 Found 225 hits with Last Name = 'ray' and Initial = 'ss'
Found 225 hits with Last Name = 'ray' and Initial = 'ss' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642632

(US20230416245, Compound 70)Show SMILES CCNc1cc(nc2n(cnc12)[C@@H]1[C@H]2C[C@@]2([C@@H](O)[C@H]1O)C(=O)NC)C#N |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642621
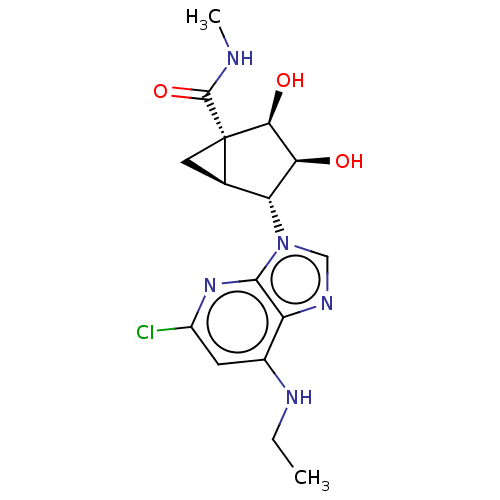
(US20230416245, Compound 59)Show SMILES CCNc1cc(Cl)nc2n(cnc12)[C@@H]1[C@H]2C[C@]2([C@@H](O)[C@H]1O)C(=O)NC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642624
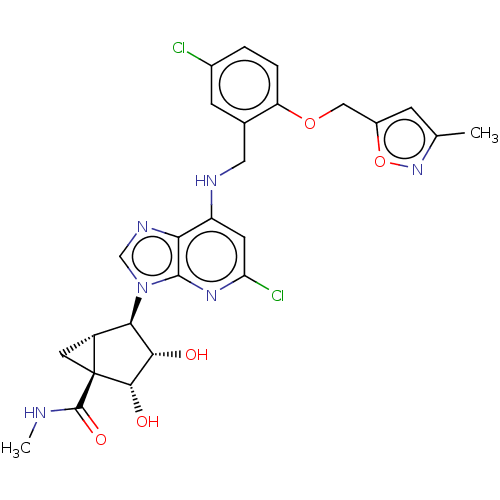
(US20230416245, Compound 62)Show SMILES CNC(=O)[C@@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCc3cc(Cl)ccc3OCc3cc(C)no3)cc(Cl)nc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642626
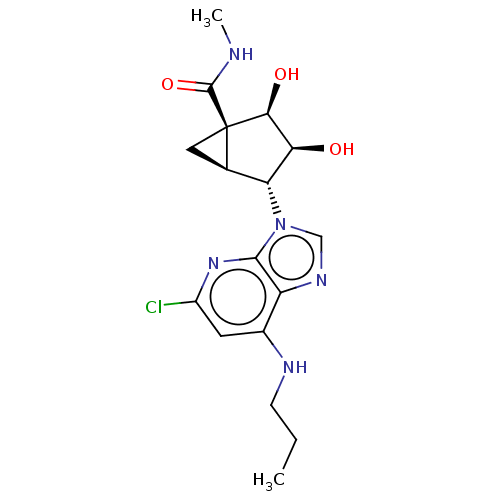
(US20230416245, Compound 64)Show SMILES CCCNc1cc(Cl)nc2n(cnc12)[C@@H]1[C@H]2C[C@@]2([C@@H](O)[C@H]1O)C(=O)NC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642625
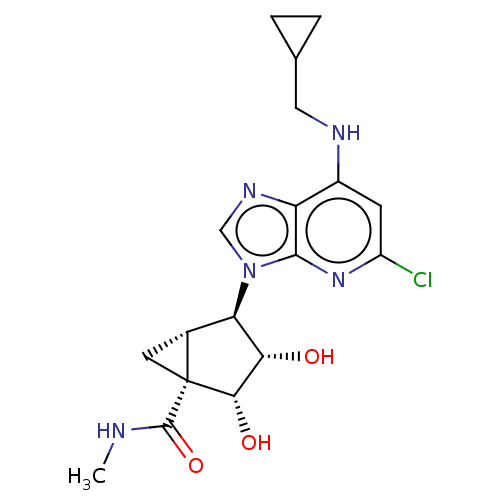
(US20230416245, Compound 63)Show SMILES CNC(=O)[C@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCC3CC3)cc(Cl)nc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642618
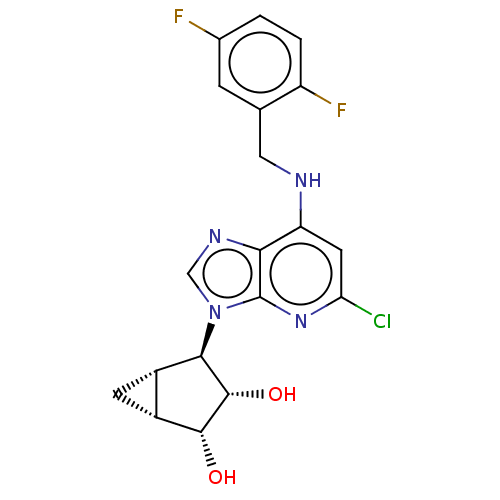
(US20230416245, Compound 45)Show SMILES O[C@@H]1[C@@H]2C[C@@H]2[C@H]([C@@H]1O)n1cnc2c(NCc3cc(F)ccc3F)cc(Cl)nc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642635

