 Found 201 hits with Last Name = 'reddy' and Initial = 'tj'
Found 201 hits with Last Name = 'reddy' and Initial = 'tj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin S
(Homo sapiens (Human)) | BDBM19516

((2S)-N-[(3S,5E)-6-(benzenesulfonyl)-4-oxo-1-phenyl...)Show SMILES CC(C)C[C@@H](NC(=O)N1CCOCC1)C(=O)N[C@H](CCc1ccccc1)C=CS(=O)(=O)c1ccccc1 |r,w:27.29| Show InChI InChI=1S/C28H37N3O5S/c1-22(2)21-26(30-28(33)31-16-18-36-19-17-31)27(32)29-24(14-13-23-9-5-3-6-10-23)15-20-37(34,35)25-11-7-4-8-12-25/h3-12,15,20,22,24,26H,13-14,16-19,21H2,1-2H3,(H,29,32)(H,30,33)/t24-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50270029
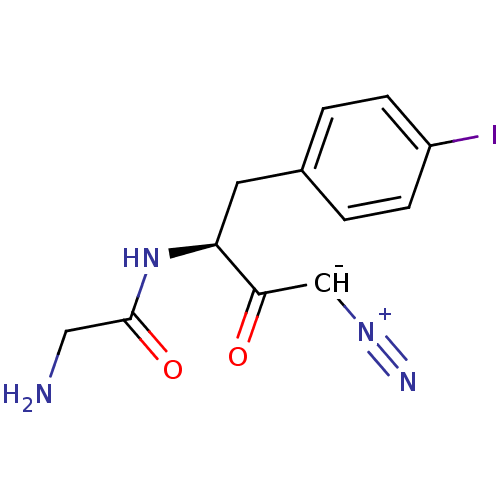
(2-Amino-N-[(S)-3-diazo-1-(4-iodo-benzyl)-2-oxo-pro...)Show SMILES NCC(=O)N[C@@H](Cc1ccc(I)cc1)C(=O)[CH-][N+]#N |r| Show InChI InChI=1S/C12H13IN4O2/c13-9-3-1-8(2-4-9)5-10(11(18)7-16-15)17-12(19)6-14/h1-4,7,10H,5-6,14H2,(H,17,19)/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin C after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Pantothenate kinase 3
(Homo sapiens (Human)) | BDBM649476

(US11891378, Example 6)Show SMILES C[C@H]1CN(CCN1C(=O)Cc1ccc(C2CC2)c(F)c1)c1ccc(Cl)nn1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Pantothenate kinase 3
(Homo sapiens (Human)) | BDBM649472

(US11891378, Example 2)Show SMILES Fc1cc(CC(=O)N2CCN(CC2)c2ccc(Cl)nn2)ccc1C1CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Pantothenate kinase 3
(Homo sapiens (Human)) | BDBM649480
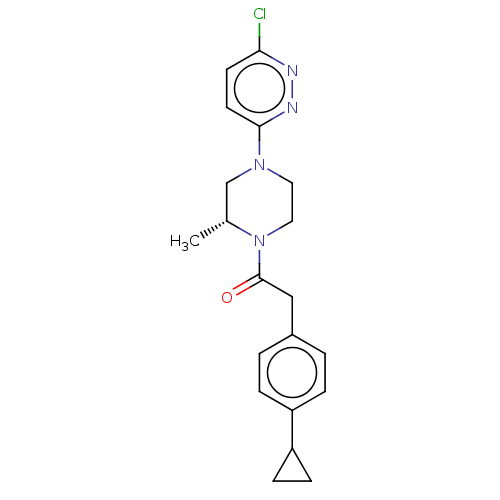
(US11891378, Example 10)Show SMILES C[C@@H]1CN(CCN1C(=O)Cc1ccc(cc1)C1CC1)c1ccc(Cl)nn1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Pantothenate kinase 3
(Homo sapiens (Human)) | BDBM649479
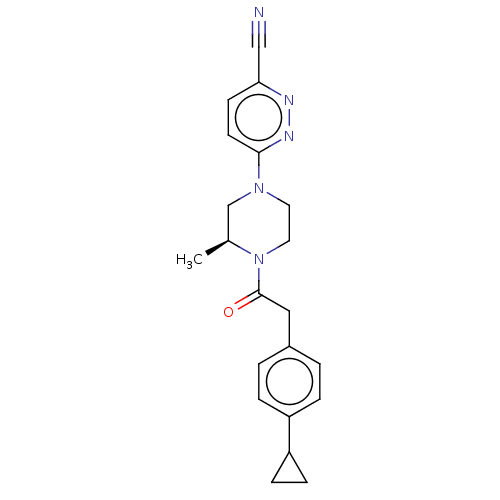
(US11891378, Example 9)Show SMILES C[C@H]1CN(CCN1C(=O)Cc1ccc(cc1)C1CC1)c1ccc(nn1)C#N |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Pantothenate kinase 3
(Homo sapiens (Human)) | BDBM649477
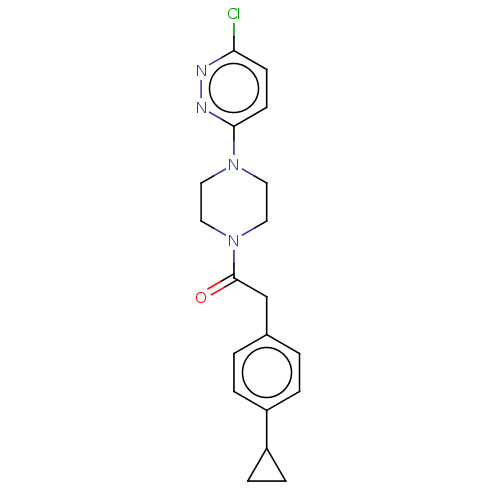
(US11891378, Example 7)Show SMILES Clc1ccc(nn1)N1CCN(CC1)C(=O)Cc1ccc(cc1)C1CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Pantothenate kinase 3
(Homo sapiens (Human)) | BDBM649473

