 Found 120 hits with Last Name = 'rivers' and Initial = 'd'
Found 120 hits with Last Name = 'rivers' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263562
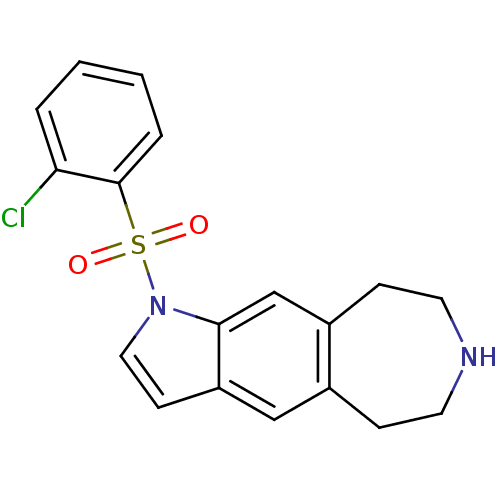
(1-(2-chlorophenylsulfonyl)-1,5,6,7,8,9-hexahydroaz...)Show InChI InChI=1S/C18H17ClN2O2S/c19-16-3-1-2-4-18(16)24(22,23)21-10-7-15-11-13-5-8-20-9-6-14(13)12-17(15)21/h1-4,7,10-12,20H,5-6,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263563

(1-(3-chlorophenylsulfonyl)-1,5,6,7,8,9-hexahydroaz...)Show InChI InChI=1S/C18H17ClN2O2S/c19-16-2-1-3-17(12-16)24(22,23)21-9-6-15-10-13-4-7-20-8-5-14(13)11-18(15)21/h1-3,6,9-12,20H,4-5,7-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263562

(1-(2-chlorophenylsulfonyl)-1,5,6,7,8,9-hexahydroaz...)Show InChI InChI=1S/C18H17ClN2O2S/c19-16-3-1-2-4-18(16)24(22,23)21-10-7-15-11-13-5-8-20-9-6-14(13)12-17(15)21/h1-4,7,10-12,20H,5-6,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using PPR substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50034557
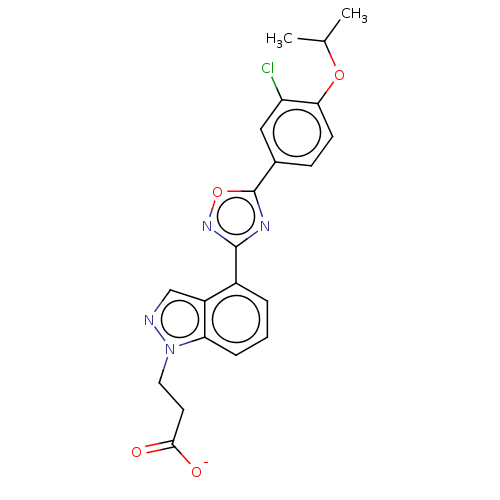
(CHEMBL3360360)Show SMILES [Na+].CC(C)Oc1ccc(cc1Cl)-c1nc(no1)-c1cccc2n(CCC([O-])=O)ncc12 Show InChI InChI=1S/C21H19ClN4O4.Na/c1-12(2)29-18-7-6-13(10-16(18)22)21-24-20(25-30-21)14-4-3-5-17-15(14)11-23-26(17)9-8-19(27)28;/h3-7,10-12H,8-9H2,1-2H3,(H,27,28);/q;+1/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263563