(US20230416245, Compound 83)Show SMILES CNC(=O)[C@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCC(C)C)cc(Cl)nc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642631
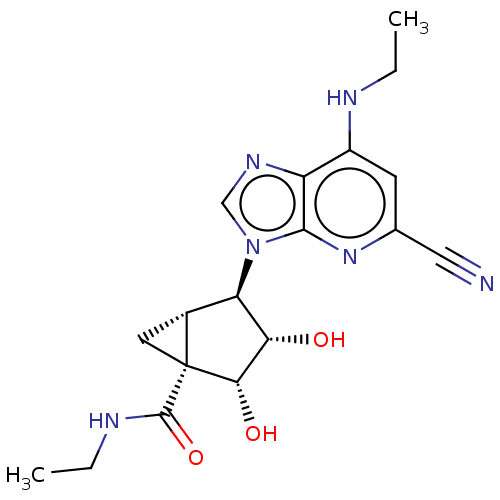
(US20230416245, Compound 69)Show SMILES CCNC(=O)[C@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCC)cc(nc12)C#N |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642620
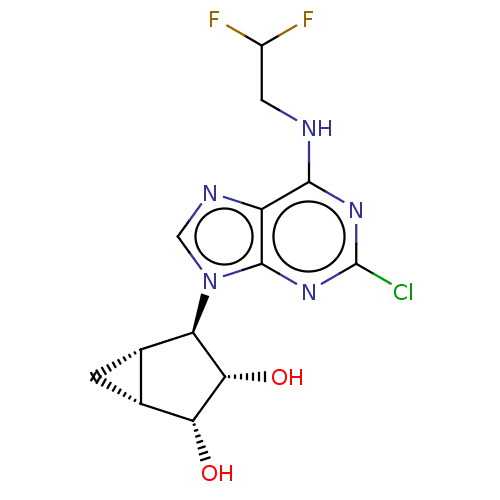
(US20230416245, Compound 35)Show SMILES O[C@@H]1[C@@H]2C[C@@H]2[C@H]([C@@H]1O)n1cnc2c(NCC(F)F)nc(Cl)nc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642615

(US20230416245, Compound 40)Show SMILES O[C@@H]1[C@@H]2C[C@@H]2[C@H]([C@@H]1O)n1cnc2c(NCC(F)F)cc(Cl)nc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642636
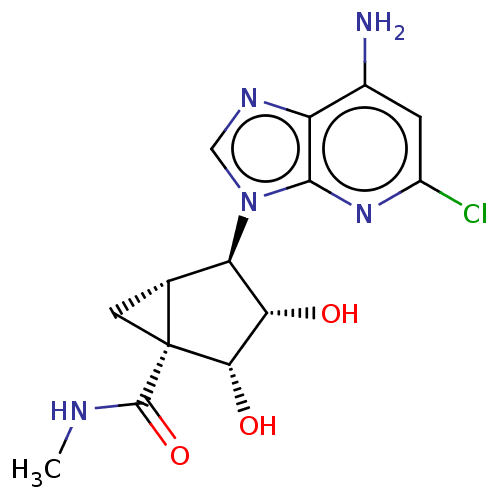
(US20230416245, Compound 90)Show SMILES CNC(=O)[C@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(N)cc(Cl)nc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642639
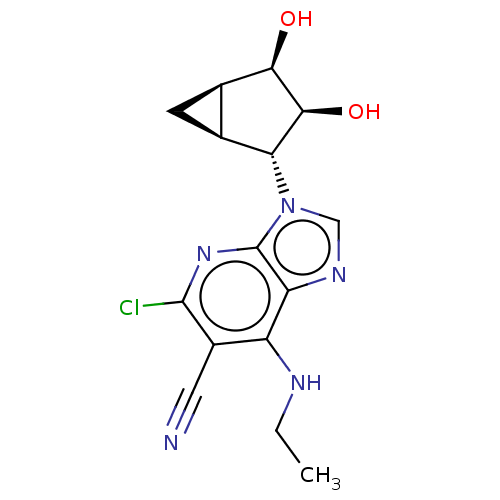
(US20230416245, Compound 93)Show SMILES CCNc1c(C#N)c(Cl)nc2n(cnc12)[C@@H]1[C@H]2C[C@H]2[C@@H](O)[C@H]1O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642627
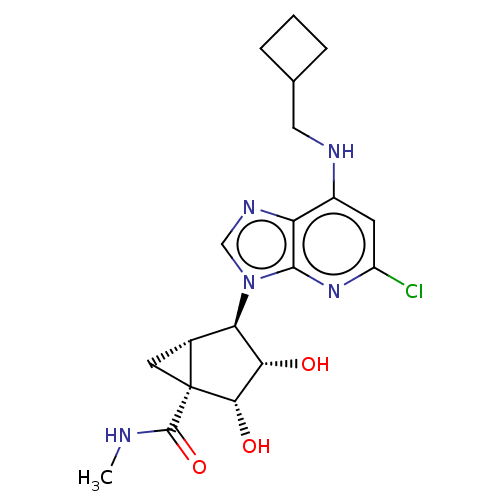
(US20230416245, Compound 65)Show SMILES CNC(=O)[C@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCC3CCC3)cc(Cl)nc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642630