(US11891378, Example 3)Show SMILES C[C@H]1CN(CCN1C(=O)Cc1ccc(C2CC2)c(F)c1)c1ccc(nn1)C#N |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Pantothenate kinase 3
(Homo sapiens (Human)) | BDBM649475

(US11891378, Example 5)Show SMILES C[C@@H]1CN(CCN1C(=O)Cc1ccc(C2CC2)c(F)c1)c1ccc(Cl)nn1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19516

((2S)-N-[(3S,5E)-6-(benzenesulfonyl)-4-oxo-1-phenyl...)Show SMILES CC(C)C[C@@H](NC(=O)N1CCOCC1)C(=O)N[C@H](CCc1ccccc1)C=CS(=O)(=O)c1ccccc1 |r,w:27.29| Show InChI InChI=1S/C28H37N3O5S/c1-22(2)21-26(30-28(33)31-16-18-36-19-17-31)27(32)29-24(14-13-23-9-5-3-6-10-23)15-20-37(34,35)25-11-7-4-8-12-25/h3-12,15,20,22,24,26H,13-14,16-19,21H2,1-2H3,(H,29,32)(H,30,33)/t24-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Pantothenate kinase 3
(Homo sapiens (Human)) | BDBM649471

(US11891378, Example 1)Show SMILES Fc1cc(CC(=O)N2CCN(CC2)c2ccc(nn2)C#N)ccc1C1CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin S
(Homo sapiens (Human)) | BDBM50270041

(4'-Iodo-biphenyl-4-carboxylic acid {1-[(S)-1-(2-di...)Show SMILES CCC[C@H](NC(=O)C(CC(C)C)NC(=O)c1ccc(cc1)-c1ccc(I)cc1)C(=O)[CH-][N+]#N |r| Show InChI InChI=1S/C25H29IN4O3/c1-4-5-21(23(31)15-28-27)29-25(33)22(14-16(2)3)30-24(32)19-8-6-17(7-9-19)18-10-12-20(26)13-11-18/h6-13,15-16,21-22H,4-5,14H2,1-3H3,(H,29,33)(H,30,32)/t21-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19492
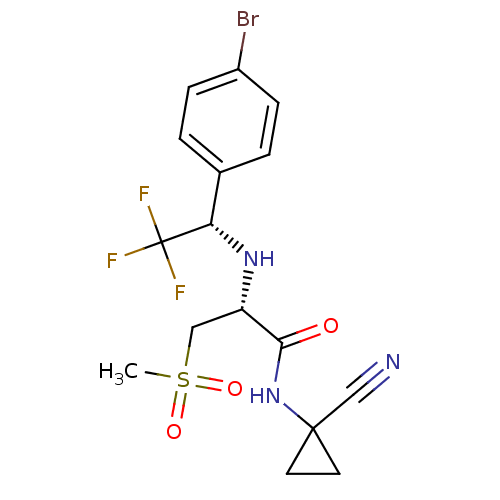
((2R)-2-{[(1S)-1-(4-bromophenyl)-2,2,2-trifluoroeth...)Show SMILES CS(=O)(=O)C[C@H](N[C@@H](c1ccc(Br)cc1)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C16H17BrF3N3O3S/c1-27(25,26)8-12(14(24)23-15(9-21)6-7-15)22-13(16(18,19)20)10-2-4-11(17)5-3-10/h2-5,12-13,22H,6-8H2,1H3,(H,23,24)/t12-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50270041

