 Found 163 hits with Last Name = 'reiser' and Initial = 'g'
Found 163 hits with Last Name = 'reiser' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019296
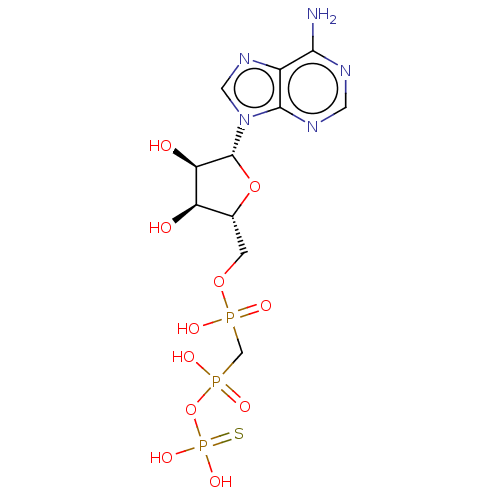
(CHEMBL3289396)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(=O)OP(O)(O)=S)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H18N5O11P3S/c12-9-6-10(14-2-13-9)16(3-15-6)11-8(18)7(17)5(26-11)1-25-28(19,20)4-29(21,22)27-30(23,24)31/h2-3,5,7-8,11,17-18H,1,4H2,(H,19,20)(H,21,22)(H2,12,13,14)(H2,23,24,31)/t5-,7-,8-,11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019294
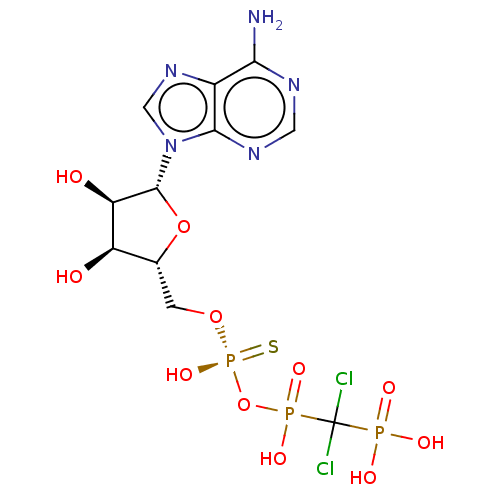
(CHEMBL3289394)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@](O)(=S)OP(O)(=O)C(Cl)(Cl)P(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H16Cl2N5O11P3S/c12-11(13,30(21,22)23)31(24,25)29-32(26,33)27-1-4-6(19)7(20)10(28-4)18-3-17-5-8(14)15-2-16-9(5)18/h2-4,6-7,10,19-20H,1H2,(H,24,25)(H,26,33)(H2,14,15,16)(H2,21,22,23)/t4-,6-,7-,10-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 685 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019292
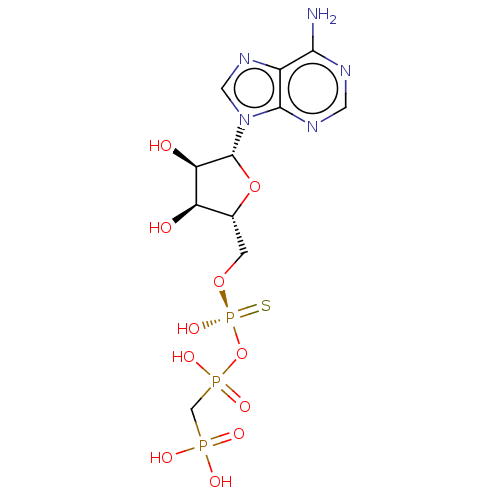
(CHEMBL3289393)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=S)OP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H18N5O11P3S/c12-9-6-10(14-2-13-9)16(3-15-6)11-8(18)7(17)5(26-11)1-25-30(24,31)27-29(22,23)4-28(19,20)21/h2-3,5,7-8,11,17-18H,1,4H2,(H,22,23)(H,24,31)(H2,12,13,14)(H2,19,20,21)/t5-,7-,8-,11-,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019291
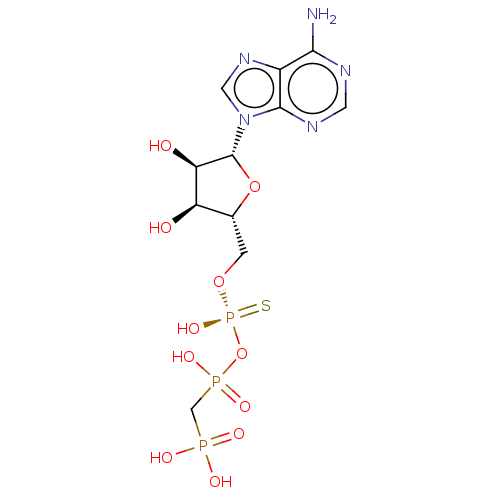
(CHEMBL3289392)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@](O)(=S)OP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H18N5O11P3S/c12-9-6-10(14-2-13-9)16(3-15-6)11-8(18)7(17)5(26-11)1-25-30(24,31)27-29(22,23)4-28(19,20)21/h2-3,5,7-8,11,17-18H,1,4H2,(H,22,23)(H,24,31)(H2,12,13,14)(H2,19,20,21)/t5-,7-,8-,11-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate assessed as dissociation constant for enzyme-inhibitor-substrate complex after 20 mins... |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019291

