

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022671 (CHEMBL3298265) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified TrkA cytoplasmic domain (unknown origin) by HTRF assay | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50446392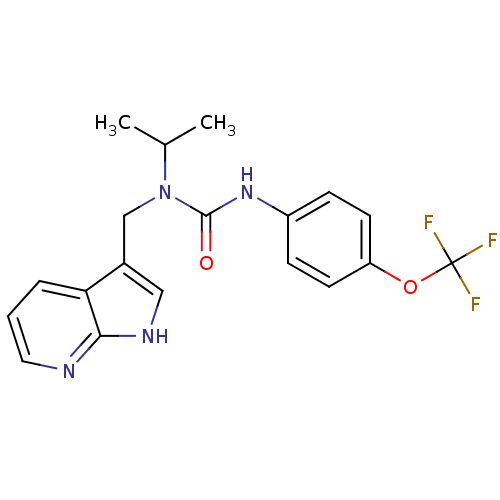 (CHEMBL3109645 | US9181261, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022675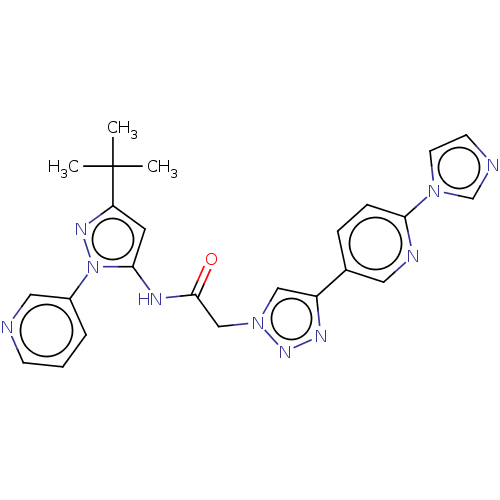 (CHEMBL3298268) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022674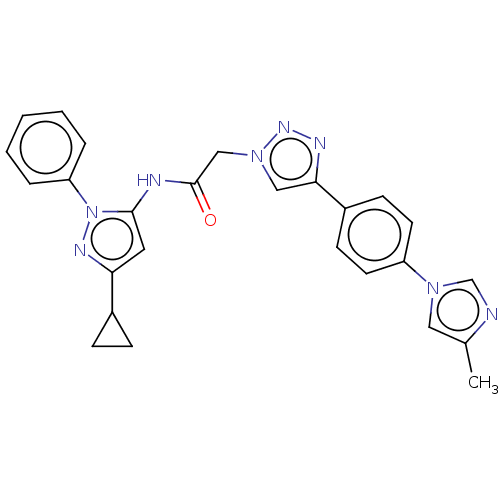 (CHEMBL3298267) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022675 (CHEMBL3298268) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified TrkA cytoplasmic domain (unknown origin) by HTRF assay | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022670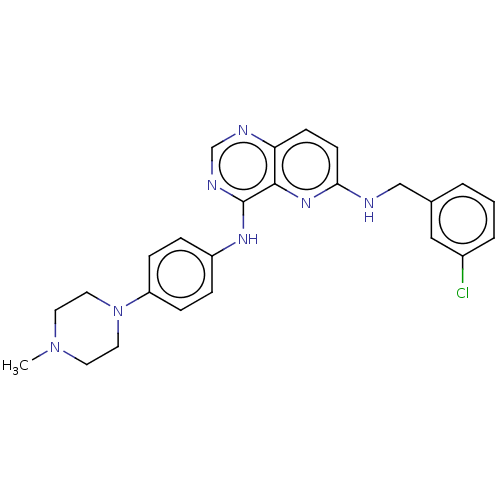 (CHEMBL3297748) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified TrkA cytoplasmic domain (unknown origin) by HTRF assay | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50446392 (CHEMBL3109645 | US9181261, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of phsophorylated TrkA (unknown origin) assessed as inhibition of fluorescently-labelled substrate phosphorylation by CALIPER enzymatic as... | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022671 (CHEMBL3298265) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of phsophorylated TrkA (unknown origin) assessed as inhibition of