(4'-Iodo-biphenyl-4-carboxylic acid {1-[(S)-1-(2-di...)Show SMILES CCC[C@H](NC(=O)C(CC(C)C)NC(=O)c1ccc(cc1)-c1ccc(I)cc1)C(=O)[CH-][N+]#N |r| Show InChI InChI=1S/C25H29IN4O3/c1-4-5-21(23(31)15-28-27)29-25(33)22(14-16(2)3)30-24(32)19-8-6-17(7-9-19)18-10-12-20(26)13-11-18/h6-13,15-16,21-22H,4-5,14H2,1-3H3,(H,29,33)(H,30,32)/t21-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Pantothenate kinase 3
(Homo sapiens (Human)) | BDBM649474

(US11891378, Example 4)Show SMILES C[C@@H]1CN(CCN1C(=O)Cc1ccc(C2CC2)c(F)c1)c1ccc(nn1)C#N |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 12.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Pantothenate kinase 3
(Homo sapiens (Human)) | BDBM649478

(US11891378, Example 8)Show SMILES C[C@@H]1CN(CCN1C(=O)Cc1ccc(cc1)C1CC1)c1ccc(nn1)C#N |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 12.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50270040
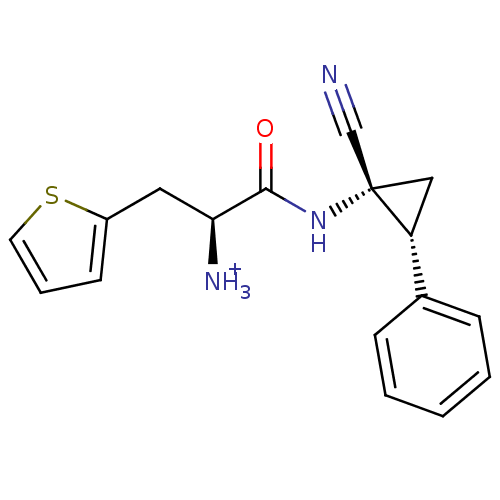
((S)-1-((1R,2R)-1-cyano-2-phenylcyclopropylamino)-1...)Show SMILES [NH3+][C@@H](Cc1cccs1)C(=O)N[C@@]1(C[C@@H]1c1ccccc1)C#N |r| Show InChI InChI=1S/C17H17N3OS/c18-11-17(10-14(17)12-5-2-1-3-6-12)20-16(21)15(19)9-13-7-4-8-22-13/h1-8,14-15H,9-10,19H2,(H,20,21)/p+1/t14-,15+,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin C after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50269860
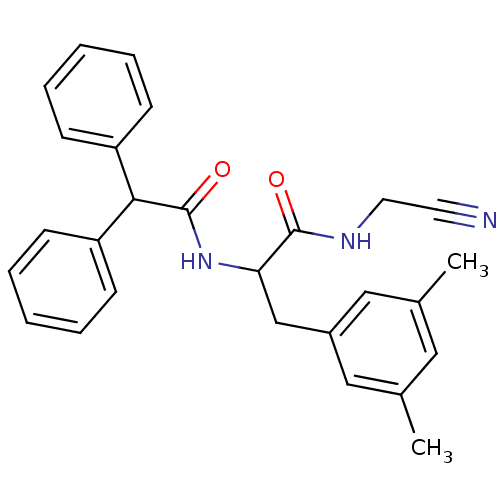
(CHEMBL477531 | N-(cyanomethyl)-3-(3,5-dimethylphen...)Show SMILES Cc1cc(C)cc(CC(NC(=O)C(c2ccccc2)c2ccccc2)C(=O)NCC#N)c1 Show InChI InChI=1S/C27H27N3O2/c1-19-15-20(2)17-21(16-19)18-24(26(31)29-14-13-28)30-27(32)25(22-9-5-3-6-10-22)23-11-7-4-8-12-23/h3-12,15-17,24-25H,14,18H2,1-2H3,(H,29,31)(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50270030
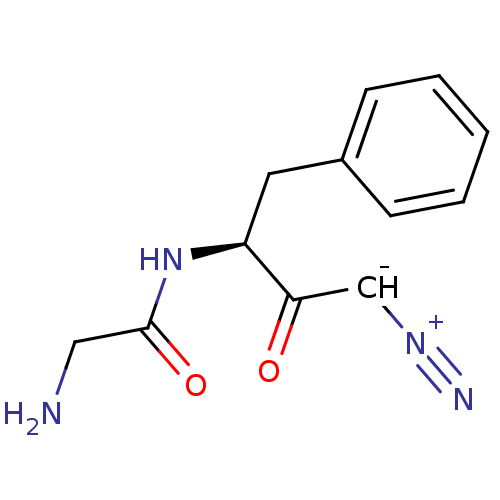
(2-Amino-N-((S)-1-benzyl-3-diazo-2-oxo-propyl)-acet...)Show SMILES NCC(=O)N[C@@H](Cc1ccccc1)C(=O)[CH-][N+]#N |r| Show InChI InChI=1S/C12H14N4O2/c13-7-12(18)16-10(11(17)8-15-14)6-9-4-2-1-3-5-9/h1-5,8,10H,6-7,13H2,(H,16,18)/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin C after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50270041