(CHEMBL3289392)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@](O)(=S)OP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H18N5O11P3S/c12-9-6-10(14-2-13-9)16(3-15-6)11-8(18)7(17)5(26-11)1-25-30(24,31)27-29(22,23)4-28(19,20)21/h2-3,5,7-8,11,17-18H,1,4H2,(H,22,23)(H,24,31)(H2,12,13,14)(H2,19,20,21)/t5-,7-,8-,11-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50347446
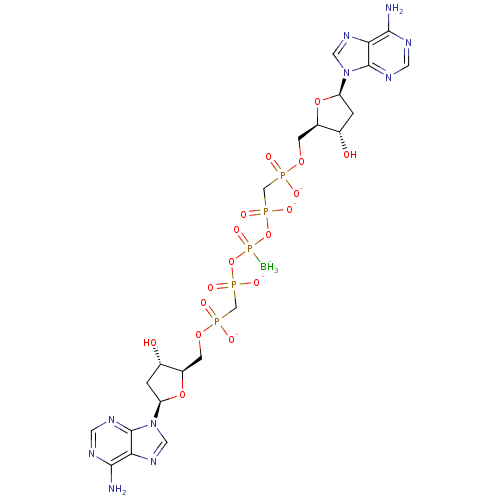
(CHEMBL1802095)Show SMILES [BH3-]P(=O)(OP([O-])(=O)CP([O-])(=O)OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2c(N)ncnc12)OP([O-])(=O)CP([O-])(=O)OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C22H35BN10O17P5/c23-55(44,49-53(40,41)9-51(36,37)45-3-13-11(34)1-15(47-13)32-7-30-17-19(24)26-5-28-21(17)32)50-54(42,43)10-52(38,39)46-4-14-12(35)2-16(48-14)33-8-31-18-20(25)27-6-29-22(18)33/h5-8,11-16,34-35H,1-4,9-10H2,23H3,(H,36,37)(H,38,39)(H,40,41)(H,42,43)(H2,24,26,28)(H2,25,27,29)/q-1/p-4/t11-,12-,13+,14+,15+,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 expressed in COS7 cells assessed as production of p-nitrophenol using pnp-TMP as the substrate after 15 mins by malachite gr... |
J Med Chem 53: 8485-97 (2010)
Article DOI: 10.1021/jm100597c
BindingDB Entry DOI: 10.7270/Q22V2GF7 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50347447
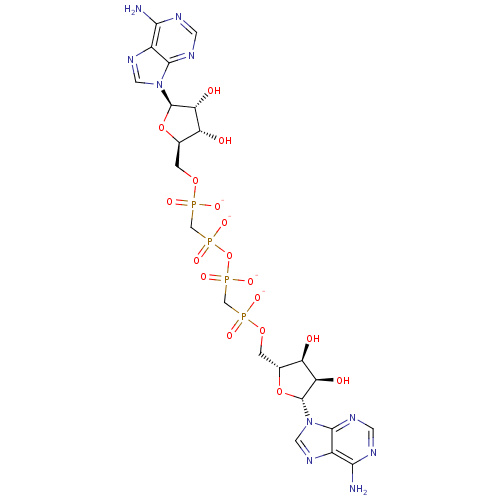
(CHEMBL1802096)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP([O-])(=O)CP([O-])(=O)OP([O-])(=O)CP([O-])(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H32N10O17P4/c23-17-11-19(27-3-25-17)31(5-29-11)21-15(35)13(33)9(47-21)1-45-50(37,38)7-52(41,42)49-53(43,44)8-51(39,40)46-2-10-14(34)16(36)22(48-10)32-6-30-12-18(24)26-4-28-20(12)32/h3-6,9-10,13-16,21-22,33-36H,1-2,7-8H2,(H,37,38)(H,39,40)(H,41,42)(H,43,44)(H2,23,25,27)(H2,24,26,28)/p-4/t9-,10-,13-,14-,15-,16-,21-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 expressed in COS7 cells assessed as production of p-nitrophenol using pnp-TMP as the substrate after 15 mins by malachite gr... |
J Med Chem 53: 8485-97 (2010)
Article DOI: 10.1021/jm100597c
BindingDB Entry DOI: 10.7270/Q22V2GF7 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019295

(CHEMBL3289395)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=S)OP(O)(=O)C(Cl)(Cl)P(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H16Cl2N5O11P3S/c12-11(13,30(21,22)23)31(24,25)29-32(26,33)27-1-4-6(19)7(20)10(28-4)18-3-17-5-8(14)15-2-16-9(5)18/h2-4,6-7,10,19-20H,1H2,(H,24,25)(H,26,33)(H2,14,15,16)(H2,21,22,23)/t4-,6-,7-,10-,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50347445

(CHEMBL1802094)Show SMILES [BH3-]P(=O)(OP([O-])(=O)CP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)OP([O-])(=O)CP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C22H35BN10O19P5/c23-57(46,51-55(42,43)7-53(38,39)47-1-9-13(34)15(36)21(49-9)32-5-30-11-17(24)26-3-28-19(11)32)52-56(44,45)8-54(40,41)48-2-10-14(35)16(37)22(50-10)33-6-31-12-18(25)27-4-29-20(12)33/h3-6,9-10,13-16,21-22,34-37H,1-2,7-8H2,23H3,(H,38,39)(H,40,41)(H,42,43)(H,44,45)(H2,24,26,28)(H2,25,27,29)/q-1/p-4/t9-,10-,13-,14-,15-,16-,21-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 expressed in COS7 cells assessed as production of p-nitrophenol using pnp-TMP as the substrate after 15 mins by malachite gr... |
J Med Chem 53: 8485-97 (2010)
Article DOI: 10.1021/jm100597c
BindingDB Entry DOI: 10.7270/Q22V2GF7 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50347445

(CHEMBL1802094)Show SMILES [BH3-]P(=O)(OP([O-])(=O)CP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)OP([O-])(=O)CP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C22H35BN10O19P5/c23-57(46,51-55(42,43)7-53(38,39)47-1-9-13(34)15(36)21(49-9)32-5-30-11-17(24)26-3-28-19(11)32)52-56(44,45)8-54(40,41)48-2-10-14(35)16(37)22(50-10)33-6-31-12-18(25)27-4-29-20(12)33/h3-6,9-10,13-16,21-22,34-37H,1-2,7-8H2,23H3,(H,38,39)(H,40,41)(H,42,43)(H,44,45)(H2,24,26,28)(H2,25,27,29)/q-1/p-4/t9-,10-,13-,14-,15-,16-,21-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 expressed in COS7 cells assessed as enzyme-substrate-inhibitor dissociation constant using pnp-TMP as the substrate after 15... |
J Med Chem 53: 8485-97 (2010)
Article DOI: 10.1021/jm100597c
BindingDB Entry DOI: 10.7270/Q22V2GF7 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50347448
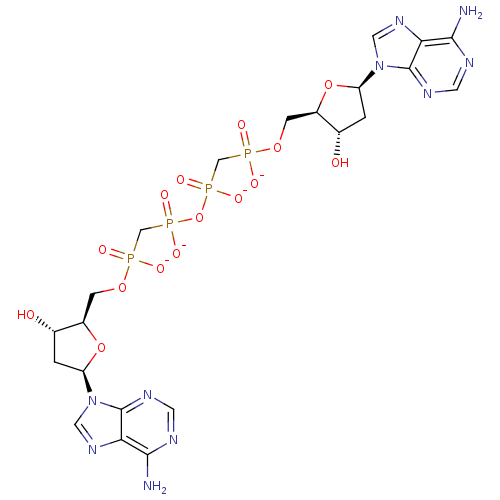
(CHEMBL1802097)Show SMILES Nc1ncnc2n(cnc12)[C@H]1C[C@H](O)[C@@H](COP([O-])(=O)CP([O-])(=O)OP([O-])(=O)CP([O-])(=O)OC[C@H]2O[C@H](C[C@@H]2O)n2cnc3c(N)ncnc23)O1 |r| Show InChI InChI=1S/C22H32N10O15P4/c23-19-17-21(27-5-25-19)31(7-29-17)15-1-11(33)13(45-15)3-43-48(35,36)9-50(39,40)47-51(41,42)10-49(37,38)44-4-14-12(34)2-16(46-14)32-8-30-18-20(24)26-6-28-22(18)32/h5-8,11-16,33-34H,1-4,9-10H2,(H,35,36)(H,37,38)(H,39,40)(H,41,42)(H2,23,25,27)(H2,24,26,28)/p-4/t11-,12-,13+,14+,15+,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 expressed in COS7 cells assessed as production of p-nitrophenol using pnp-TMP as the substrate after 15 mins by malachite gr... |
J Med Chem 53: 8485-97 (2010)
Article DOI: 10.1021/jm100597c
BindingDB Entry DOI: 10.7270/Q22V2GF7 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50347448

