(21(32)20(30)26-17)12-15-4-3-5-16(23)10-15/h3-7,10-11,13H,8-9,12H2,1-2H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505991

(CHEMBL4470113)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cc2C(=O)N(Cc3cccc(Cl)c3)CCn2c1 Show InChI InChI=1S/C23H22ClN7O/c1-15-12-25-23(27-20-6-7-26-29(20)2)28-21(15)17-11-19-22(32)31(9-8-30(19)14-17)13-16-4-3-5-18(24)10-16/h3-7,10-12,14H,8-9,13H2,1-2H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505994

(CHEMBL4440109)Show SMILES C[C@H]1Cn2cc(nc2C(=O)N1Cc1ccc(F)c(F)c1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C23H22F2N8O/c1-13-9-26-23(29-19-6-7-27-31(19)3)30-20(13)18-12-32-10-14(2)33(22(34)21(32)28-18)11-15-4-5-16(24)17(25)8-15/h4-9,12,14H,10-11H2,1-3H3,(H,26,29,30)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505992

(CHEMBL4452853)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cn2CCN(Cc3cccc(Cl)c3)C(=O)c2n1 Show InChI InChI=1S/C22H21ClN8O/c1-14-11-24-22(27-18-6-7-25-29(18)2)28-19(14)17-13-30-8-9-31(21(32)20(30)26-17)12-15-4-3-5-16(23)10-15/h3-7,10-11,13H,8-9,12H2,1-2H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505993

(CHEMBL4587118)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cc2C(=O)N(Cc3ccc(F)c(F)c3)CCn2c1 Show InChI InChI=1S/C23H21F2N7O/c1-14-11-26-23(28-20-5-6-27-30(20)2)29-21(14)16-10-19-22(33)32(8-7-31(19)13-16)12-15-3-4-17(24)18(25)9-15/h3-6,9-11,13H,7-8,12H2,1-2H3,(H,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265959

(CHEMBL4071576)Show SMILES C[C@@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H26N8O/c1-15-11-25-24(28-21-8-9-26-30(21)4)29-22(15)18-10-20-23(33)32(17(3)12-31(20)13-18)14-19-7-5-6-16(2)27-19/h5-11,13,17H,12,14H2,1-4H3,(H,25,28,29)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505989

(CHEMBL4551714)Show SMILES COC[C@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C25H28N8O2/c1-16-11-26-25(29-22-8-9-27-31(22)3)30-23(16)18-10-21-24(34)33(13-19-7-5-6-17(2)28-19)20(15-35-4)14-32(21)12-18/h5-12,20H,13-15H2,1-4H3,(H,26,29,30)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505988

(CHEMBL4482864)Show SMILES COC[C@H]1Cn2cc(nc2C(=O)N1Cc1ccc(F)c(F)c1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H24F2N8O2/c1-14-9-27-24(30-20-6-7-28-32(20)2)31-21(14)19-12-33-11-16(13-36-3)34(23(35)22(33)29-19)10-15-4-5-17(25)18(26)8-15/h4-9,12,16H,10-11,13H2,1-3H3,(H,27,30,31)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50563635
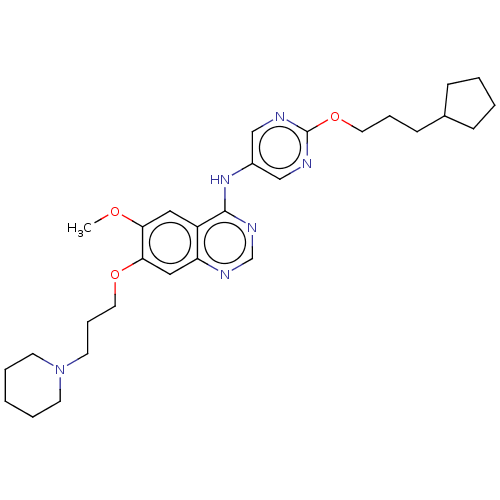
(CHEMBL4777640)Show SMILES COc1cc2c(Nc3cnc(OCCCC4CCCC4)nc3)ncnc2cc1OCCCN1CCCCC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of 6His/thrombin cleavage site-fused Avi-tagged dephosphorylated MER (unknown origin) (R528 to M999 residues) using Axltide (CKKSRGDYMTMQJ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01904
BindingDB Entry DOI: 10.7270/Q2XP78NC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505990
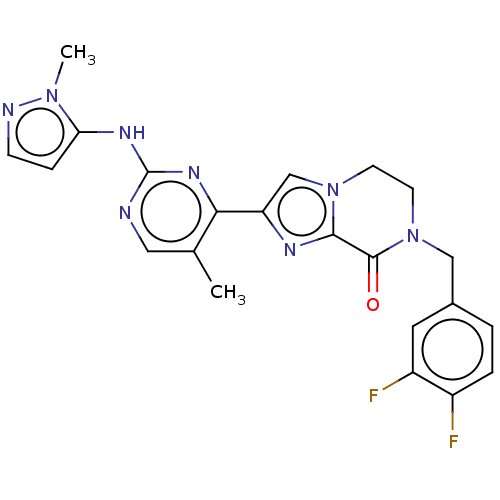
(CHEMBL4458562)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cn2CCN(Cc3ccc(F)c(F)c3)C(=O)c2n1 Show InChI InChI=1S/C22H20F2N8O/c1-13-10-25-22(28-18-5-6-26-30(18)2)29-19(13)17-12-31-7-8-32(21(33)20(31)27-17)11-14-3-4-15(23)16(24)9-14/h3-6,9-10,12H,7-8,11H2,1-2H3,(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21190