(1-(3-chlorophenylsulfonyl)-1,5,6,7,8,9-hexahydroaz...)Show InChI InChI=1S/C18H17ClN2O2S/c19-16-2-1-3-17(12-16)24(22,23)21-9-6-15-10-13-4-7-20-8-5-14(13)11-18(15)21/h1-3,6,9-12,20H,4-5,7-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using PPR substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263617
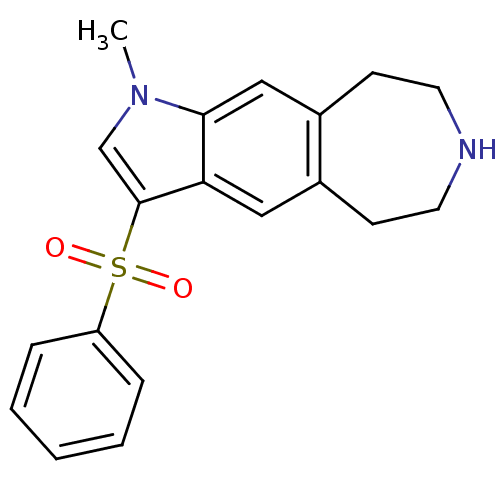
(1-methyl-3-(phenylsulfonyl)-1,5,6,7,8,9-hexahydroa...)Show InChI InChI=1S/C19H20N2O2S/c1-21-13-19(24(22,23)16-5-3-2-4-6-16)17-11-14-7-9-20-10-8-15(14)12-18(17)21/h2-6,11-13,20H,7-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263566
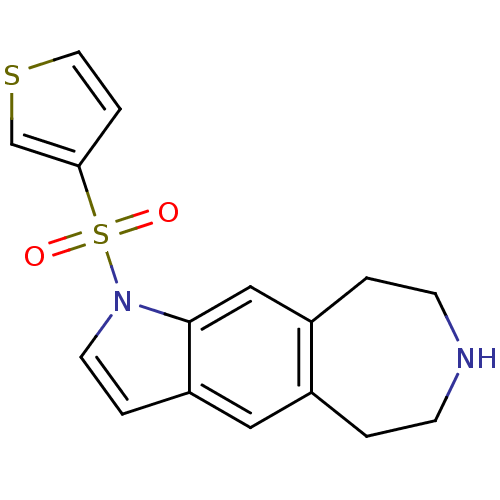
(1-(thiophen-3-ylsulfonyl)-1,5,6,7,8,9-hexahydroaze...)Show InChI InChI=1S/C16H16N2O2S2/c19-22(20,15-4-8-21-11-15)18-7-3-14-9-12-1-5-17-6-2-13(12)10-16(14)18/h3-4,7-11,17H,1-2,5-6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263518

(1-(phenylsulfonyl)-1,5,6,7,8,9-hexahydroazepino[4,...)Show InChI InChI=1S/C18H18N2O2S/c21-23(22,17-4-2-1-3-5-17)20-11-8-16-12-14-6-9-19-10-7-15(14)13-18(16)20/h1-5,8,11-13,19H,6-7,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263614
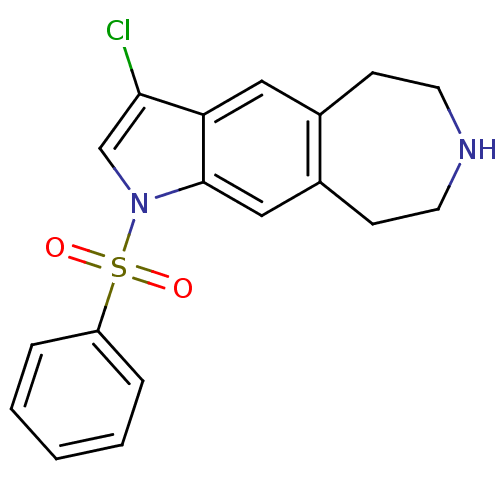
(3-chloro-1-(phenylsulfonyl)-1,5,6,7,8,9-hexahydroa...)Show InChI InChI=1S/C18H17ClN2O2S/c19-17-12-21(24(22,23)15-4-2-1-3-5-15)18-11-14-7-9-20-8-6-13(14)10-16(17)18/h1-5,10-12,20H,6-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263615

(3-chloro-1-(3-chlorophenylsulfonyl)-1,5,6,7,8,9-he...)Show SMILES Clc1cn(c2cc3CCNCCc3cc12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C18H16Cl2N2O2S/c19-14-2-1-3-15(10-14)25(23,24)22-11-17(20)16-8-12-4-6-21-7-5-13(12)9-18(16)22/h1-3,8-11,21H,4-7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263617