(CHEMBL1802097)Show SMILES Nc1ncnc2n(cnc12)[C@H]1C[C@H](O)[C@@H](COP([O-])(=O)CP([O-])(=O)OP([O-])(=O)CP([O-])(=O)OC[C@H]2O[C@H](C[C@@H]2O)n2cnc3c(N)ncnc23)O1 |r| Show InChI InChI=1S/C22H32N10O15P4/c23-19-17-21(27-5-25-19)31(7-29-17)15-1-11(33)13(45-15)3-43-48(35,36)9-50(39,40)47-51(41,42)10-49(37,38)44-4-14-12(34)2-16(46-14)32-8-30-18-20(24)26-6-28-22(18)32/h5-8,11-16,33-34H,1-4,9-10H2,(H,35,36)(H,37,38)(H,39,40)(H,41,42)(H2,23,25,27)(H2,24,26,28)/p-4/t11-,12-,13+,14+,15+,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 expressed in COS7 cells assessed as enzyme-substrate-inhibitor dissociation constant using pnp-TMP as the substrate after 15... |
J Med Chem 53: 8485-97 (2010)
Article DOI: 10.1021/jm100597c
BindingDB Entry DOI: 10.7270/Q22V2GF7 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50347446

(CHEMBL1802095)Show SMILES [BH3-]P(=O)(OP([O-])(=O)CP([O-])(=O)OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2c(N)ncnc12)OP([O-])(=O)CP([O-])(=O)OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C22H35BN10O17P5/c23-55(44,49-53(40,41)9-51(36,37)45-3-13-11(34)1-15(47-13)32-7-30-17-19(24)26-5-28-21(17)32)50-54(42,43)10-52(38,39)46-4-14-12(35)2-16(48-14)33-8-31-18-20(25)27-6-29-22(18)33/h5-8,11-16,34-35H,1-4,9-10H2,23H3,(H,36,37)(H,38,39)(H,40,41)(H,42,43)(H2,24,26,28)(H2,25,27,29)/q-1/p-4/t11-,12-,13+,14+,15+,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 expressed in COS7 cells assessed as enzyme-substrate-inhibitor dissociation constant using pnp-TMP as the substrate after 15... |
J Med Chem 53: 8485-97 (2010)
Article DOI: 10.1021/jm100597c
BindingDB Entry DOI: 10.7270/Q22V2GF7 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50347447

(CHEMBL1802096)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP([O-])(=O)CP([O-])(=O)OP([O-])(=O)CP([O-])(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H32N10O17P4/c23-17-11-19(27-3-25-17)31(5-29-11)21-15(35)13(33)9(47-21)1-45-50(37,38)7-52(41,42)49-53(43,44)8-51(39,40)46-2-10-14(34)16(36)22(48-10)32-6-30-12-18(24)26-4-28-20(12)32/h3-6,9-10,13-16,21-22,33-36H,1-2,7-8H2,(H,37,38)(H,39,40)(H,41,42)(H,43,44)(H2,23,25,27)(H2,24,26,28)/p-4/t9-,10-,13-,14-,15-,16-,21-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.47E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 expressed in COS7 cells assessed as enzyme-substrate-inhibitor dissociation constant using pnp-TMP as the substrate after 15... |
J Med Chem 53: 8485-97 (2010)
Article DOI: 10.1021/jm100597c
BindingDB Entry DOI: 10.7270/Q22V2GF7 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019295