(4'-Iodo-biphenyl-4-carboxylic acid {1-[(S)-1-(2-di...)Show SMILES CCC[C@H](NC(=O)C(CC(C)C)NC(=O)c1ccc(cc1)-c1ccc(I)cc1)C(=O)[CH-][N+]#N |r| Show InChI InChI=1S/C25H29IN4O3/c1-4-5-21(23(31)15-28-27)29-25(33)22(14-16(2)3)30-24(32)19-8-6-17(7-9-19)18-10-12-20(26)13-11-18/h6-13,15-16,21-22H,4-5,14H2,1-3H3,(H,29,33)(H,30,32)/t21-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50270031
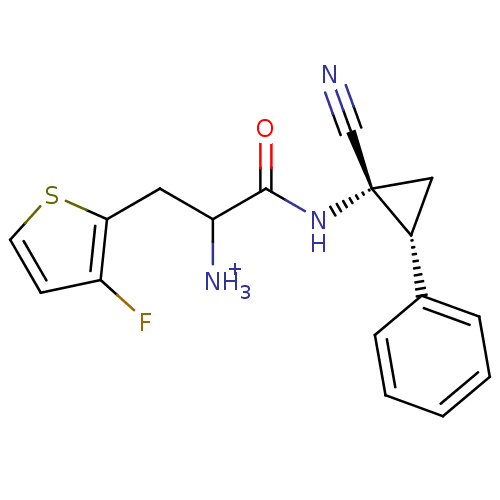
(1-((1R,2R)-1-cyano-2-phenylcyclopropylamino)-3-(3-...)Show SMILES [NH3+]C(Cc1sccc1F)C(=O)N[C@@]1(C[C@@H]1c1ccccc1)C#N |r| Show InChI InChI=1S/C17H16FN3OS/c18-13-6-7-23-15(13)8-14(20)16(22)21-17(10-19)9-12(17)11-4-2-1-3-5-11/h1-7,12,14H,8-9,20H2,(H,21,22)/p+1/t12-,14?,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of cathepsin G processing in human U937 cells by densitometry |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50269860

(CHEMBL477531 | N-(cyanomethyl)-3-(3,5-dimethylphen...)Show SMILES Cc1cc(C)cc(CC(NC(=O)C(c2ccccc2)c2ccccc2)C(=O)NCC#N)c1 Show InChI InChI=1S/C27H27N3O2/c1-19-15-20(2)17-21(16-19)18-24(26(31)29-14-13-28)30-27(32)25(22-9-5-3-6-10-22)23-11-7-4-8-12-23/h3-12,15-17,24-25H,14,18H2,1-2H3,(H,29,31)(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Myeloblastin
(Mus musculus) | BDBM50270040

((S)-1-((1R,2R)-1-cyano-2-phenylcyclopropylamino)-1...)Show SMILES [NH3+][C@@H](Cc1cccs1)C(=O)N[C@@]1(C[C@@H]1c1ccccc1)C#N |r| Show InChI InChI=1S/C17H17N3OS/c18-11-17(10-14(17)12-5-2-1-3-6-12)20-16(21)15(19)9-13-7-4-8-22-13/h1-8,14-15H,9-10,19H2,(H,20,21)/p+1/t14-,15+,17+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of proteinase-3 activation in beta-estradiol differentiated mouse EcoM-G cells after 24 hrs |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50270040

((S)-1-((1R,2R)-1-cyano-2-phenylcyclopropylamino)-1...)Show SMILES [NH3+][C@@H](Cc1cccs1)C(=O)N[C@@]1(C[C@@H]1c1ccccc1)C#N |r| Show InChI InChI=1S/C17H17N3OS/c18-11-17(10-14(17)12-5-2-1-3-6-12)20-16(21)15(19)9-13-7-4-8-22-13/h1-8,14-15H,9-10,19H2,(H,20,21)/p+1/t14-,15+,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of cathepsin G processing in human U937 cells by densitometry |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Myeloblastin
(Mus musculus) | BDBM50270031