(US20230416245, Compound 68)Show SMILES O[C@@H]1[C@@H]2C[C@@H]2[C@H]([C@@H]1O)n1cnc2c(NCc3cc(Cl)ccc3Cl)cc(Cl)nc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642634
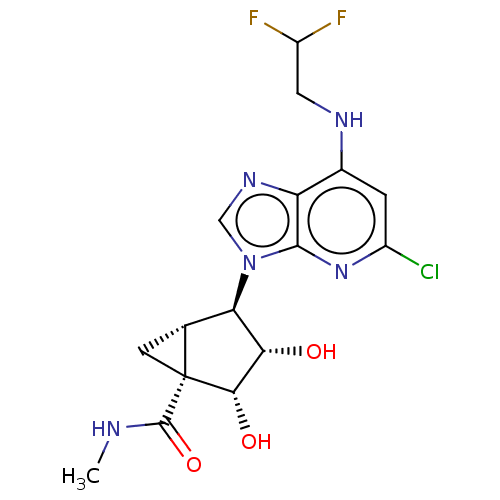
(US20230416245, Compound 82)Show SMILES CNC(=O)[C@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCC(F)F)cc(Cl)nc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642637
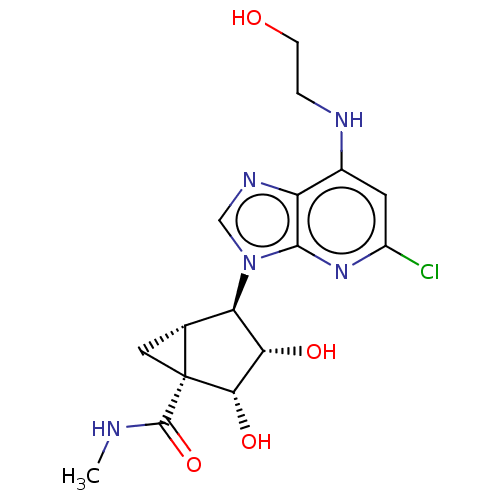
(US20230416245, Compound 91)Show SMILES CNC(=O)[C@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCCO)cc(Cl)nc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642623

(US20230416245, Compound 61)Show SMILES O[C@@H]1[C@@H]2C[C@@H]2[C@H]([C@@H]1O)n1cnc2c(NCC(F)F)c(F)c(Cl)nc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 7.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642619
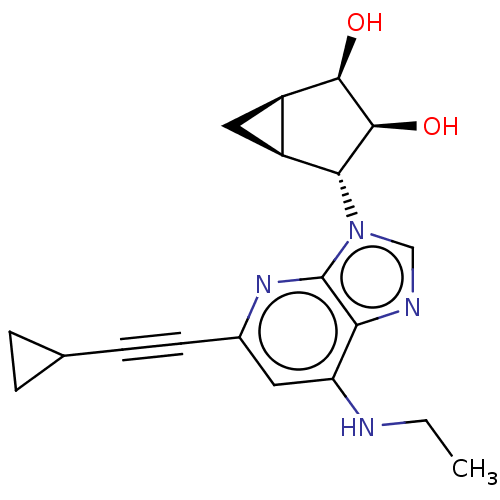
(US20230416245, Compound 46)Show SMILES CCNc1cc(nc2n(cnc12)[C@@H]1[C@H]2C[C@H]2[C@@H](O)[C@H]1O)C#CC1CC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 21.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642638
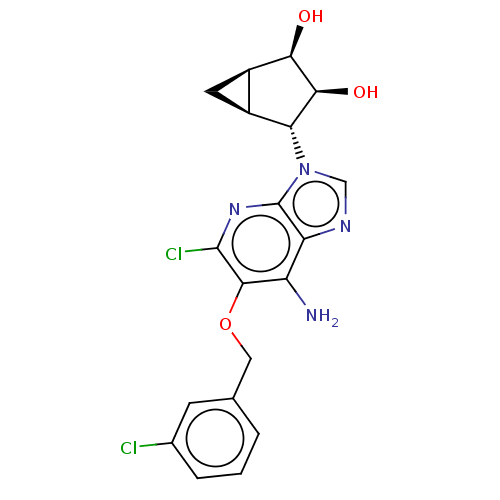
(US20230416245, Compound 92)Show SMILES Nc1c(OCc2cccc(Cl)c2)c(Cl)nc2n(cnc12)[C@@H]1[C@H]2C[C@H]2[C@@H](O)[C@H]1O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 26.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642617

(US20230416245, Compound 44)Show SMILES O[C@@H]1[C@@H]2C[C@@H]2[C@H]([C@@H]1O)n1cnc2c(NC3CCC3)cc(Cl)nc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 28.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642629
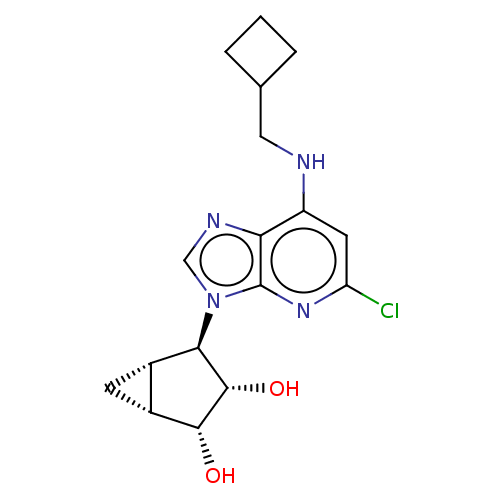
(US20230416245, Compound 67)Show SMILES O[C@@H]1[C@@H]2C[C@@H]2[C@H]([C@@H]1O)n1cnc2c(NCC3CCC3)cc(Cl)nc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 94.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642628