(CHEMBL3289395)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=S)OP(O)(=O)C(Cl)(Cl)P(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H16Cl2N5O11P3S/c12-11(13,30(21,22)23)31(24,25)29-32(26,33)27-1-4-6(19)7(20)10(28-4)18-3-17-5-8(14)15-2-16-9(5)18/h2-4,6-7,10,19-20H,1H2,(H,24,25)(H,26,33)(H2,14,15,16)(H2,21,22,23)/t4-,6-,7-,10-,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate assessed as dissociation constant for enzyme-inhibitor-substrate complex after 20 mins... |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Inositol 1,4,5-trisphosphate receptor type 3
(Homo sapiens (Human)) | BDBM50075651
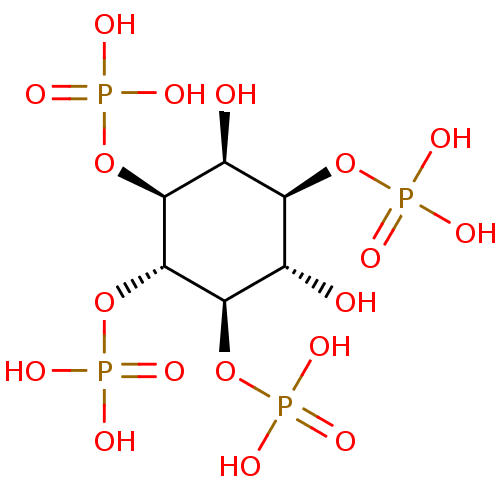
(1D-myo-inositol 1,3,4,5-tetrakis(dihydrogen phosph...)Show SMILES O[C@H]1[C@@H](OP(O)(O)=O)[C@H](O)[C@@H](OP(O)(O)=O)[C@H](OP(O)(O)=O)[C@H]1OP(O)(O)=O Show InChI InChI=1S/C6H16O18P4/c7-1-3(21-25(9,10)11)2(8)5(23-27(15,16)17)6(24-28(18,19)20)4(1)22-26(12,13)14/h1-8H,(H2,9,10,11)(H2,12,13,14)(H2,15,16,17)(H2,18,19,20)/t1-,2-,3-,4+,5-,6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bergische Universität Wuppertal
Curated by ChEMBL
| Assay Description
Inhibition of Ins(1,3,4,5)P4-PtdIns(3,4,5)P3-specific receptor from Pig cerebellum membrane |
J Med Chem 42: 1262-73 (1999)
Article DOI: 10.1021/jm981113k
BindingDB Entry DOI: 10.7270/Q2CF9P8S |
More data for this
Ligand-Target Pair | |
Inositol 1,4,5-trisphosphate receptor type 3
(Homo sapiens (Human)) | BDBM50075647
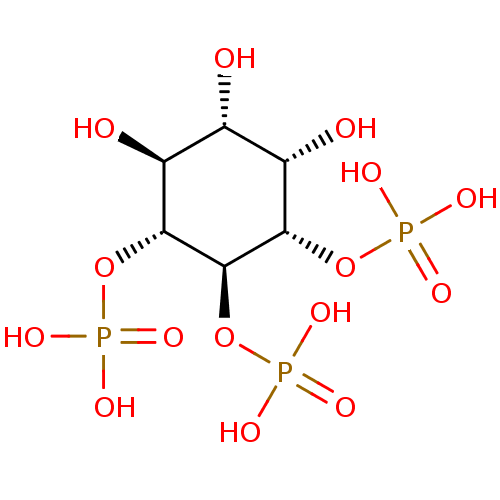
(CHEMBL283791 | Phosphoric acid mono-((1R,2S,3R,4S,...)Show SMILES O[C@@H]1[C@H](O)[C@H](OP(O)(O)=O)[C@@H](OP(O)(O)=O)[C@H](OP(O)(O)=O)[C@H]1O Show InChI InChI=1S/C6H15O15P3/c7-1-2(8)4(19-22(10,11)12)6(21-24(16,17)18)5(3(1)9)20-23(13,14)15/h1-9H,(H2,10,11,12)(H2,13,14,15)(H2,16,17,18)/t1-,2-,3-,4+,5-,6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bergische Universität Wuppertal
Curated by ChEMBL
| Assay Description
Inhibition of Ins(1,3,4,5)P4-PtdIns(3,4,5)P3-specific receptor from Pig cerebellum membrane |
J Med Chem 42: 1262-73 (1999)
Article DOI: 10.1021/jm981113k
BindingDB Entry DOI: 10.7270/Q2CF9P8S |
More data for this
Ligand-Target Pair | |
Inositol 1,4,5-trisphosphate receptor type 3
(Homo sapiens (Human)) | BDBM50075648
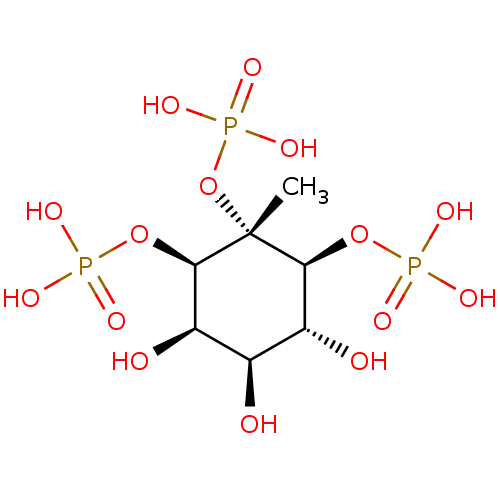
((1R,3S,4R,6R)-4,5,6-trihydroxy-2-methyl-2,3-bis(ph...)Show SMILES C[C@@]1(OP(O)(O)=O)[C@@H](OP(O)(O)=O)[C@H](O)[C@@H](O)[C@@H](O)[C@H]1OP(O)(O)=O Show InChI InChI=1S/C7H17O15P3/c1-7(22-25(17,18)19)5(20-23(11,12)13)3(9)2(8)4(10)6(7)21-24(14,15)16/h2-6,8-10H,1H3,(H2,11,12,13)(H2,14,15,16)(H2,17,18,19)/t2-,3-,4-,5+,6-,7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Bergische Universität Wuppertal
Curated by ChEMBL
| Assay Description
Inhibition of Ins(1,3,4,5)P4-PtdIns(3,4,5)P3-specific receptor from Pig cerebellum membrane |
J Med Chem 42: 1262-73 (1999)
Article DOI: 10.1021/jm981113k
BindingDB Entry DOI: 10.7270/Q2CF9P8S |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019296

(CHEMBL3289396)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(=O)OP(O)(O)=S)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H18N5O11P3S/c12-9-6-10(14-2-13-9)16(3-15-6)11-8(18)7(17)5(26-11)1-25-28(19,20)4-29(21,22)27-30(23,24)31/h2-3,5,7-8,11,17-18H,1,4H2,(H,19,20)(H,21,22)(H2,12,13,14)(H2,23,24,31)/t5-,7-,8-,11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Inositol 1,4,5-trisphosphate receptor type 3
(Homo sapiens (Human)) | BDBM50075649
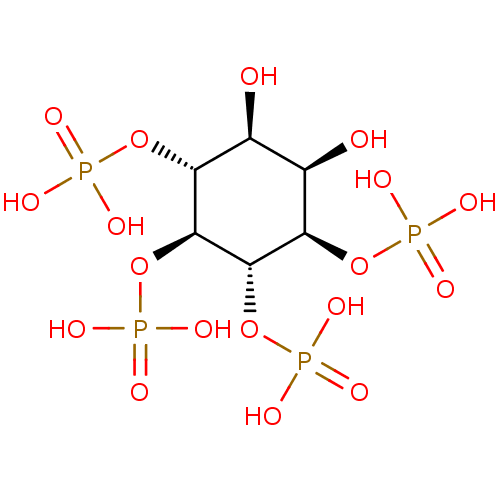
(1D-myo-inositol 1,4,5,6-tetrakis(dihydrogen phosph...)Show SMILES O[C@H]1[C@@H](O)[C@@H](OP(O)(O)=O)[C@H](OP(O)(O)=O)[C@@H](OP(O)(O)=O)[C@@H]1OP(O)(O)=O Show InChI InChI=1S/C6H16O18P4/c7-1-2(8)4(22-26(12,13)14)6(24-28(18,19)20)5(23-27(15,16)17)3(1)21-25(9,10)11/h1-8H,(H2,9,10,11)(H2,12,13,14)(H2,15,16,17)(H2,18,19,20)/t1-,2+,3-,4-,5+,6+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Bergische Universität Wuppertal
Curated by ChEMBL
| Assay Description
Inhibition of Ins(1,3,4,5)P4-PtdIns(3,4,5)P3-specific receptor from Pig cerebellum membrane |
J Med Chem 42: 1262-73 (1999)
Article DOI: 10.1021/jm981113k
BindingDB Entry DOI: 10.7270/Q2CF9P8S |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019294