(1-((1R,2R)-1-cyano-2-phenylcyclopropylamino)-3-(3-...)Show SMILES [NH3+]C(Cc1sccc1F)C(=O)N[C@@]1(C[C@@H]1c1ccccc1)C#N |r| Show InChI InChI=1S/C17H16FN3OS/c18-13-6-7-23-15(13)8-14(20)16(22)21-17(10-19)9-12(17)11-4-2-1-3-5-11/h1-7,12,14H,8-9,20H2,(H,21,22)/p+1/t12-,14?,17+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of proteinase-3 activation in beta-estradiol differentiated mouse EcoM-G cells after 24 hrs |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Mus musculus) | BDBM50270031

(1-((1R,2R)-1-cyano-2-phenylcyclopropylamino)-3-(3-...)Show SMILES [NH3+]C(Cc1sccc1F)C(=O)N[C@@]1(C[C@@H]1c1ccccc1)C#N |r| Show InChI InChI=1S/C17H16FN3OS/c18-13-6-7-23-15(13)8-14(20)16(22)21-17(10-19)9-12(17)11-4-2-1-3-5-11/h1-7,12,14H,8-9,20H2,(H,21,22)/p+1/t12-,14?,17+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of neutrophil elastase activation in beta-estradiol differentiated mouse EcoM-G cells after 24 hrs |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Mus musculus) | BDBM50270040

((S)-1-((1R,2R)-1-cyano-2-phenylcyclopropylamino)-1...)Show SMILES [NH3+][C@@H](Cc1cccs1)C(=O)N[C@@]1(C[C@@H]1c1ccccc1)C#N |r| Show InChI InChI=1S/C17H17N3OS/c18-11-17(10-14(17)12-5-2-1-3-6-12)20-16(21)15(19)9-13-7-4-8-22-13/h1-8,14-15H,9-10,19H2,(H,20,21)/p+1/t14-,15+,17+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of neutrophil elastase activation in beta-estradiol differentiated mouse EcoM-G cells after 24 hrs |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50270031

(1-((1R,2R)-1-cyano-2-phenylcyclopropylamino)-3-(3-...)Show SMILES [NH3+]C(Cc1sccc1F)C(=O)N[C@@]1(C[C@@H]1c1ccccc1)C#N |r| Show InChI InChI=1S/C17H16FN3OS/c18-13-6-7-23-15(13)8-14(20)16(22)21-17(10-19)9-12(17)11-4-2-1-3-5-11/h1-7,12,14H,8-9,20H2,(H,21,22)/p+1/t12-,14?,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of neutrophil elastase processing in human U937 cells after 7 days by fluorogenic substrate cleavage assay |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Pro-cathepsin H
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin H after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50270040

((S)-1-((1R,2R)-1-cyano-2-phenylcyclopropylamino)-1...)Show SMILES [NH3+][C@@H](Cc1cccs1)C(=O)N[C@@]1(C[C@@H]1c1ccccc1)C#N |r| Show InChI InChI=1S/C17H17N3OS/c18-11-17(10-14(17)12-5-2-1-3-6-12)20-16(21)15(19)9-13-7-4-8-22-13/h1-8,14-15H,9-10,19H2,(H,20,21)/p+1/t14-,15+,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Blockade of neutrophil elastase processing in human U937 cells after 7 days by fluorogenic substrate cleavage assay |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50269858
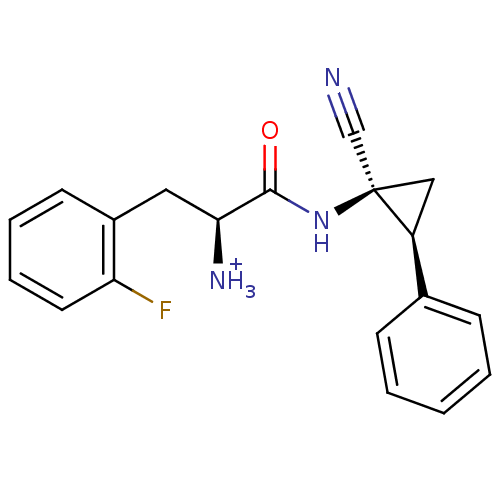
(Trifluoro-acetate(S)-1-((1S,2S)-1-cyano-2-phenyl-c...)Show SMILES [NH3+][C@@H](Cc1ccccc1F)C(=O)N[C@]1(C[C@H]1c1ccccc1)C#N |r| Show InChI InChI=1S/C19H18FN3O/c20-16-9-5-4-8-14(16)10-17(22)18(24)23-19(12-21)11-15(19)13-6-2-1-3-7-13/h1-9,15,17H,10-11,22H2,(H,23,24)/p+1/t15-,17-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin C after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM19492