(4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...)Show InChI InChI=1S/C16H15N7O2/c17-14-20-15(18-8-7-10-3-5-11(24)6-4-10)21-16-19-13(22-23(14)16)12-2-1-9-25-12/h1-6,9,24H,7-8H2,(H3,17,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00099
BindingDB Entry DOI: 10.7270/Q23200WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50573136

(CHEMBL4875486)Show SMILES CN(Cc1ccc2nccn2c1)c1nnc(o1)-c1ccc(cc1C)-c1c(C)nn(C)c1C#N | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MER (unknown origin) by LC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00920
BindingDB Entry DOI: 10.7270/Q2S18686 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50172077

(CHEMBL3810063)Show SMILES Cc1c(C(=O)Nc2ccc(Oc3ccnc4[nH]cc(-c5ccccc5)c34)c(F)c2)c(=O)n(-c2ccc(F)cc2)n1C Show InChI InChI=1S/C31H23F2N5O3/c1-18-27(31(40)38(37(18)2)22-11-8-20(32)9-12-22)30(39)36-21-10-13-25(24(33)16-21)41-26-14-15-34-29-28(26)23(17-35-29)19-6-4-3-5-7-19/h3-17H,1-2H3,(H,34,35)(H,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of 6His/thrombin cleavage site-fused Avi-tagged dephosphorylated MER (unknown origin) (R528 to M999 residues) using Axltide (CKKSRGDYMTMQJ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01904
BindingDB Entry DOI: 10.7270/Q2XP78NC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1/3
(Homo sapiens (Human)) | BDBM50505988

(CHEMBL4482864)Show SMILES COC[C@H]1Cn2cc(nc2C(=O)N1Cc1ccc(F)c(F)c1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H24F2N8O2/c1-14-9-27-24(30-20-6-7-28-32(20)2)31-21(14)19-12-33-11-16(13-36-3)34(23(35)22(33)29-19)10-15-4-5-17(25)18(26)8-15/h4-9,12,16H,10-11,13H2,1-3H3,(H,27,30,31)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK1/2 in human A375 cells assessed as inhibition of ERK phosphorylation after 2 hrs |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50573135

(CHEMBL4873217)Show SMILES CN(Cc1ccc2nccn2c1)c1nnc(o1)-c1ccc(cc1C)-c1cnn(C)c1C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MER (unknown origin) by LC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00920
BindingDB Entry DOI: 10.7270/Q2S18686 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1/3
(Homo sapiens (Human)) | BDBM50094464