fluorescently-labelled substrate phosphorylation by CALIPER enzymatic as... | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022720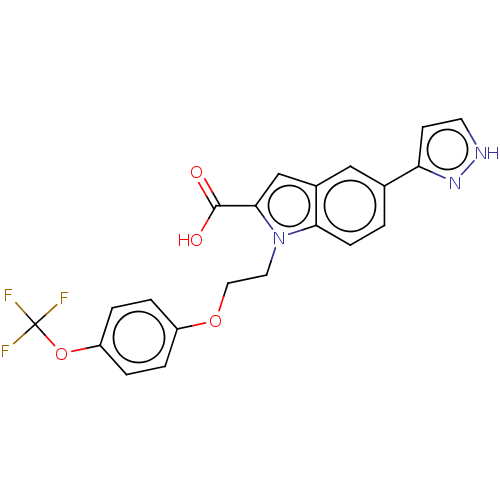 (CHEMBL3298161) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50231986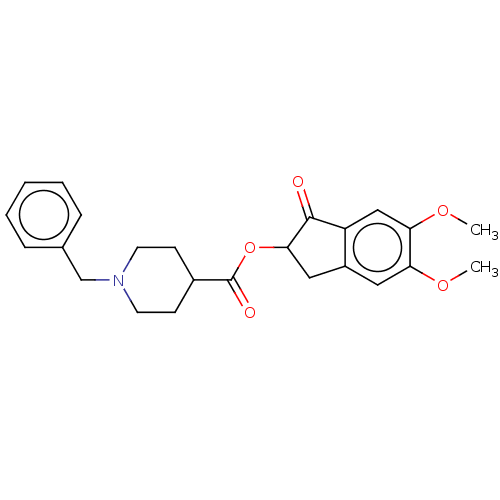 (CHEMBL4083213) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 127: 671-690 (2017) Article DOI: 10.1016/j.ejmech.2016.10.036 BindingDB Entry DOI: 10.7270/Q2P84F48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022672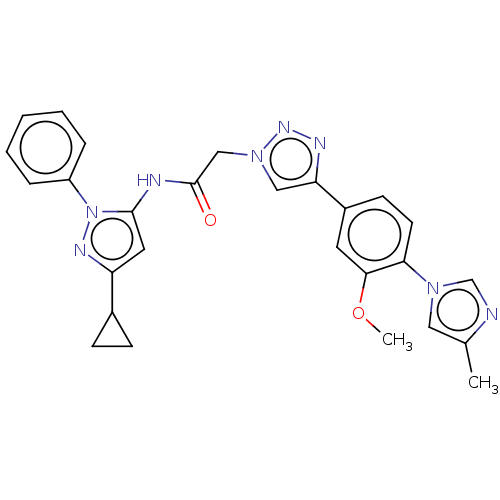 (CHEMBL3298266) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 127: 671-690 (2017) Article DOI: 10.1016/j.ejmech.2016.10.036 BindingDB Entry DOI: 10.7270/Q2P84F48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022720 (CHEMBL3298161) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of phsophorylated TrkA (unknown origin) assessed as inhibition of fluorescently-labelled substrate phosphorylation by CALIPER enzymatic as... | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022677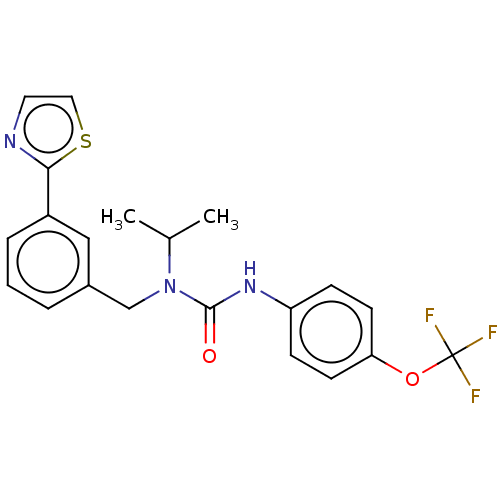 (CHEMBL3298270 | US9181261, 17) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022679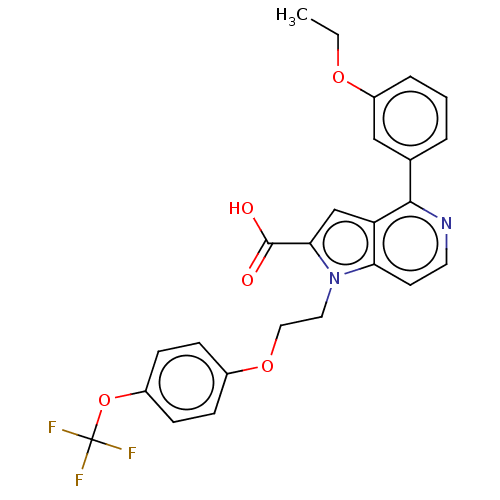 (CHEMBL3298365) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022674 (CHEMBL3298267) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified TrkA cytoplasmic domain (unknown origin) by HTRF assay | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 179: 680-693 (2019) Article DOI: 10.1016/j.ejmech.2019.06.088 BindingDB Entry DOI: 10.7270/Q2ZK5M1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022757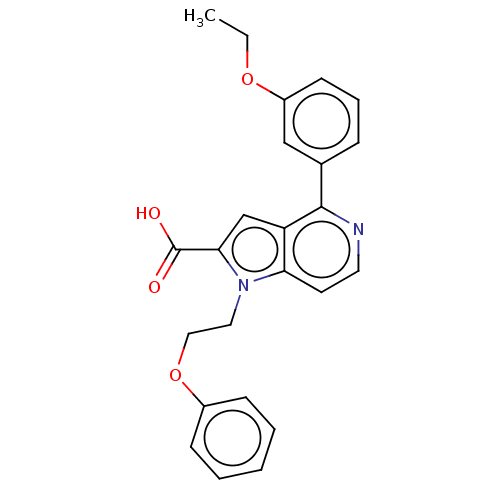 (CHEMBL3298163) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022681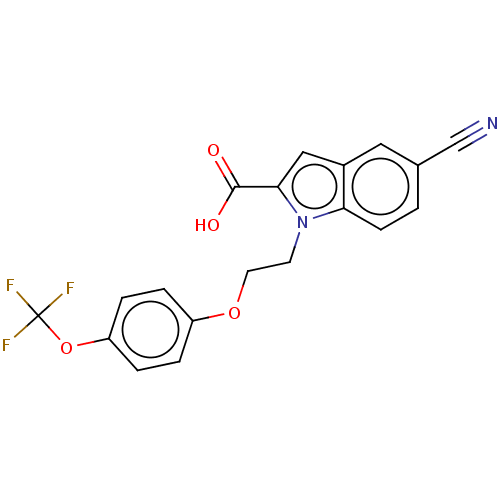 (CHEMBL3298535) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of phsophorylated TrkA (unknown origin) assessed as inhibition of fluorescently-labelled substrate phosphorylation by CALIPER enzymatic as... | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022677 (CHEMBL3298270 | US9181261, 17) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of phsophorylated TrkA (unknown origin) assessed as inhibition of fluorescently-labelled substrate phosphorylation by CALIPER enzymatic as... | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022681 (CHEMBL3298535) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022756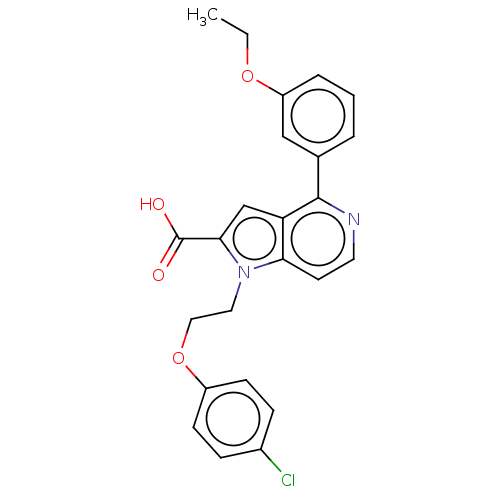 (CHEMBL3298164) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022679 (CHEMBL3298365) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 348 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of phsophorylated TrkA (unknown origin) assessed as inhibition of fluorescently-labelled substrate phosphorylation by CALIPER enzymatic as... | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50513701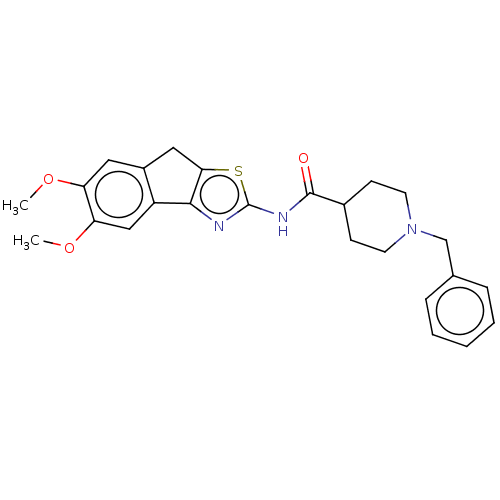 (CHEMBL4434663) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 179: 680-693 (2019) Article DOI: 10.1016/j.ejmech.2019.06.088 BindingDB Entry DOI: 10.7270/Q2ZK5M1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50280646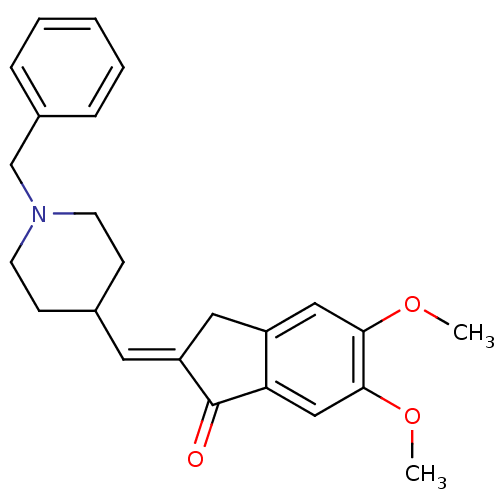 (2-[1-(1-Benzyl-piperidin-4-yl)-meth-(E)-ylidene]-5...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 127: 671-690 (2017) Article DOI: 10.1016/j.ejmech.2016.10.036 BindingDB Entry DOI: 10.7270/Q2P84F48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50231971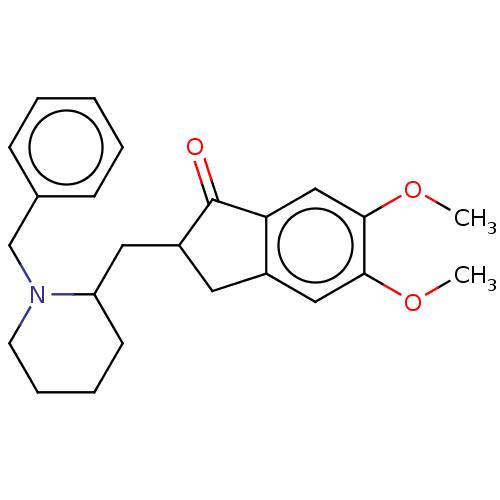 (CHEMBL4101144) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 127: 671-690 (2017) Article DOI: 10.1016/j.ejmech.2016.10.036 BindingDB Entry DOI: 10.7270/Q2P84F48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50231968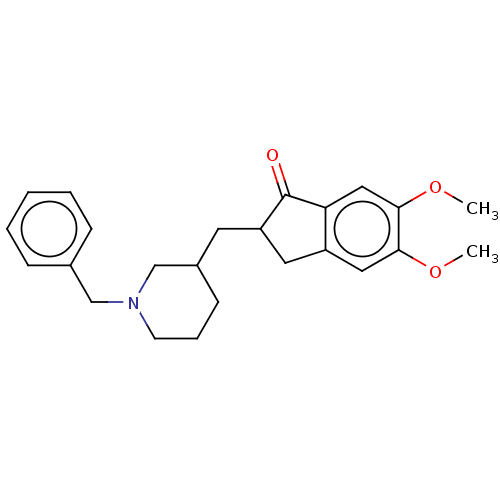 (CHEMBL4067672) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 127: 671-690 (2017) Article DOI: 10.1016/j.ejmech.2016.10.036 BindingDB Entry DOI: 10.7270/Q2P84F48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50231974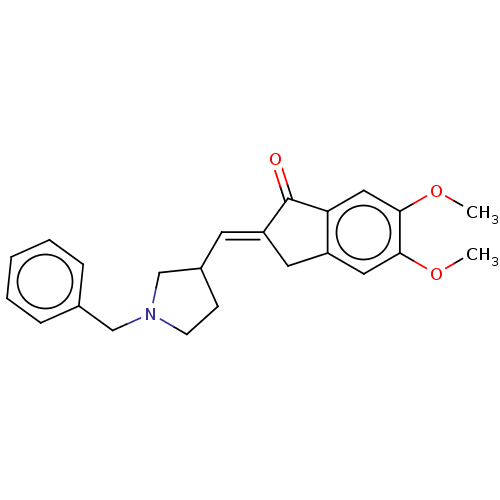 (CHEMBL4090024) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 127: 671-690 (2017) Article DOI: 10.1016/j.ejmech.2016.10.036 BindingDB Entry DOI: 10.7270/Q2P84F48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022676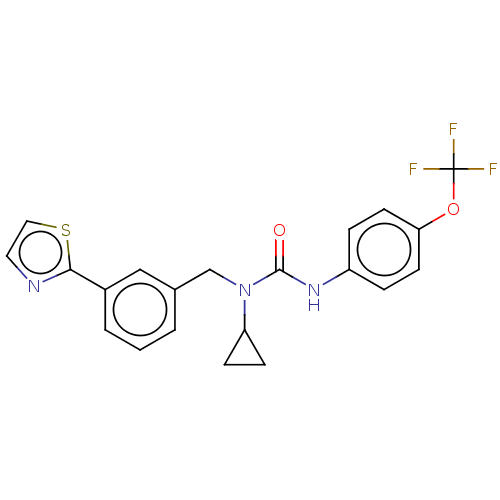 (CHEMBL3298269) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 