(CHEMBL3289394)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@](O)(=S)OP(O)(=O)C(Cl)(Cl)P(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H16Cl2N5O11P3S/c12-11(13,30(21,22)23)31(24,25)29-32(26,33)27-1-4-6(19)7(20)10(28-4)18-3-17-5-8(14)15-2-16-9(5)18/h2-4,6-7,10,19-20H,1H2,(H,24,25)(H,26,33)(H2,14,15,16)(H2,21,22,23)/t4-,6-,7-,10-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Inositol 1,4,5-trisphosphate receptor type 3
(Homo sapiens (Human)) | BDBM50075650
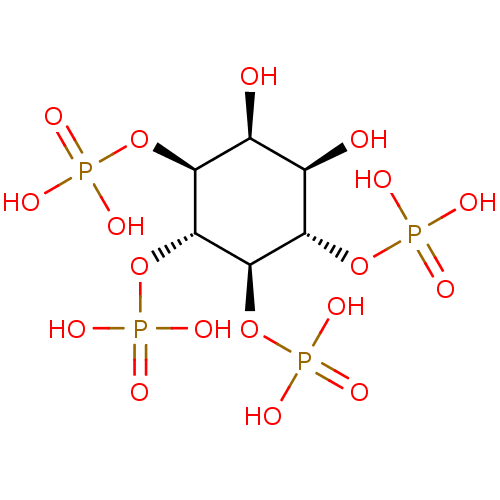
(1D-myo-inositol 3,4,5,6-tetrakisphosphate | 1L-myo...)Show SMILES O[C@H]1[C@@H](O)[C@H](OP(O)(O)=O)[C@@H](OP(O)(O)=O)[C@H](OP(O)(O)=O)[C@H]1OP(O)(O)=O Show InChI InChI=1S/C6H16O18P4/c7-1-2(8)4(22-26(12,13)14)6(24-28(18,19)20)5(23-27(15,16)17)3(1)21-25(9,10)11/h1-8H,(H2,9,10,11)(H2,12,13,14)(H2,15,16,17)(H2,18,19,20)/t1-,2+,3-,4-,5+,6+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Bergische Universität Wuppertal
Curated by ChEMBL
| Assay Description
Inhibition of Ins(1,3,4,5)P4-PtdIns(3,4,5)P3-specific receptor from Pig cerebellum membrane |
J Med Chem 42: 1262-73 (1999)
Article DOI: 10.1021/jm981113k
BindingDB Entry DOI: 10.7270/Q2CF9P8S |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50347446

(CHEMBL1802095)Show SMILES [BH3-]P(=O)(OP([O-])(=O)CP([O-])(=O)OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2c(N)ncnc12)OP([O-])(=O)CP([O-])(=O)OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C22H35BN10O17P5/c23-55(44,49-53(40,41)9-51(36,37)45-3-13-11(34)1-15(47-13)32-7-30-17-19(24)26-5-28-21(17)32)50-54(42,43)10-52(38,39)46-4-14-12(35)2-16(48-14)33-8-31-18-20(25)27-6-29-22(18)33/h5-8,11-16,34-35H,1-4,9-10H2,23H3,(H,36,37)(H,38,39)(H,40,41)(H,42,43)(H2,24,26,28)(H2,25,27,29)/q-1/p-4/t11-,12-,13+,14+,15+,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 expressed in COS7 cells assessed as production of p-nitrophenol using pnp-TMP as the substrate after 15 mins by malachite gr... |
J Med Chem 53: 8485-97 (2010)
Article DOI: 10.1021/jm100597c
BindingDB Entry DOI: 10.7270/Q22V2GF7 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019291

(CHEMBL3289392)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@](O)(=S)OP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H18N5O11P3S/c12-9-6-10(14-2-13-9)16(3-15-6)11-8(18)7(17)5(26-11)1-25-30(24,31)27-29(22,23)4-28(19,20)21/h2-3,5,7-8,11,17-18H,1,4H2,(H,22,23)(H,24,31)(H2,12,13,14)(H2,19,20,21)/t5-,7-,8-,11-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019292

(CHEMBL3289393)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=S)OP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H18N5O11P3S/c12-9-6-10(14-2-13-9)16(3-15-6)11-8(18)7(17)5(26-11)1-25-30(24,31)27-29(22,23)4-28(19,20)21/h2-3,5,7-8,11,17-18H,1,4H2,(H,22,23)(H,24,31)(H2,12,13,14)(H2,19,20,21)/t5-,7-,8-,11-,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019295

(CHEMBL3289395)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=S)OP(O)(=O)C(Cl)(Cl)P(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H16Cl2N5O11P3S/c12-11(13,30(21,22)23)31(24,25)29-32(26,33)27-1-4-6(19)7(20)10(28-4)18-3-17-5-8(14)15-2-16-9(5)18/h2-4,6-7,10,19-20H,1H2,(H,24,25)(H,26,33)(H2,14,15,16)(H2,21,22,23)/t4-,6-,7-,10-,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50347447

(CHEMBL1802096)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP([O-])(=O)CP([O-])(=O)OP([O-])(=O)CP([O-])(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H32N10O17P4/c23-17-11-19(27-3-25-17)31(5-29-11)21-15(35)13(33)9(47-21)1-45-50(37,38)7-52(41,42)49-53(43,44)8-51(39,40)46-2-10-14(34)16(36)22(48-10)32-6-30-12-18(24)26-4-28-20(12)32/h3-6,9-10,13-16,21-22,33-36H,1-2,7-8H2,(H,37,38)(H,39,40)(H,41,42)(H,43,44)(H2,23,25,27)(H2,24,26,28)/p-4/t9-,10-,13-,14-,15-,16-,21-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 expressed in COS7 cells assessed as production of p-nitrophenol using pnp-TMP as the substrate after 15 mins by malachite gr... |
J Med Chem 53: 8485-97 (2010)
Article DOI: 10.1021/jm100597c
BindingDB Entry DOI: 10.7270/Q22V2GF7 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50347448