(CHEMBL3590107 | US10525036, Example SCH772984 | US...)Show SMILES O=C(CN1CC[C@H](C1)C(=O)Nc1ccc2[nH]nc(-c3ccncc3)c2c1)N1CCN(CC1)c1ccc(cc1)-c1ncccn1 |r| Show InChI InChI=1S/C22H27NO3/c1-2-15-23-16-13-20(14-17-23)26-21(24)22(25,18-9-5-3-6-10-18)19-11-7-4-8-12-19/h3-12,20,25H,2,13-17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK1/2 in human A375 cells assessed as inhibition of ERK phosphorylation after 2 hrs |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50573128
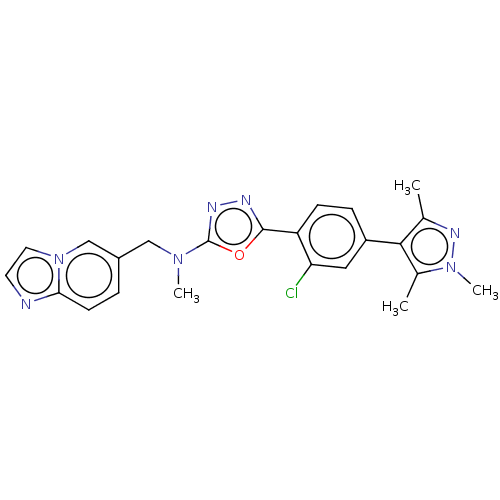
(CHEMBL4865752)Show SMILES CN(Cc1ccc2nccn2c1)c1nnc(o1)-c1ccc(cc1Cl)-c1c(C)nn(C)c1C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MER (unknown origin) by LC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00920
BindingDB Entry DOI: 10.7270/Q2S18686 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505990

(CHEMBL4458562)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cn2CCN(Cc3ccc(F)c(F)c3)C(=O)c2n1 Show InChI InChI=1S/C22H20F2N8O/c1-13-10-25-22(28-18-5-6-26-30(18)2)29-19(13)17-12-31-7-8-32(21(33)20(31)27-17)11-14-3-4-15(23)16(24)9-14/h3-6,9-10,12H,7-8,11H2,1-2H3,(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50575300
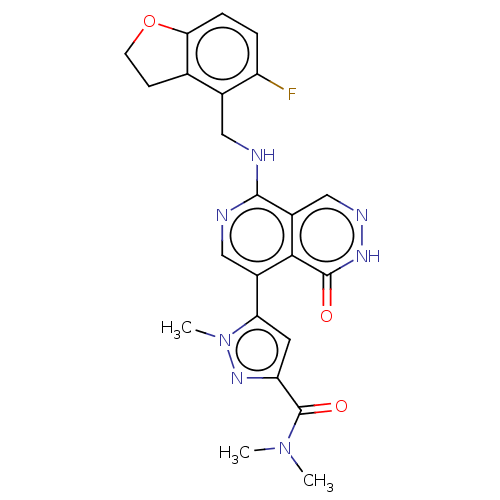
(CHEMBL4851414)Show SMILES CN(C)C(=O)c1cc(-c2cnc(NCc3c4CCOc4ccc3F)c3cn[nH]c(=O)c23)n(C)n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EED in human G-401 cells assessed as reduction in H3K27 trimethylation incubated for 48 hrs by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01161
BindingDB Entry DOI: 10.7270/Q2MP5737 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50573130
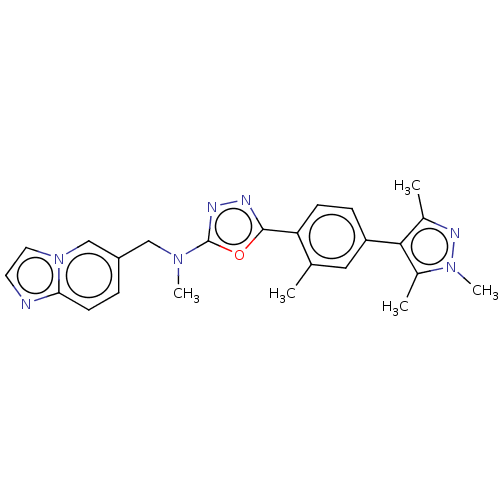
(CHEMBL4855381)Show SMILES CN(Cc1ccc2nccn2c1)c1nnc(o1)-c1ccc(cc1C)-c1c(C)nn(C)c1C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MER (unknown origin) by LC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00920
BindingDB Entry DOI: 10.7270/Q2S18686 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50563640