549 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human TrkA expressed in human U2OS cells assessed as inhibition of NGF-induced maximum response after 1 hr by beta-galactosidase assay | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022756 (CHEMBL3298164) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 653 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of phsophorylated TrkA (unknown origin) assessed as inhibition of fluorescently-labelled substrate phosphorylation by CALIPER enzymatic as... | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50231982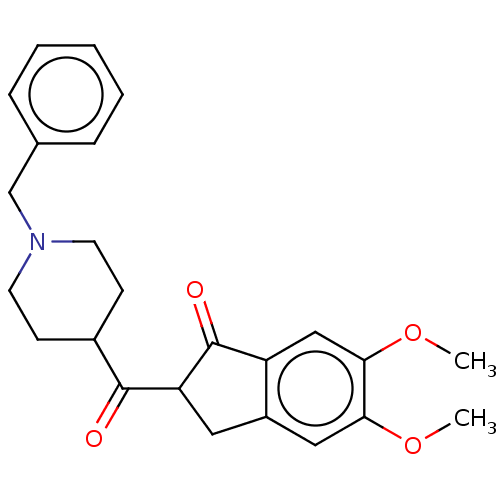 (CHEMBL4101215) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 127: 671-690 (2017) Article DOI: 10.1016/j.ejmech.2016.10.036 BindingDB Entry DOI: 10.7270/Q2P84F48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022672 (CHEMBL3298266) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 662 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified TrkA cytoplasmic domain (unknown origin) by HTRF assay | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50231989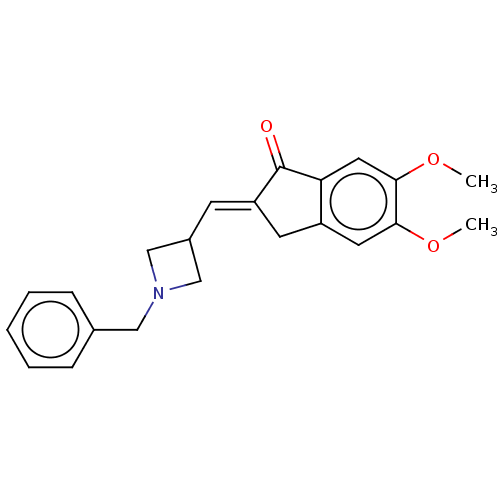 (CHEMBL4078831) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 127: 671-690 (2017) Article DOI: 10.1016/j.ejmech.2016.10.036 BindingDB Entry DOI: 10.7270/Q2P84F48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50231984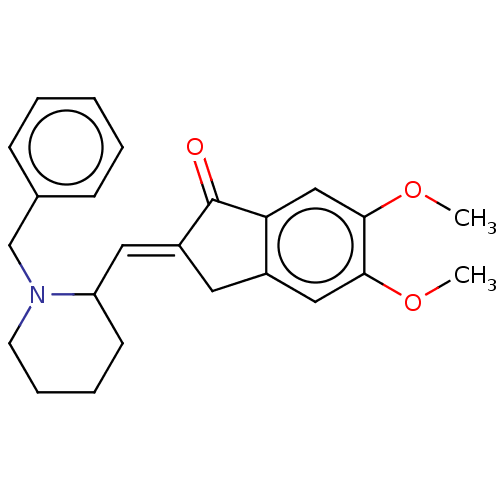 (CHEMBL4060269) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 127: 671-690 (2017) Article DOI: 10.1016/j.ejmech.2016.10.036 BindingDB Entry DOI: 10.7270/Q2P84F48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022669 (CHEMBL3298264) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 997 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified TrkA cytoplasmic domain (unknown origin) by HTRF assay | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50231977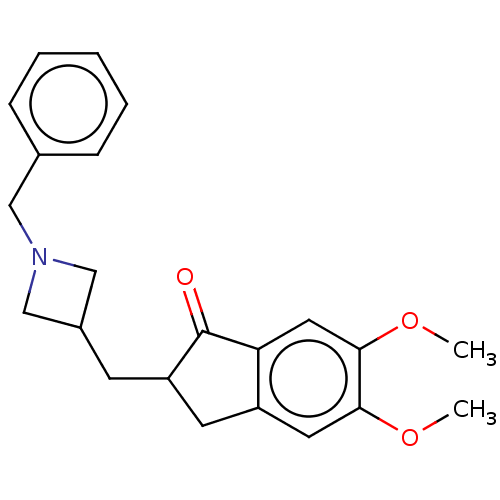 (CHEMBL4090929) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 127: 671-690 (2017) Article DOI: 10.1016/j.ejmech.2016.10.036 BindingDB Entry DOI: 10.7270/Q2P84F48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50231973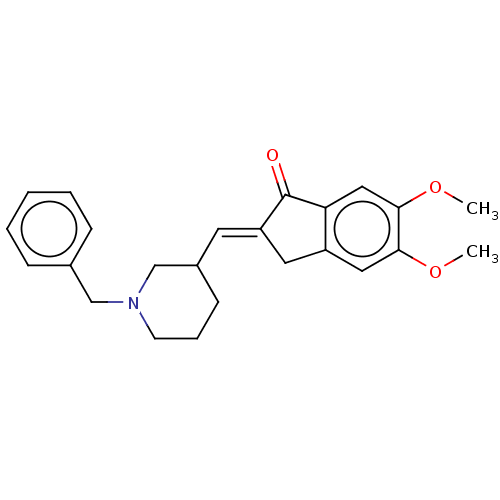 (CHEMBL4082178) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 127: 671-690 (2017) Article DOI: 10.1016/j.ejmech.2016.10.036 BindingDB Entry DOI: 10.7270/Q2P84F48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022670 (CHEMBL3297748) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of phsophorylated TrkA (unknown origin) assessed as inhibition of fluorescently-labelled substrate phosphorylation by CALIPER enzymatic as... | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50231972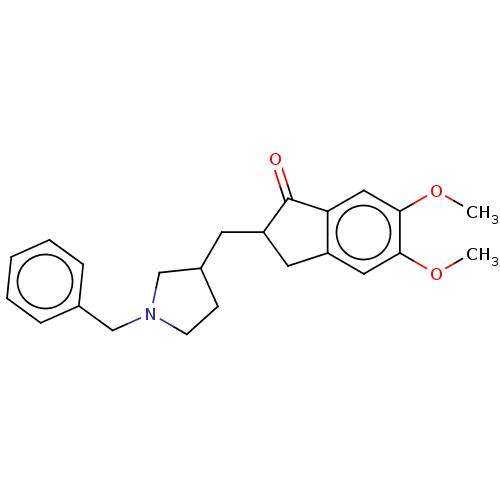 (CHEMBL4083147) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 127: 671-690 (2017) Article DOI: 10.1016/j.ejmech.2016.10.036 