(1-methyl-3-(phenylsulfonyl)-1,5,6,7,8,9-hexahydroa...)Show InChI InChI=1S/C19H20N2O2S/c1-21-13-19(24(22,23)16-5-3-2-4-6-16)17-11-14-7-9-20-10-8-15(14)12-18(17)21/h2-6,11-13,20H,7-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using PPR substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263616
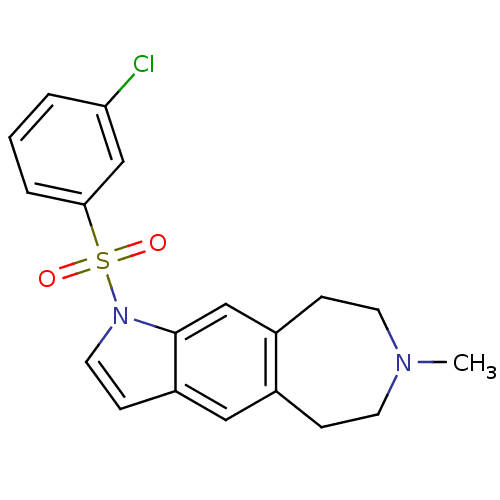
(1-(3-chlorophenylsulfonyl)-7-methyl-1,5,6,7,8,9-he...)Show SMILES CN1CCc2cc3ccn(c3cc2CC1)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H19ClN2O2S/c1-21-8-5-14-11-16-7-10-22(19(16)12-15(14)6-9-21)25(23,24)18-4-2-3-17(20)13-18/h2-4,7,10-13H,5-6,8-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263564
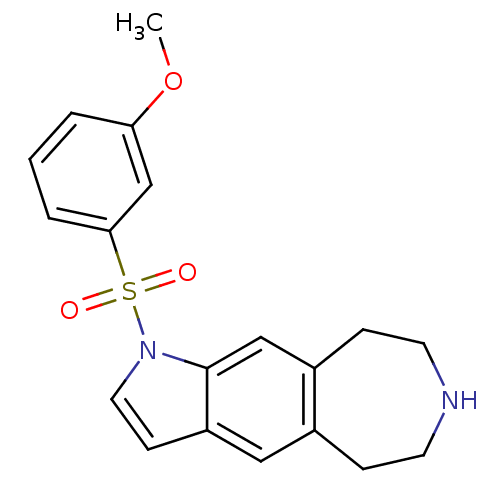
(1-(3-methoxyphenylsulfonyl)-1,5,6,7,8,9-hexahydroa...)Show InChI InChI=1S/C19H20N2O3S/c1-24-17-3-2-4-18(13-17)25(22,23)21-10-7-16-11-14-5-8-20-9-6-15(14)12-19(16)21/h2-4,7,10-13,20H,5-6,8-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263614

(3-chloro-1-(phenylsulfonyl)-1,5,6,7,8,9-hexahydroa...)Show InChI InChI=1S/C18H17ClN2O2S/c19-17-12-21(24(22,23)15-4-2-1-3-5-15)18-11-14-7-9-20-8-6-13(14)10-16(17)18/h1-5,10-12,20H,6-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using PPR substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263518

(1-(phenylsulfonyl)-1,5,6,7,8,9-hexahydroazepino[4,...)Show InChI InChI=1S/C18H18N2O2S/c21-23(22,17-4-2-1-3-5-17)20-11-8-16-12-14-6-9-19-10-7-15(14)13-18(16)20/h1-5,8,11-13,19H,6-7,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using PPR substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263615

(3-chloro-1-(3-chlorophenylsulfonyl)-1,5,6,7,8,9-he...)Show SMILES Clc1cn(c2cc3CCNCCc3cc12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C18H16Cl2N2O2S/c19-14-2-1-3-15(10-14)25(23,24)22-11-17(20)16-8-12-4-6-21-7-5-13(12)9-18(16)22/h1-3,8-11,21H,4-7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using PPR substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263566