(US20230416245, Compound 66)Show SMILES CCCNc1cc(Cl)nc2n(cnc12)[C@@H]1[C@H]2C[C@H]2[C@@H](O)[C@H]1O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642633
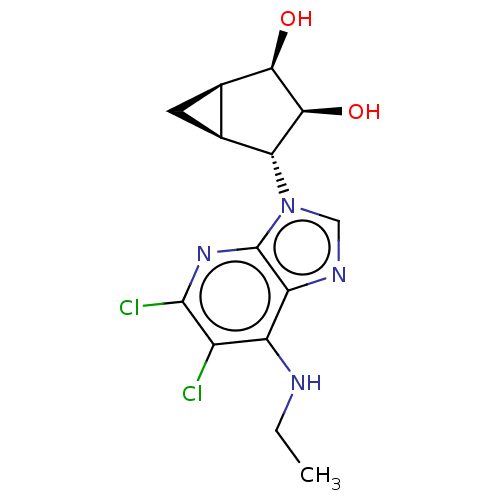
(US20230416245, Compound 71)Show SMILES CCNc1c(Cl)c(Cl)nc2n(cnc12)[C@@H]1[C@H]2C[C@H]2[C@@H](O)[C@H]1O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 456 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642622
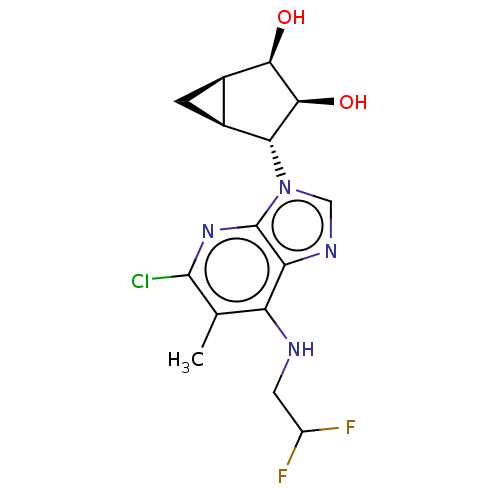
(US20230416245, Compound 60)Show SMILES Cc1c(Cl)nc2n(cnc2c1NCC(F)F)[C@@H]1[C@H]2C[C@H]2[C@@H](O)[C@H]1O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acid ceramidase
(Homo sapiens (Human)) | BDBM50556798
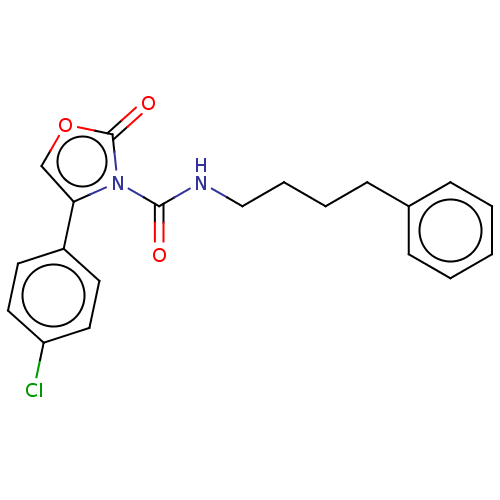
(CHEMBL4742096) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human acid ceramidase using N-lauroyl ceramide incubated for 1 hr by LC/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01561
BindingDB Entry DOI: 10.7270/Q2G73JC2 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase
(Cryptosporidium parvum) | BDBM50365429
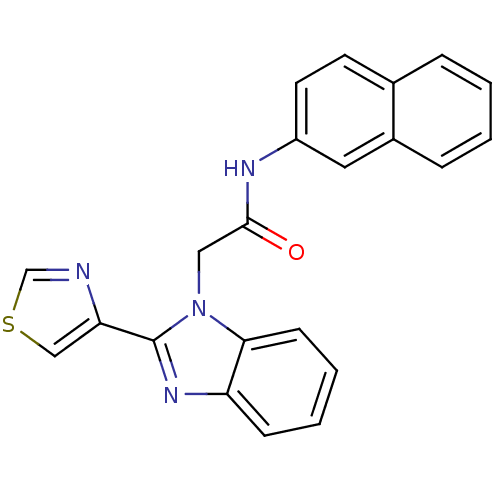
(CHEMBL1450808)Show SMILES O=C(Cn1c(nc2ccccc12)-c1cscn1)Nc1ccc2ccccc2c1 Show InChI InChI=1S/C22H16N4OS/c27-21(24-17-10-9-15-5-1-2-6-16(15)11-17)12-26-20-8-4-3-7-18(20)25-22(26)19-13-28-14-23-19/h1-11,13-14H,12H2,(H,24,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Brandeis University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cryptosporidium parvum IMPDH expressed in Escherichia coli assessed as NADH production preincubated for 5 mins by fluoresce... |
Bioorg Med Chem Lett 22: 1985-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.029
BindingDB Entry DOI: 10.7270/Q2959JJB |
More data for this
Ligand-Target Pair | |
Acid ceramidase
(Homo sapiens (Human)) | BDBM50431253
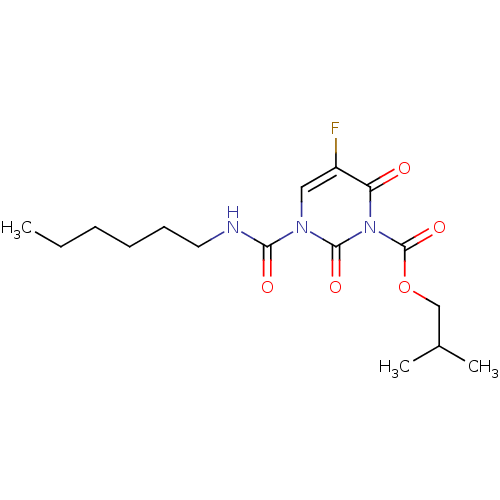
(CHEMBL2333055)Show SMILES CCCCCCNC(=O)n1cc(F)c(=O)n(C(=O)OCC(C)C)c1=O Show InChI InChI=1S/C16H24FN3O5/c1-4-5-6-7-8-18-14(22)19-9-12(17)13(21)20(15(19)23)16(24)25-10-11(2)3/h9,11H,4-8,10H2,1-3H3,(H,18,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human acid ceramidase |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01561
BindingDB Entry DOI: 10.7270/Q2G73JC2 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase
(Cryptosporidium parvum) | BDBM50365452
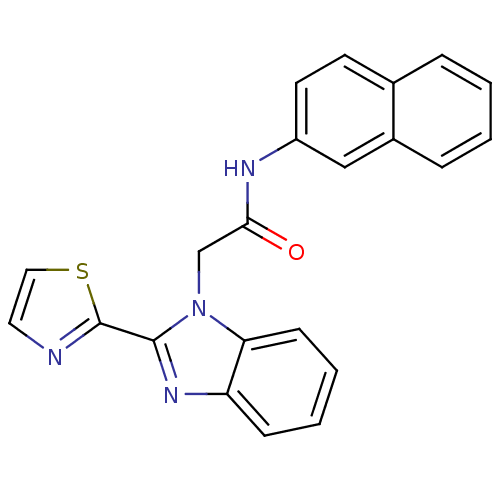
(CHEMBL1957252)Show SMILES O=C(Cn1c(nc2ccccc12)-c1nccs1)Nc1ccc2ccccc2c1 Show InChI InChI=1S/C22H16N4OS/c27-20(24-17-10-9-15-5-1-2-6-16(15)13-17)14-26-19-8-4-3-7-18(19)25-21(26)22-23-11-12-28-22/h1-13H,14H2,(H,24,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Brandeis University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cryptosporidium parvum IMPDH expressed in Escherichia coli assessed as NADH production preincubated for 5 mins by fluoresce... |
Bioorg Med Chem Lett 22: 1985-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.029
BindingDB Entry DOI: 10.7270/Q2959JJB |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase
(Cryptosporidium parvum) | BDBM81428