(CHEMBL1802097)Show SMILES Nc1ncnc2n(cnc12)[C@H]1C[C@H](O)[C@@H](COP([O-])(=O)CP([O-])(=O)OP([O-])(=O)CP([O-])(=O)OC[C@H]2O[C@H](C[C@@H]2O)n2cnc3c(N)ncnc23)O1 |r| Show InChI InChI=1S/C22H32N10O15P4/c23-19-17-21(27-5-25-19)31(7-29-17)15-1-11(33)13(45-15)3-43-48(35,36)9-50(39,40)47-51(41,42)10-49(37,38)44-4-14-12(34)2-16(46-14)32-8-30-18-20(24)26-6-28-22(18)32/h5-8,11-16,33-34H,1-4,9-10H2,(H,35,36)(H,37,38)(H,39,40)(H,41,42)(H2,23,25,27)(H2,24,26,28)/p-4/t11-,12-,13+,14+,15+,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 expressed in COS7 cells assessed as production of p-nitrophenol using pnp-TMP as the substrate after 15 mins by malachite gr... |
J Med Chem 53: 8485-97 (2010)
Article DOI: 10.1021/jm100597c
BindingDB Entry DOI: 10.7270/Q22V2GF7 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50347445

(CHEMBL1802094)Show SMILES [BH3-]P(=O)(OP([O-])(=O)CP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)OP([O-])(=O)CP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C22H35BN10O19P5/c23-57(46,51-55(42,43)7-53(38,39)47-1-9-13(34)15(36)21(49-9)32-5-30-11-17(24)26-3-28-19(11)32)52-56(44,45)8-54(40,41)48-2-10-14(35)16(37)22(50-10)33-6-31-12-18(25)27-4-29-20(12)33/h3-6,9-10,13-16,21-22,34-37H,1-2,7-8H2,23H3,(H,38,39)(H,40,41)(H,42,43)(H,44,45)(H2,24,26,28)(H2,25,27,29)/q-1/p-4/t9-,10-,13-,14-,15-,16-,21-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 expressed in COS7 cells assessed as production of p-nitrophenol using pnp-TMP as the substrate after 15 mins by malachite gr... |
J Med Chem 53: 8485-97 (2010)
Article DOI: 10.1021/jm100597c
BindingDB Entry DOI: 10.7270/Q22V2GF7 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50366480
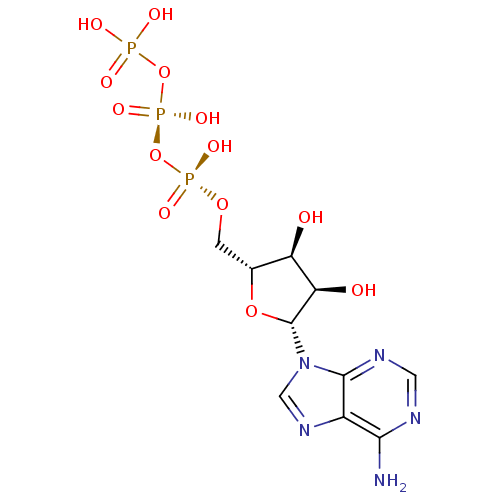
(ADENOSINE TRIPHOSPHATE | ATP)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@](O)(=O)O[P@@](O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H16N5O13P3/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(26-10)1-25-30(21,22)28-31(23,24)27-29(18,19)20/h2-4,6-7,10,16-17H,1H2,(H,21,22)(H,23,24)(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 850 | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Agonist activity at human P2Y1 receptor expressed in human 1321N1 cells assessed as increase of intracellular calcium level after 30 mins using fura-... |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 11
(Homo sapiens (Human)) | BDBM50019292

(CHEMBL3289393)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=S)OP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H18N5O11P3S/c12-9-6-10(14-2-13-9)16(3-15-6)11-8(18)7(17)5(26-11)1-25-30(24,31)27-29(22,23)4-28(19,20)21/h2-3,5,7-8,11,17-18H,1,4H2,(H,22,23)(H,24,31)(H2,12,13,14)(H2,19,20,21)/t5-,7-,8-,11-,30-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Agonist activity at human P2Y11 receptor expressed in human 1321N1 cells assessed as increase of intracellular calcium level after 30 mins using fura... |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 11
(Homo sapiens (Human)) | BDBM50131064
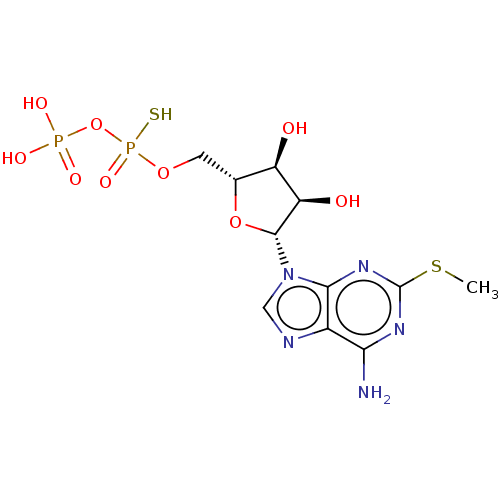
(CHEMBL3634182)Show SMILES CSc1nc(N)c2ncn([C@@H]3O[C@H](COP(S)(=O)OP(O)(O)=O)[C@@H](O)[C@H]3O)c2n1 |r| Show InChI InChI=1S/C11H17N5O9P2S2/c1-29-11-14-8(12)5-9(15-11)16(3-13-5)10-7(18)6(17)4(24-10)2-23-27(22,28)25-26(19,20)21/h3-4,6-7,10,17-18H,2H2,1H3,(H,22,28)(H2,12,14,15)(H2,19,20,21)/t4-,6-,7-,10-,27?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-U-69,593 binding to Opioid receptor kappa 1 |
J Med Chem 58: 8427-43 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00575
BindingDB Entry DOI: 10.7270/Q2736SRG |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50131062
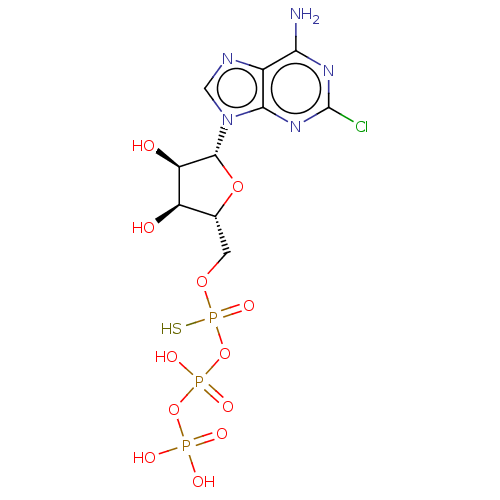
(CHEMBL3634184)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1O[C@H](COP(S)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H15ClN5O12P3S/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(18)5(17)3(26-9)1-25-31(24,32)28-30(22,23)27-29(19,20)21/h2-3,5-6,9,17-18H,1H2,(H,22,23)(H,24,32)(H2,12,14,15)(H2,19,20,21)/t3-,5-,6-,9-,31?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 880 | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Cathepsin B activity |
J Med Chem 58: 8427-43 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00575
BindingDB Entry DOI: 10.7270/Q2736SRG |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 11
(Homo sapiens (Human)) | BDBM50131063