((2R)-2-{[(1S)-1-(4-bromophenyl)-2,2,2-trifluoroeth...)Show SMILES CS(=O)(=O)C[C@H](N[C@@H](c1ccc(Br)cc1)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C16H17BrF3N3O3S/c1-27(25,26)8-12(14(24)23-15(9-21)6-7-15)22-13(16(18,19)20)10-2-4-11(17)5-3-10/h2-5,12-13,22H,6-8H2,1H3,(H,23,24)/t12-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin B after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50139666
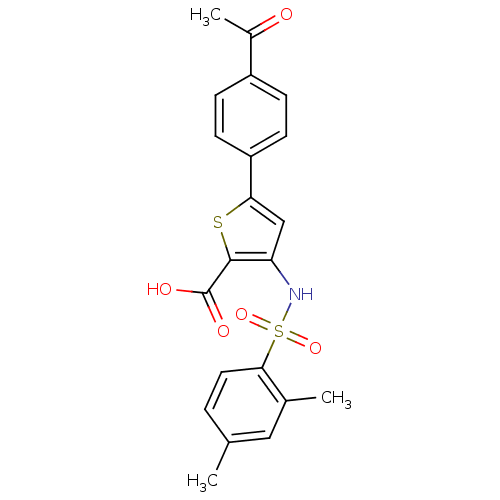
(5-(4-Acetyl-phenyl)-3-(2,4-dimethyl-benzenesulfony...)Show SMILES CC(=O)c1ccc(cc1)-c1cc(NS(=O)(=O)c2ccc(C)cc2C)c(s1)C(O)=O Show InChI InChI=1S/C21H19NO5S2/c1-12-4-9-19(13(2)10-12)29(26,27)22-17-11-18(28-20(17)21(24)25)16-7-5-15(6-8-16)14(3)23/h4-11,22H,1-3H3,(H,24,25) | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS5B polymerase |
Bioorg Med Chem Lett 14: 793-6 (2004)
BindingDB Entry DOI: 10.7270/Q2PV6JSN |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19492

((2R)-2-{[(1S)-1-(4-bromophenyl)-2,2,2-trifluoroeth...)Show SMILES CS(=O)(=O)C[C@H](N[C@@H](c1ccc(Br)cc1)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C16H17BrF3N3O3S/c1-27(25,26)8-12(14(24)23-15(9-21)6-7-15)22-13(16(18,19)20)10-2-4-11(17)5-3-10/h2-5,12-13,22H,6-8H2,1H3,(H,23,24)/t12-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50270041

(4'-Iodo-biphenyl-4-carboxylic acid {1-[(S)-1-(2-di...)Show SMILES CCC[C@H](NC(=O)C(CC(C)C)NC(=O)c1ccc(cc1)-c1ccc(I)cc1)C(=O)[CH-][N+]#N |r| Show InChI InChI=1S/C25H29IN4O3/c1-4-5-21(23(31)15-28-27)29-25(33)22(14-16(2)3)30-24(32)19-8-6-17(7-9-19)18-10-12-20(26)13-11-18/h6-13,15-16,21-22H,4-5,14H2,1-3H3,(H,29,33)(H,30,32)/t21-,22?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin C after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50269860

(CHEMBL477531 | N-(cyanomethyl)-3-(3,5-dimethylphen...)Show SMILES Cc1cc(C)cc(CC(NC(=O)C(c2ccccc2)c2ccccc2)C(=O)NCC#N)c1 Show InChI InChI=1S/C27H27N3O2/c1-19-15-20(2)17-21(16-19)18-24(26(31)29-14-13-28)30-27(32)25(22-9-5-3-6-10-22)23-11-7-4-8-12-23/h3-12,15-17,24-25H,14,18H2,1-2H3,(H,29,31)(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Mus musculus) | BDBM50270040

((S)-1-((1R,2R)-1-cyano-2-phenylcyclopropylamino)-1...)Show SMILES [NH3+][C@@H](Cc1cccs1)C(=O)N[C@@]1(C[C@@H]1c1ccccc1)C#N |r| Show InChI InChI=1S/C17H17N3OS/c18-11-17(10-14(17)12-5-2-1-3-6-12)20-16(21)15(19)9-13-7-4-8-22-13/h1-8,14-15H,9-10,19H2,(H,20,21)/p+1/t14-,15+,17+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin G activation in beta-estradiol differentiated mouse EcoM-G cells after 24 hrs |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50269858