(CHEMBL4746916)Show SMILES FC(F)(F)c1cc(NC(=O)Nc2ccc(Oc3cc(NC(=O)C4CC4)ncn3)cc2)ccc1CN1CCn2ccnc2C1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of His6/TEV fused-GST-tagged Flt3 (unknown origin) (H564 to S993 residues) using Axltide (CKKSRGDYMTMQJ-acid) peptide as substrate preincu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01904
BindingDB Entry DOI: 10.7270/Q2XP78NC |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50575300

(CHEMBL4851414)Show SMILES CN(C)C(=O)c1cc(-c2cnc(NCc3c4CCOc4ccc3F)c3cn[nH]c(=O)c23)n(C)n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EED in human G-401 cells assessed as reduction in H3K27 trimethylation incubated for 48 hrs by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01161
BindingDB Entry DOI: 10.7270/Q2MP5737 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50172077

(CHEMBL3810063)Show SMILES Cc1c(C(=O)Nc2ccc(Oc3ccnc4[nH]cc(-c5ccccc5)c34)c(F)c2)c(=O)n(-c2ccc(F)cc2)n1C Show InChI InChI=1S/C31H23F2N5O3/c1-18-27(31(40)38(37(18)2)22-11-8-20(32)9-12-22)30(39)36-21-10-13-25(24(33)16-21)41-26-14-15-34-29-28(26)23(17-35-29)19-6-4-3-5-7-19/h3-17H,1-2H3,(H,34,35)(H,36,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-tagged AXL (unknown origin) (464 to 485 residues) using Axltide (CKKSRGDYMTMQJG-acid) peptide as substrate preincubated for 30 mins... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01904
BindingDB Entry DOI: 10.7270/Q2XP78NC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1/3
(Homo sapiens (Human)) | BDBM50505994

(CHEMBL4440109)Show SMILES C[C@H]1Cn2cc(nc2C(=O)N1Cc1ccc(F)c(F)c1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C23H22F2N8O/c1-13-9-26-23(29-19-6-7-27-31(19)3)30-20(13)18-12-32-10-14(2)33(22(34)21(32)28-18)11-15-4-5-16(24)17(25)8-15/h4-9,12,14H,10-11H2,1-3H3,(H,26,29,30)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK1/2 in human A375 cells assessed as inhibition of ERK phosphorylation after 2 hrs |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50575298
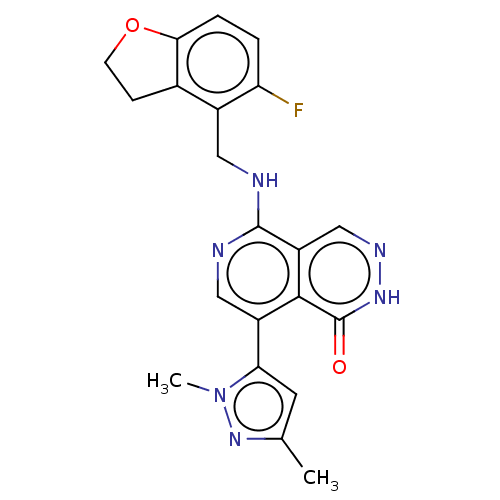
(CHEMBL4859723)Show SMILES Cc1cc(-c2cnc(NCc3c4CCOc4ccc3F)c3cn[nH]c(=O)c23)n(C)n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EED in human G-401 cells assessed as reduction in H3K27 trimethylation incubated for 48 hrs by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01161
BindingDB Entry DOI: 10.7270/Q2MP5737 |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50575298