(N-(naphthalen-2-yl)-2-[2-(pyridin-2-yl)-1H-1,3-ben...)Show SMILES O=C(Cn1c(nc2ccccc12)-c1ccccn1)Nc1ccc2ccccc2c1 Show InChI InChI=1S/C24H18N4O/c29-23(26-19-13-12-17-7-1-2-8-18(17)15-19)16-28-22-11-4-3-9-20(22)27-24(28)21-10-5-6-14-25-21/h1-15H,16H2,(H,26,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Brandeis University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cryptosporidium parvum IMPDH expressed in Escherichia coli assessed as NADH production preincubated for 5 mins by fluoresce... |
Bioorg Med Chem Lett 22: 1985-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.029
BindingDB Entry DOI: 10.7270/Q2959JJB |
More data for this
Ligand-Target Pair | |
Acid ceramidase
(Rattus norvegicus (Rat)) | BDBM50431271
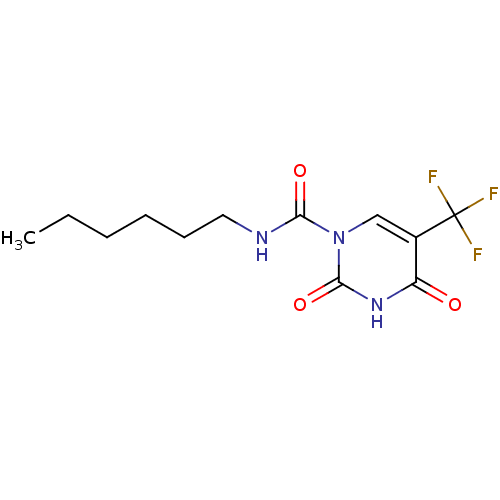
(CHEMBL2333033 | US9428465, 4)Show InChI InChI=1S/C12H16F3N3O3/c1-2-3-4-5-6-16-10(20)18-7-8(12(13,14)15)9(19)17-11(18)21/h7H,2-6H2,1H3,(H,16,20)(H,17,19,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat acid ceramidase |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01561
BindingDB Entry DOI: 10.7270/Q2G73JC2 |
More data for this
Ligand-Target Pair | |
Acid ceramidase
(Homo sapiens (Human)) | BDBM50556790
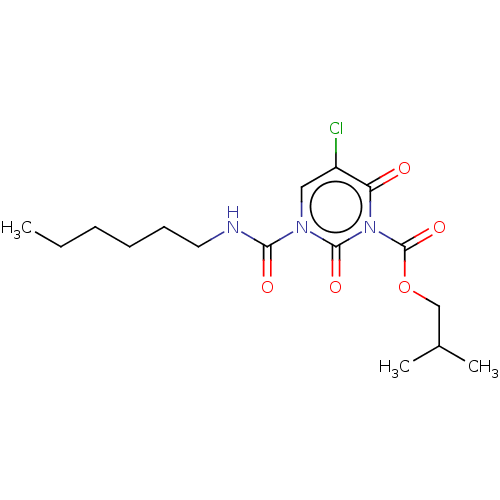
(CHEMBL4777626)Show SMILES CCCCCCNC(=O)n1cc(Cl)c(=O)n(C(=O)OCC(C)C)c1=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human acid ceramidase |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01561
BindingDB Entry DOI: 10.7270/Q2G73JC2 |
More data for this
Ligand-Target Pair | |
Acid ceramidase
(Homo sapiens (Human)) | BDBM50556791
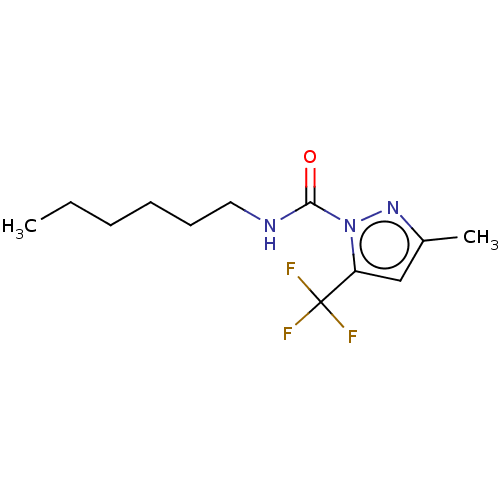
(CHEMBL4754069) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human acid ceramidase |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01561
BindingDB Entry DOI: 10.7270/Q2G73JC2 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase
(Cryptosporidium parvum) | BDBM81428