BindingDB Entry DOI: 10.7270/Q2P84F48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022757 (CHEMBL3298163) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of phsophorylated TrkA (unknown origin) assessed as inhibition of fluorescently-labelled substrate phosphorylation by CALIPER enzymatic as... | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022755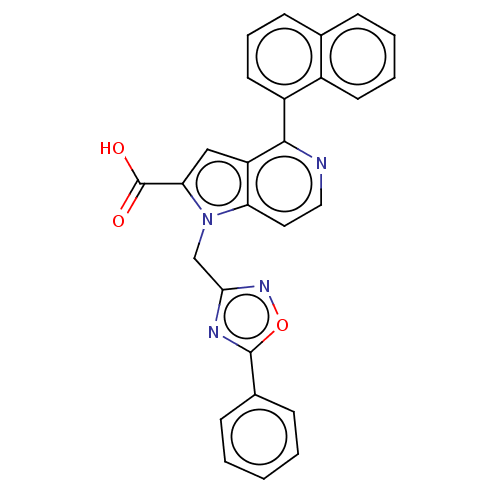 (CHEMBL3298162) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of non-phosphorylated TrkA (unknown origin) assessed as inhibition of fluorescently-labelled substrate phosphorylation by CALIPER enzymati... | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50231976 (CHEMBL4083925) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 127: 671-690 (2017) Article DOI: 10.1016/j.ejmech.2016.10.036 BindingDB Entry DOI: 10.7270/Q2P84F48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50231966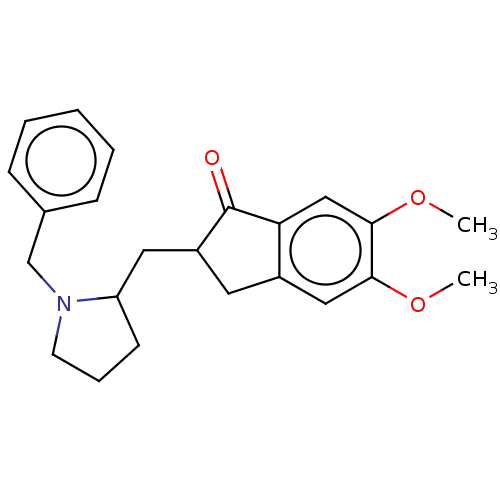 (CHEMBL4063021) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 127: 671-690 (2017) Article DOI: 10.1016/j.ejmech.2016.10.036 BindingDB Entry DOI: 10.7270/Q2P84F48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 127: 671-690 (2017) Article DOI: 10.1016/j.ejmech.2016.10.036 BindingDB Entry DOI: 10.7270/Q2P84F48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 179: 680-693 (2019) Article DOI: 10.1016/j.ejmech.2019.06.088 BindingDB Entry DOI: 10.7270/Q2ZK5M1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022676 (CHEMBL3298269) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of phsophorylated TrkA (unknown origin) assessed as inhibition of fluorescently-labelled substrate phosphorylation by CALIPER enzymatic as... | J Med Chem 57: 5800-16 (2014) Article DOI: 10.1021/jm5006429 BindingDB Entry DOI: 10.7270/Q2BV7J69 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using Butyrylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 179: 680-693 (2019) Article DOI: 10.1016/j.ejmech.2019.06.088 BindingDB Entry DOI: 10.7270/Q2ZK5M1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50513709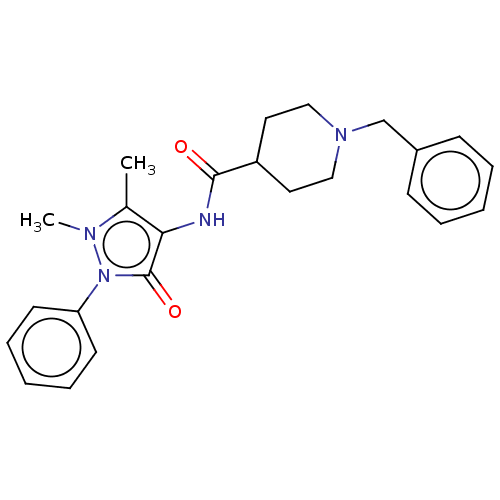 (CHEMBL4435317) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 179: 680-693 (2019) Article DOI: 10.1016/j.ejmech.2019.06.088 BindingDB Entry DOI: 10.7270/Q2ZK5M1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50231981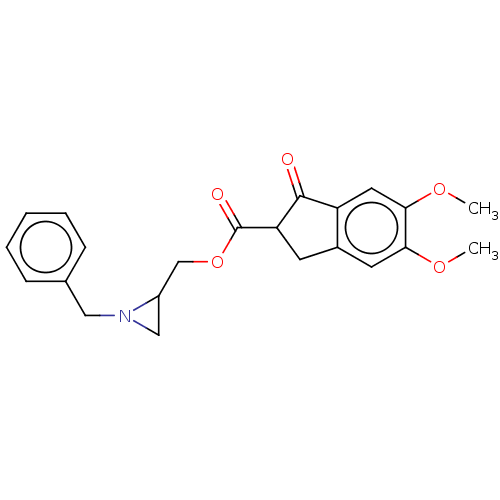 (CHEMBL4095084) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 127: 671-690 (2017) Article DOI: 10.1016/j.ejmech.2016.10.036 BindingDB Entry DOI: 10.7270/Q2P84F48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50231979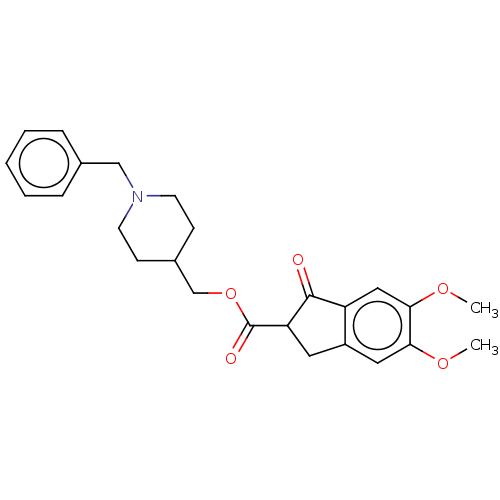 (CHEMBL4101917) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 127: 671-690 (2017) Article DOI: 10.1016/j.ejmech.2016.10.036 BindingDB Entry DOI: 10.7270/Q2P84F48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 98 total ) | Next | Last >> |