(CHEMBL3634183)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1O[C@H](COP(S)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H14ClN5O9P2S/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(18)5(17)3(24-9)1-23-27(22,28)25-26(19,20)21/h2-3,5-6,9,17-18H,1H2,(H,22,28)(H2,12,14,15)(H2,19,20,21)/t3-,5-,6-,9-,27?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Cathepsin B activity |
J Med Chem 58: 8427-43 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00575
BindingDB Entry DOI: 10.7270/Q2736SRG |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50131059
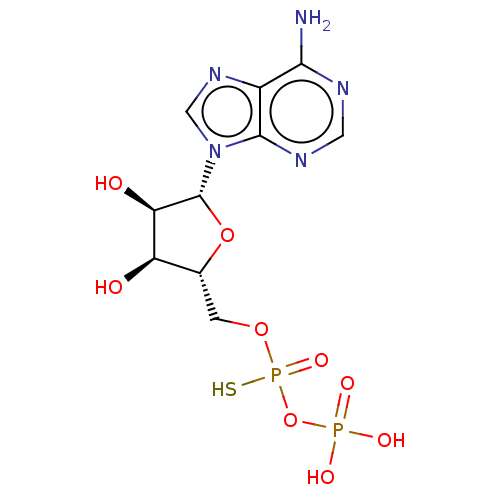
(CHEMBL575257)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(S)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H15N5O9P2S/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(23-10)1-22-26(21,27)24-25(18,19)20/h2-4,6-7,10,16-17H,1H2,(H,21,27)(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-,26?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Cathepsin B activity |
J Med Chem 58: 8427-43 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00575
BindingDB Entry DOI: 10.7270/Q2736SRG |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 11
(Homo sapiens (Human)) | BDBM50131059

(CHEMBL575257)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(S)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H15N5O9P2S/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(23-10)1-22-26(21,27)24-25(18,19)20/h2-4,6-7,10,16-17H,1H2,(H,21,27)(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-,26?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against interleukin-2 binding |
J Med Chem 58: 8427-43 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00575
BindingDB Entry DOI: 10.7270/Q2736SRG |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 11
(Homo sapiens (Human)) | BDBM50131062

(CHEMBL3634184)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1O[C@H](COP(S)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H15ClN5O12P3S/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(18)5(17)3(26-9)1-25-31(24,32)28-30(22,23)27-29(19,20)21/h2-3,5-6,9,17-18H,1H2,(H,22,23)(H,24,32)(H2,12,14,15)(H2,19,20,21)/t3-,5-,6-,9-,31?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against interleukin-2 binding |
J Med Chem 58: 8427-43 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00575
BindingDB Entry DOI: 10.7270/Q2736SRG |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50131063

(CHEMBL3634183)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1O[C@H](COP(S)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H14ClN5O9P2S/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(18)5(17)3(24-9)1-23-27(22,28)25-26(19,20)21/h2-3,5-6,9,17-18H,1H2,(H,22,28)(H2,12,14,15)(H2,19,20,21)/t3-,5-,6-,9-,27?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against interleukin-2 binding |
J Med Chem 58: 8427-43 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00575
BindingDB Entry DOI: 10.7270/Q2736SRG |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50131064

(CHEMBL3634182)Show SMILES CSc1nc(N)c2ncn([C@@H]3O[C@H](COP(S)(=O)OP(O)(O)=O)[C@@H](O)[C@H]3O)c2n1 |r| Show InChI InChI=1S/C11H17N5O9P2S2/c1-29-11-14-8(12)5-9(15-11)16(3-13-5)10-7(18)6(17)4(24-10)2-23-27(22,28)25-26(19,20)21/h3-4,6-7,10,17-18H,2H2,1H3,(H,22,28)(H2,12,14,15)(H2,19,20,21)/t4-,6-,7-,10-,27?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against interleukin-2 binding |
J Med Chem 58: 8427-43 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00575
BindingDB Entry DOI: 10.7270/Q2736SRG |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 11
(Homo sapiens (Human)) | BDBM50118230

(5'-O-[(R)-HYDROXY(THIOPHOSPHONOOXY)PHOSPHORYL]ADEN...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)OP(O)(S)=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H15N5O9P2S/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(23-10)1-22-25(18,19)24-26(20,21)27/h2-4,6-7,10,16-17H,1H2,(H,18,19)(H2,11,12,13)(H2,20,21,27)/t4-,6-,7-,10-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.27E+4 | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Agonist activity at GFP-tagged human P2Y11R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-bas... |
J Med Chem 58: 8427-43 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00575
BindingDB Entry DOI: 10.7270/Q2736SRG |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 11
(Homo sapiens (Human)) | BDBM50368125

(ADENOSINE DIPHOSPHATE | ADP)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H15N5O10P2/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(24-10)1-23-27(21,22)25-26(18,19)20/h2-4,6-7,10,16-17H,1H2,(H,21,22)(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Agonist activity at GFP-tagged human P2Y11R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-bas... |
J Med Chem 58: 8427-43 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00575
BindingDB Entry DOI: 10.7270/Q2736SRG |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 11
(Homo sapiens (Human)) | BDBM50366480