(N-(naphthalen-2-yl)-2-[2-(pyridin-2-yl)-1H-1,3-ben...)Show SMILES O=C(Cn1c(nc2ccccc12)-c1ccccn1)Nc1ccc2ccccc2c1 Show InChI InChI=1S/C24H18N4O/c29-23(26-19-13-12-17-7-1-2-8-18(17)15-19)16-28-22-11-4-3-9-20(22)27-24(28)21-10-5-6-14-25-21/h1-15H,16H2,(H,26,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Brandeis University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cryptosporidium parvum IMPDH expressed in Escherichia coli assessed as NADH production preincubated for 5 mins by fluoresce... |
Bioorg Med Chem Lett 22: 1985-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.029
BindingDB Entry DOI: 10.7270/Q2959JJB |
More data for this
Ligand-Target Pair | |
Acid ceramidase
(Rattus norvegicus (Rat)) | BDBM50431253

(CHEMBL2333055)Show SMILES CCCCCCNC(=O)n1cc(F)c(=O)n(C(=O)OCC(C)C)c1=O Show InChI InChI=1S/C16H24FN3O5/c1-4-5-6-7-8-18-14(22)19-9-12(17)13(21)20(15(19)23)16(24)25-10-11(2)3/h9,11H,4-8,10H2,1-3H3,(H,18,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat acid ceramidase |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01561
BindingDB Entry DOI: 10.7270/Q2G73JC2 |
More data for this
Ligand-Target Pair | |
Acid ceramidase
(Homo sapiens (Human)) | BDBM50556813

(CHEMBL4777275) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human acid ceramidase using N-lauroyl ceramide incubated for 1 hr by LC/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01561
BindingDB Entry DOI: 10.7270/Q2G73JC2 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase
(Cryptosporidium parvum) | BDBM50365453

(CHEMBL1957251)Show SMILES Clc1ccc(NC(=O)Cn2c(nc3ccccc23)-c2nccs2)cc1Cl Show InChI InChI=1S/C18H12Cl2N4OS/c19-12-6-5-11(9-13(12)20)22-16(25)10-24-15-4-2-1-3-14(15)23-17(24)18-21-7-8-26-18/h1-9H,10H2,(H,22,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Brandeis University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cryptosporidium parvum IMPDH expressed in Escherichia coli assessed as NADH production preincubated for 5 mins by fluoresce... |
Bioorg Med Chem Lett 22: 1985-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.029
BindingDB Entry DOI: 10.7270/Q2959JJB |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase
(Cryptosporidium parvum) | BDBM50365450

(CHEMBL1957254)Show InChI InChI=1S/C19H14ClN3OS/c20-13-7-9-14(10-8-13)21-18(24)12-23-16-5-2-1-4-15(16)22-19(23)17-6-3-11-25-17/h1-11H,12H2,(H,21,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Brandeis University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cryptosporidium parvum IMPDH expressed in Escherichia coli assessed as NADH production preincubated for 5 mins by fluoresce... |
Bioorg Med Chem Lett 22: 1985-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.029
BindingDB Entry DOI: 10.7270/Q2959JJB |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase
(Cryptosporidium parvum) | BDBM50365452

(CHEMBL1957252)Show SMILES O=C(Cn1c(nc2ccccc12)-c1nccs1)Nc1ccc2ccccc2c1 Show InChI InChI=1S/C22H16N4OS/c27-20(24-17-10-9-15-5-1-2-6-16(15)13-17)14-26-19-8-4-3-7-18(19)25-21(26)22-23-11-12-28-22/h1-13H,14H2,(H,24,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Brandeis University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cryptosporidium parvum IMPDH expressed in Escherichia coli assessed as NADH production preincubated for 5 mins by fluoresce... |
Bioorg Med Chem Lett 22: 1985-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.029
BindingDB Entry DOI: 10.7270/Q2959JJB |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase
(Cryptosporidium parvum) | BDBM50365429