(Trifluoro-acetate(S)-1-((1S,2S)-1-cyano-2-phenyl-c...)Show SMILES [NH3+][C@@H](Cc1ccccc1F)C(=O)N[C@]1(C[C@H]1c1ccccc1)C#N |r| Show InChI InChI=1S/C19H18FN3O/c20-16-9-5-4-8-14(16)10-17(22)18(24)23-19(12-21)11-15(19)13-6-2-1-3-7-13/h1-9,15,17H,10-11,22H2,(H,23,24)/p+1/t15-,17-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50126676
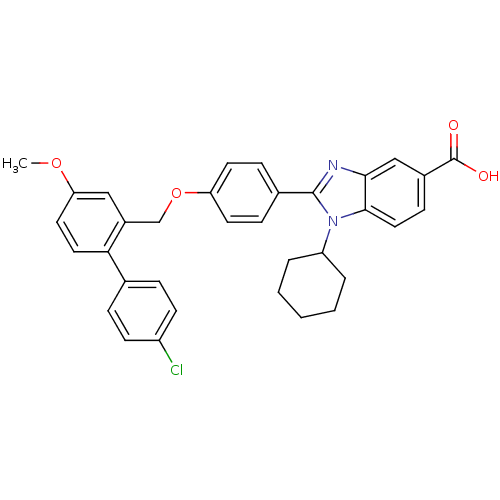
(2-[4-(4'-Chloro-4-methoxy-biphenyl-2-ylmethoxy)-ph...)Show SMILES COc1ccc(c(COc2ccc(cc2)-c2nc3cc(ccc3n2C2CCCCC2)C(O)=O)c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C34H31ClN2O4/c1-40-29-16-17-30(22-7-12-26(35)13-8-22)25(19-29)21-41-28-14-9-23(10-15-28)33-36-31-20-24(34(38)39)11-18-32(31)37(33)27-5-3-2-4-6-27/h7-20,27H,2-6,21H2,1H3,(H,38,39) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C RNA dependent RNA polymerase Nonstructural protein 5B (NS5B polymerase) expressed from baculovirus-infected S... |
J Med Chem 46: 1283-5 (2003)
Article DOI: 10.1021/jm0340400
BindingDB Entry DOI: 10.7270/Q2XP74BP |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Mus musculus) | BDBM50270031

(1-((1R,2R)-1-cyano-2-phenylcyclopropylamino)-3-(3-...)Show SMILES [NH3+]C(Cc1sccc1F)C(=O)N[C@@]1(C[C@@H]1c1ccccc1)C#N |r| Show InChI InChI=1S/C17H16FN3OS/c18-13-6-7-23-15(13)8-14(20)16(22)21-17(10-19)9-12(17)11-4-2-1-3-5-11/h1-7,12,14H,8-9,20H2,(H,21,22)/p+1/t12-,14?,17+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin G activation in beta-estradiol differentiated mouse EcoM-G cells after 24 hrs |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50126679
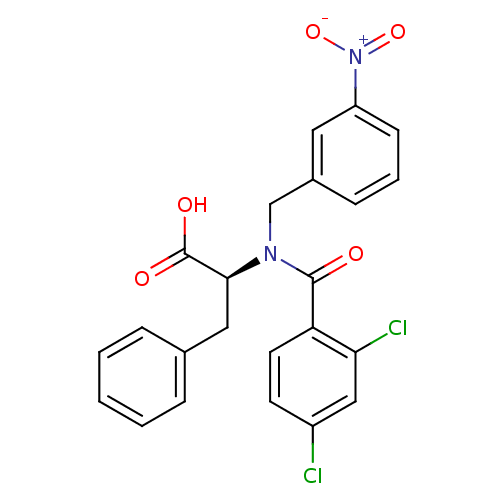
((S)-2-(2,4-dichloro-N-(3-nitrobenzyl)benzamido)-3-...)Show SMILES OC(=O)[C@H](Cc1ccccc1)N(Cc1cccc(c1)[N+]([O-])=O)C(=O)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C23H18Cl2N2O5/c24-17-9-10-19(20(25)13-17)22(28)26(14-16-7-4-8-18(11-16)27(31)32)21(23(29)30)12-15-5-2-1-3-6-15/h1-11,13,21H,12,14H2,(H,29,30)/t21-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C RNA dependent RNA polymerase Nonstructural protein 5B (NS5B polymerase) expressed from baculovirus-infected S... |
J Med Chem 46: 1283-5 (2003)
Article DOI: 10.1021/jm0340400
BindingDB Entry DOI: 10.7270/Q2XP74BP |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50139669
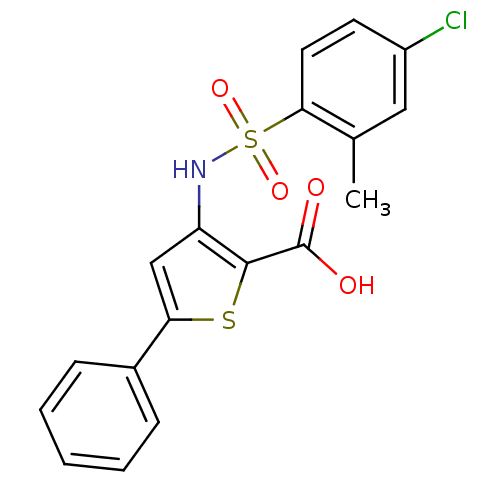
(3-(4-Chloro-2-methyl-benzenesulfonylamino)-5-pheny...)Show SMILES Cc1cc(Cl)ccc1S(=O)(=O)Nc1cc(sc1C(O)=O)-c1ccccc1 Show InChI InChI=1S/C18H14ClNO4S2/c1-11-9-13(19)7-8-16(11)26(23,24)20-14-10-15(25-17(14)18(21)22)12-5-3-2-4-6-12/h2-10,20H,1H3,(H,21,22) | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS5B polymerase |
Bioorg Med Chem Lett 14: 793-6 (2004)
BindingDB Entry DOI: 10.7270/Q2PV6JSN |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin C after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50269859