(CHEMBL4859723)Show SMILES Cc1cc(-c2cnc(NCc3c4CCOc4ccc3F)c3cn[nH]c(=O)c23)n(C)n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EED in human G-401 cells assessed as reduction in H3K27 trimethylation incubated for 48 hrs by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01161
BindingDB Entry DOI: 10.7270/Q2MP5737 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50563644
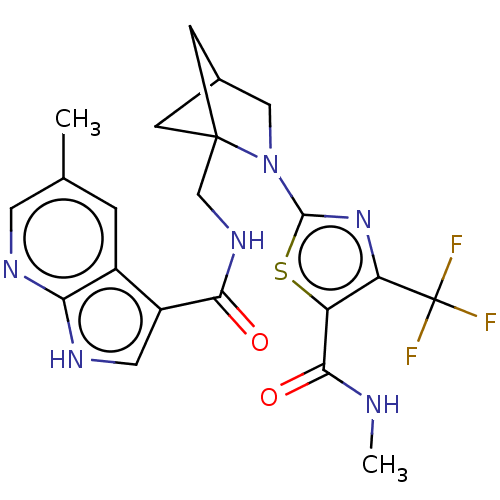
(CHEMBL4782810)Show SMILES CNC(=O)c1sc(nc1C(F)(F)F)N1CC2CC1(CNC(=O)c1c[nH]c3ncc(C)cc13)C2 |(45.05,-5.8,;43.51,-5.8,;42.74,-7.13,;43.5,-8.46,;41.2,-7.12,;40.3,-5.87,;38.82,-6.34,;38.82,-7.88,;40.29,-8.36,;40.75,-9.82,;42.29,-9.82,;41.52,-11.16,;39.72,-10.97,;37.58,-5.43,;37.59,-3.89,;36.13,-3.41,;35.22,-4.66,;36.12,-5.9,;35.64,-7.37,;34.13,-7.68,;33.1,-6.53,;33.58,-5.07,;31.6,-6.85,;30.97,-8.24,;29.44,-8.08,;29.13,-6.58,;27.79,-5.81,;27.79,-4.27,;29.12,-3.5,;29.11,-1.96,;30.45,-4.26,;30.46,-5.81,;36.52,-4.89,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-tagged AXL (unknown origin) (464 to 485 residues) using Axltide (CKKSRGDYMTMQJG-acid) peptide as substrate preincubated for 30 mins... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01904
BindingDB Entry DOI: 10.7270/Q2XP78NC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1/3
(Homo sapiens (Human)) | BDBM50505992

(CHEMBL4452853)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cn2CCN(Cc3cccc(Cl)c3)C(=O)c2n1 Show InChI InChI=1S/C22H21ClN8O/c1-14-11-24-22(27-18-6-7-25-29(18)2)28-19(14)17-13-30-8-9-31(21(32)20(30)26-17)12-15-4-3-5-16(23)10-15/h3-7,10-11,13H,8-9,12H2,1-2H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK1/2 in human A375 cells assessed as inhibition of ERK phosphorylation after 2 hrs |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM291687
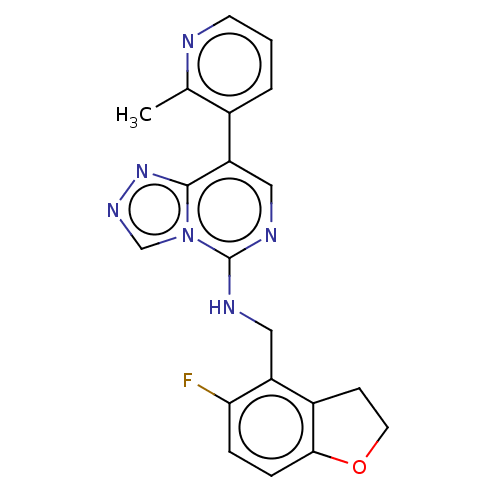
(N-((5-Fluoro-2,3-dihydrobenzofuran-4-yl)methyl)-8-...)Show InChI InChI=1S/C20H17FN6O/c1-12-13(3-2-7-22-12)16-10-24-20(27-11-25-26-19(16)27)23-9-15-14-6-8-28-18(14)5-4-17(15)21/h2-5,7,10-11H,6,8-9H2,1H3,(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EED in human G-401 cells assessed as reduction in H3K27 trimethylation incubated for 48 hrs by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01161
BindingDB Entry DOI: 10.7270/Q2MP5737 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50563643

(CHEMBL4754609)Show SMILES CN(Cc1ccc2nccn2c1)c1nnc(o1)-c1ccc(cc1)-c1ccccc1Cl | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MER (unknown origin) by LC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00920
BindingDB Entry DOI: 10.7270/Q2S18686 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50563642

(CHEMBL4746146)Show SMILES CNC(=O)C[C@@H](NC(=O)[C@@H](CCc1ccccc1)NC(=O)c1c(C)noc1-c1snnc1C)c1ccc(cc1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of 6His/thrombin cleavage site-fused Avi-tagged dephosphorylated MER (unknown origin) (R528 to M999 residues) using Axltide (CKKSRGDYMTMQJ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01904
BindingDB Entry DOI: 10.7270/Q2XP78NC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data