(CHEMBL1450808)Show SMILES O=C(Cn1c(nc2ccccc12)-c1cscn1)Nc1ccc2ccccc2c1 Show InChI InChI=1S/C22H16N4OS/c27-21(24-17-10-9-15-5-1-2-6-16(15)11-17)12-26-20-8-4-3-7-18(20)25-22(26)19-13-28-14-23-19/h1-11,13-14H,12H2,(H,24,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Brandeis University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cryptosporidium parvum IMPDH expressed in Escherichia coli assessed as NADH production preincubated for 5 mins by fluoresce... |
Bioorg Med Chem Lett 22: 1985-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.029
BindingDB Entry DOI: 10.7270/Q2959JJB |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase
(Cryptosporidium parvum) | BDBM50365446
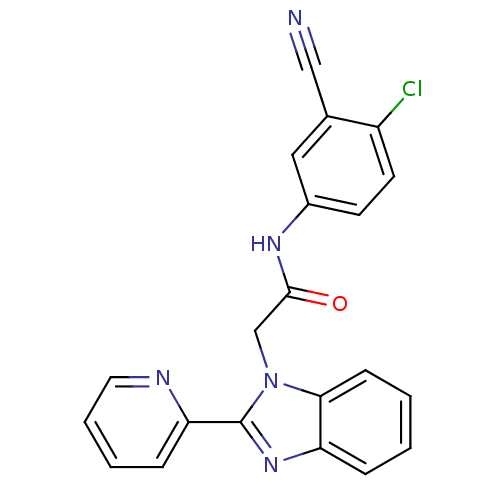
(CHEMBL1957259)Show SMILES Clc1ccc(NC(=O)Cn2c(nc3ccccc23)-c2ccccn2)cc1C#N Show InChI InChI=1S/C21H14ClN5O/c22-16-9-8-15(11-14(16)12-23)25-20(28)13-27-19-7-2-1-5-17(19)26-21(27)18-6-3-4-10-24-18/h1-11H,13H2,(H,25,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Brandeis University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cryptosporidium parvum IMPDH expressed in Escherichia coli assessed as NADH production preincubated for 5 mins by fluoresce... |
Bioorg Med Chem Lett 22: 1985-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.029
BindingDB Entry DOI: 10.7270/Q2959JJB |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase
(Cryptosporidium parvum) | BDBM50365447
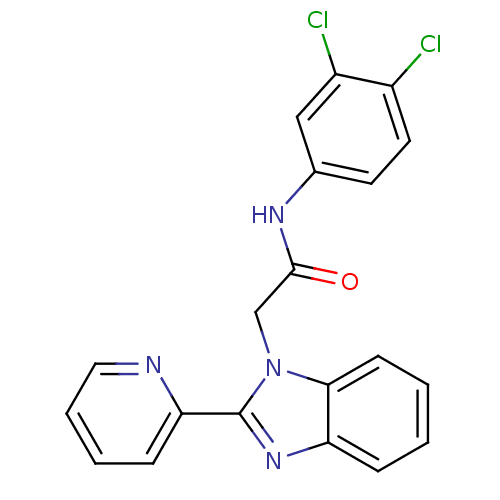
(CHEMBL1957257)Show SMILES Clc1ccc(NC(=O)Cn2c(nc3ccccc23)-c2ccccn2)cc1Cl Show InChI InChI=1S/C20H14Cl2N4O/c21-14-9-8-13(11-15(14)22)24-19(27)12-26-18-7-2-1-5-16(18)25-20(26)17-6-3-4-10-23-17/h1-11H,12H2,(H,24,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Brandeis University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cryptosporidium parvum IMPDH expressed in Escherichia coli assessed as NADH production preincubated for 5 mins by fluoresce... |
Bioorg Med Chem Lett 22: 1985-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.029
BindingDB Entry DOI: 10.7270/Q2959JJB |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase
(Cryptosporidium parvum) | BDBM50365454

(CHEMBL1957250)Show SMILES CC(C(=O)Nc1ccc(Cl)cc1)n1c(nc2ccccc12)-c1nccs1 Show InChI InChI=1S/C19H15ClN4OS/c1-12(18(25)22-14-8-6-13(20)7-9-14)24-16-5-3-2-4-15(16)23-17(24)19-21-10-11-26-19/h2-12H,1H3,(H,22,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Brandeis University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cryptosporidium parvum IMPDH expressed in Escherichia coli assessed as NADH production preincubated for 5 mins by fluoresce... |
Bioorg Med Chem Lett 22: 1985-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.029
BindingDB Entry DOI: 10.7270/Q2959JJB |
More data for this
Ligand-Target Pair | |
Acid ceramidase
(Homo sapiens (Human)) | BDBM50556811

(CHEMBL4783041) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human acid ceramidase using N-lauroyl ceramide incubated for 1 hr by LC/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01561
BindingDB Entry DOI: 10.7270/Q2G73JC2 |
More data for this
Ligand-Target Pair | |
Acid ceramidase
(Homo sapiens (Human)) | BDBM50556796

(CHEMBL4753638) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human acid ceramidase using N-lauroyl ceramide incubated for 1 hr by LC/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01561
BindingDB Entry DOI: 10.7270/Q2G73JC2 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase
(Cryptosporidium parvum) | BDBM50365455