(ADENOSINE TRIPHOSPHATE | ATP)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@](O)(=O)O[P@@](O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H16N5O13P3/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(26-10)1-25-30(21,22)28-31(23,24)27-29(18,19)20/h2-4,6-7,10,16-17H,1H2,(H,21,22)(H,23,24)(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Agonist activity at GFP-tagged human P2Y11R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-bas... |
J Med Chem 58: 8427-43 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00575
BindingDB Entry DOI: 10.7270/Q2736SRG |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50131060
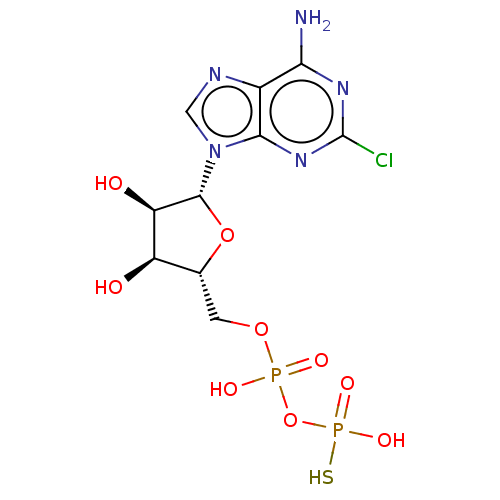
(CHEMBL3634186)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)OP(O)(S)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H14ClN5O9P2S/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(18)5(17)3(24-9)1-23-26(19,20)25-27(21,22)28/h2-3,5-6,9,17-18H,1H2,(H,19,20)(H2,12,14,15)(H2,21,22,28)/t3-,5-,6-,9-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Agonist activity at GFP-tagged human P2Y1R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-base... |
J Med Chem 58: 8427-43 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00575
BindingDB Entry DOI: 10.7270/Q2736SRG |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50131061
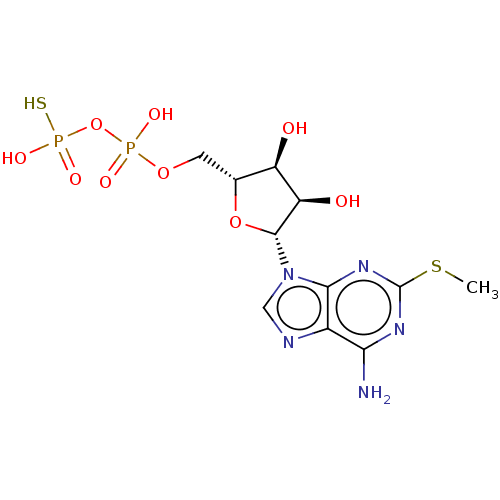
(CHEMBL3634185)Show SMILES CSc1nc(N)c2ncn([C@@H]3O[C@H](COP(O)(=O)OP(O)(S)=O)[C@@H](O)[C@H]3O)c2n1 |r| Show InChI InChI=1S/C11H17N5O9P2S2/c1-29-11-14-8(12)5-9(15-11)16(3-13-5)10-7(18)6(17)4(24-10)2-23-26(19,20)25-27(21,22)28/h3-4,6-7,10,17-18H,2H2,1H3,(H,19,20)(H2,12,14,15)(H2,21,22,28)/t4-,6-,7-,10-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Agonist activity at GFP-tagged human P2Y1R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-base... |
J Med Chem 58: 8427-43 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00575
BindingDB Entry DOI: 10.7270/Q2736SRG |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50131062

(CHEMBL3634184)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1O[C@H](COP(S)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H15ClN5O12P3S/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(18)5(17)3(26-9)1-25-31(24,32)28-30(22,23)27-29(19,20)21/h2-3,5-6,9,17-18H,1H2,(H,22,23)(H,24,32)(H2,12,14,15)(H2,19,20,21)/t3-,5-,6-,9-,31?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 880 | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Agonist activity at GFP-tagged human P2Y1R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-base... |
J Med Chem 58: 8427-43 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00575
BindingDB Entry DOI: 10.7270/Q2736SRG |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50131063

(CHEMBL3634183)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1O[C@H](COP(S)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H14ClN5O9P2S/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(18)5(17)3(24-9)1-23-27(22,28)25-26(19,20)21/h2-3,5-6,9,17-18H,1H2,(H,22,28)(H2,12,14,15)(H2,19,20,21)/t3-,5-,6-,9-,27?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Agonist activity at GFP-tagged human P2Y1R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-base... |
J Med Chem 58: 8427-43 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00575
BindingDB Entry DOI: 10.7270/Q2736SRG |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50131064

(CHEMBL3634182)Show SMILES CSc1nc(N)c2ncn([C@@H]3O[C@H](COP(S)(=O)OP(O)(O)=O)[C@@H](O)[C@H]3O)c2n1 |r| Show InChI InChI=1S/C11H17N5O9P2S2/c1-29-11-14-8(12)5-9(15-11)16(3-13-5)10-7(18)6(17)4(24-10)2-23-27(22,28)25-26(19,20)21/h3-4,6-7,10,17-18H,2H2,1H3,(H,22,28)(H2,12,14,15)(H2,19,20,21)/t4-,6-,7-,10-,27?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Agonist activity at GFP-tagged human P2Y1R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-base... |
J Med Chem 58: 8427-43 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00575
BindingDB Entry DOI: 10.7270/Q2736SRG |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50131059

(CHEMBL575257)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(S)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H15N5O9P2S/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(23-10)1-22-26(21,27)24-25(18,19)20/h2-4,6-7,10,16-17H,1H2,(H,21,27)(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-,26?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Agonist activity at GFP-tagged human P2Y1R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-base... |
J Med Chem 58: 8427-43 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00575
BindingDB Entry DOI: 10.7270/Q2736SRG |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50118230

(5'-O-[(R)-HYDROXY(THIOPHOSPHONOOXY)PHOSPHORYL]ADEN...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)OP(O)(S)=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H15N5O9P2S/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(23-10)1-22-25(18,19)24-26(20,21)27/h2-4,6-7,10,16-17H,1H2,(H,18,19)(H2,11,12,13)(H2,20,21,27)/t4-,6-,7-,10-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Agonist activity at GFP-tagged human P2Y1R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-base... |
J Med Chem 58: 8427-43 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00575
BindingDB Entry DOI: 10.7270/Q2736SRG |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50118242
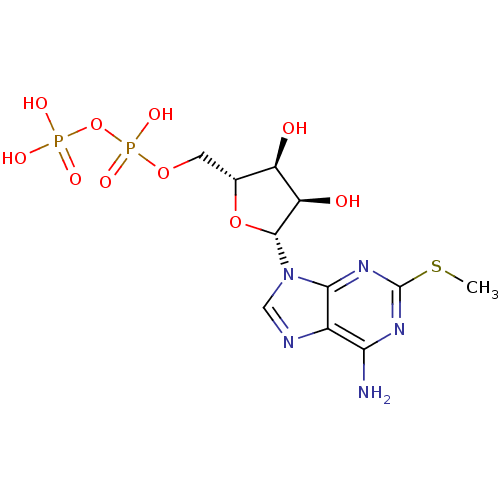
(((2R,3S,4R,5R)-5-(6-amino-2-(methylthio)-9H-purin-...)Show SMILES CSc1nc(N)c2ncn([C@@H]3O[C@H](COP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]3O)c2n1 |r| Show InChI InChI=1S/C11H17N5O10P2S/c1-29-11-14-8(12)5-9(15-11)16(3-13-5)10-7(18)6(17)4(25-10)2-24-28(22,23)26-27(19,20)21/h3-4,6-7,10,17-18H,2H2,1H3,(H,22,23)(H2,12,14,15)(H2,19,20,21)/t4-,6-,7-,10-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Agonist activity at GFP-tagged human P2Y1R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-base... |
J Med Chem 58: 8427-43 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00575
BindingDB Entry DOI: 10.7270/Q2736SRG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data