(2,2,2-trifluoroacetate; 5-(2-azaniumyl-2-{[(1S,2S)...)Show SMILES [NH3+]C(Cc1c[nH+]cs1)C(=O)N[C@]1(C[C@H]1c1ccccc1)C#N |r| Show InChI InChI=1S/C16H16N4OS/c17-9-16(7-13(16)11-4-2-1-3-5-11)20-15(21)14(18)6-12-8-19-10-22-12/h1-5,8,10,13-14H,6-7,18H2,(H,20,21)/p+2/t13-,14?,16+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin C after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50126666
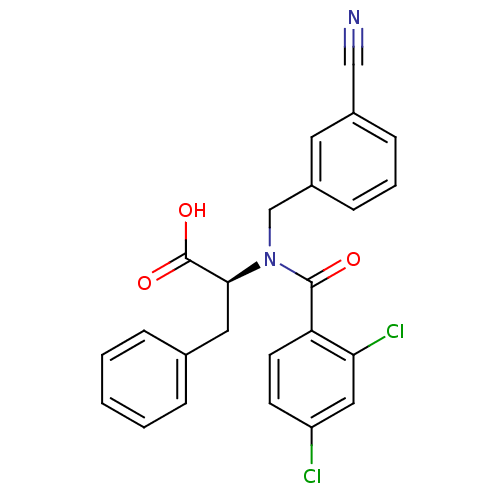
(2-[(3-Cyano-benzyl)-(2,4-dichloro-benzoyl)-amino]-...)Show SMILES OC(=O)[C@H](Cc1ccccc1)N(Cc1cccc(c1)C#N)C(=O)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C24H18Cl2N2O3/c25-19-9-10-20(21(26)13-19)23(29)28(15-18-8-4-7-17(11-18)14-27)22(24(30)31)12-16-5-2-1-3-6-16/h1-11,13,22H,12,15H2,(H,30,31) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hepatitis C RNA dependent RNA polymerase Nonstructural protein 5B (NS5B polymerase) expressed from baculovirus-infected S... |
J Med Chem 46: 1283-5 (2003)
Article DOI: 10.1021/jm0340400
BindingDB Entry DOI: 10.7270/Q2XP74BP |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50139657

(3-(2,4-Dimethyl-benzenesulfonylamino)-5-phenyl-thi...)Show SMILES Cc1ccc(c(C)c1)S(=O)(=O)Nc1cc(sc1C(O)=O)-c1ccccc1 Show InChI InChI=1S/C19H17NO4S2/c1-12-8-9-17(13(2)10-12)26(23,24)20-15-11-16(25-18(15)19(21)22)14-6-4-3-5-7-14/h3-11,20H,1-2H3,(H,21,22) | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS5B polymerase |
Bioorg Med Chem Lett 14: 793-6 (2004)
BindingDB Entry DOI: 10.7270/Q2PV6JSN |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50139657

(3-(2,4-Dimethyl-benzenesulfonylamino)-5-phenyl-thi...)Show SMILES Cc1ccc(c(C)c1)S(=O)(=O)Nc1cc(sc1C(O)=O)-c1ccccc1 Show InChI InChI=1S/C19H17NO4S2/c1-12-8-9-17(13(2)10-12)26(23,24)20-15-11-16(25-18(15)19(21)22)14-6-4-3-5-7-14/h3-11,20H,1-2H3,(H,21,22) | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Hepatitis C polymerase. |
Bioorg Med Chem Lett 14: 797-800 (2004)
BindingDB Entry DOI: 10.7270/Q2K35T2N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data