(CHEMBL1231618)Show InChI InChI=1S/C18H13BrN4OS/c19-12-5-7-13(8-6-12)21-16(24)11-23-15-4-2-1-3-14(15)22-17(23)18-20-9-10-25-18/h1-10H,11H2,(H,21,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Brandeis University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cryptosporidium parvum IMPDH expressed in Escherichia coli assessed as NADH production preincubated for 5 mins by fluoresce... |
Bioorg Med Chem Lett 22: 1985-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.029
BindingDB Entry DOI: 10.7270/Q2959JJB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Inosine-5'-monophosphate dehydrogenase
(Cryptosporidium parvum) | BDBM50365455

(CHEMBL1231618)Show InChI InChI=1S/C18H13BrN4OS/c19-12-5-7-13(8-6-12)21-16(24)11-23-15-4-2-1-3-14(15)22-17(23)18-20-9-10-25-18/h1-10H,11H2,(H,21,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Brandeis University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cryptosporidium parvum IMPDH expressed in Escherichia coli assessed as NADH production preincubated for 5 mins by fluoresce... |
Bioorg Med Chem Lett 22: 1985-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.029
BindingDB Entry DOI: 10.7270/Q2959JJB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acid ceramidase
(Rattus norvegicus (Rat)) | BDBM50431275
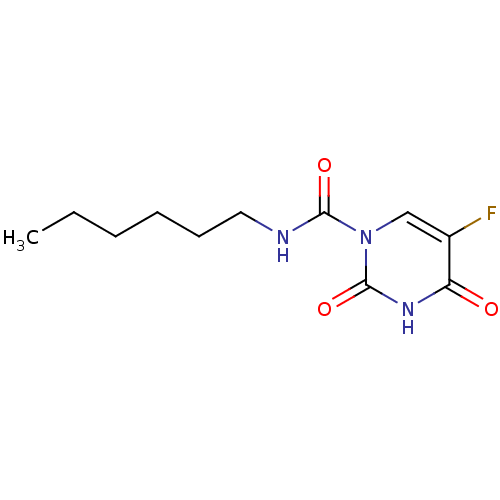
(CARMOFUR | Carm-ofur | Mifurol | med.21724, Compou...)Show InChI InChI=1S/C11H16FN3O3/c1-2-3-4-5-6-13-10(17)15-7-8(12)9(16)14-11(15)18/h7H,2-6H2,1H3,(H,13,17)(H,14,16,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat acid ceramidase |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01561
BindingDB Entry DOI: 10.7270/Q2G73JC2 |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase
(Cryptosporidium parvum) | BDBM50365430
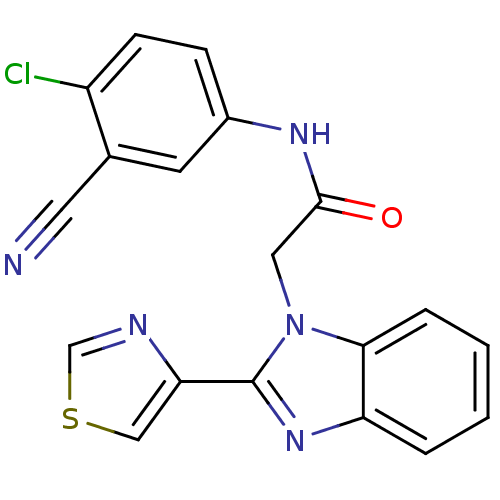
(CHEMBL1957244)Show SMILES Clc1ccc(NC(=O)Cn2c(nc3ccccc23)-c2cscn2)cc1C#N Show InChI InChI=1S/C19H12ClN5OS/c20-14-6-5-13(7-12(14)8-21)23-18(26)9-25-17-4-2-1-3-15(17)24-19(25)16-10-27-11-22-16/h1-7,10-11H,9H2,(H,23,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Brandeis University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cryptosporidium parvum IMPDH expressed in Escherichia coli assessed as NADH production preincubated for 5 mins by fluoresce... |
Bioorg Med Chem Lett 22: 1985-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.029
BindingDB Entry DOI: 10.7270/Q2959JJB |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase
(Cryptosporidium parvum) | BDBM50365456

(CHEMBL1957249)Show InChI InChI=1S/C18H13ClN4OS/c19-12-5-7-13(8-6-12)21-16(24)11-23-15-4-2-1-3-14(15)22-17(23)18-20-9-10-25-18/h1-10H,11H2,(H,21,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Brandeis University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cryptosporidium parvum IMPDH expressed in Escherichia coli assessed as NADH production preincubated for 5 mins by fluoresce... |
Bioorg Med Chem Lett 22: 1985-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.029
BindingDB Entry DOI: 10.7270/Q2959JJB |
More data for this
Ligand-Target Pair | |
Inosine-5'-monophosphate dehydrogenase
(Cryptosporidium parvum) | BDBM50365454

(CHEMBL1957250)Show SMILES CC(C(=O)Nc1ccc(Cl)cc1)n1c(nc2ccccc12)-c1nccs1 Show InChI InChI=1S/C19H15ClN4OS/c1-12(18(25)22-14-8-6-13(20)7-9-14)24-16-5-3-2-4-15(16)23-17(24)19-21-10-11-26-19/h2-12H,1H3,(H,22,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Brandeis University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Cryptosporidium parvum IMPDH expressed in Escherichia coli assessed as NADH production preincubated for 5 mins by fluoresce... |
Bioorg Med Chem Lett 22: 1985-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.029
BindingDB Entry DOI: 10.7270/Q2959JJB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data