(1-(thiophen-3-ylsulfonyl)-1,5,6,7,8,9-hexahydroaze...)Show InChI InChI=1S/C16H16N2O2S2/c19-22(20,15-4-8-21-11-15)18-7-3-14-9-12-1-5-17-6-2-13(12)10-16(14)18/h3-4,7-11,17H,1-2,5-6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using PPR substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50034556
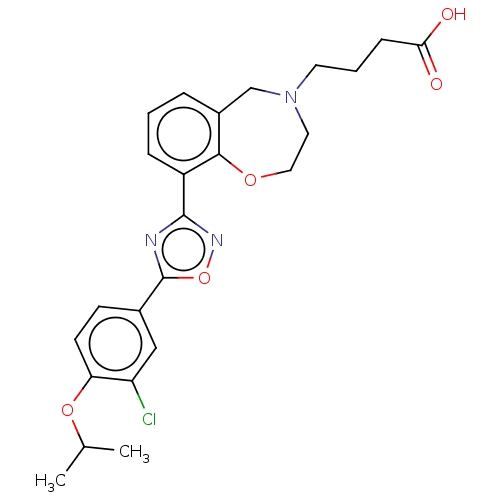
(CHEMBL3359844)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nc(no1)-c1cccc2CN(CCCC(O)=O)CCOc12 Show InChI InChI=1S/C24H26ClN3O5/c1-15(2)32-20-9-8-16(13-19(20)25)24-26-23(27-33-24)18-6-3-5-17-14-28(10-4-7-21(29)30)11-12-31-22(17)18/h3,5-6,8-9,13,15H,4,7,10-12,14H2,1-2H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263564

(1-(3-methoxyphenylsulfonyl)-1,5,6,7,8,9-hexahydroa...)Show InChI InChI=1S/C19H20N2O3S/c1-24-17-3-2-4-18(13-17)25(22,23)21-10-7-16-11-14-5-8-20-9-6-15(14)12-19(16)21/h2-4,7,10-13,20H,5-6,8-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using PPR substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263565

(1-(pyridin-2-ylsulfonyl)-1,5,6,7,8,9-hexahydroazep...)Show InChI InChI=1S/C17H17N3O2S/c21-23(22,17-3-1-2-7-19-17)20-10-6-15-11-13-4-8-18-9-5-14(13)12-16(15)20/h1-3,6-7,10-12,18H,4-5,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using N-N,diethyl-formamide as substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50034554
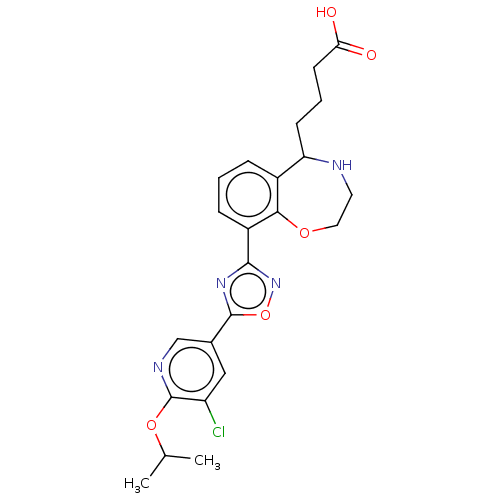
(CHEMBL3359854)Show SMILES CC(C)Oc1ncc(cc1Cl)-c1nc(no1)-c1cccc2C(CCCC(O)=O)NCCOc12 Show InChI InChI=1S/C23H25ClN4O5/c1-13(2)32-23-17(24)11-14(12-26-23)22-27-21(28-33-22)16-6-3-5-15-18(7-4-8-19(29)30)25-9-10-31-20(15)16/h3,5-6,11-13,18,25H,4,7-10H2,1-2H3,(H,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50034554

(CHEMBL3359854)Show SMILES CC(C)Oc1ncc(cc1Cl)-c1nc(no1)-c1cccc2C(CCCC(O)=O)NCCOc12 Show InChI InChI=1S/C23H25ClN4O5/c1-13(2)32-23-17(24)11-14(12-26-23)22-27-21(28-33-22)16-6-3-5-15-18(7-4-8-19(29)30)25-9-10-31-20(15)16/h3,5-6,11-13,18,25H,4,7-10H2,1-2H3,(H,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50034555

(CHEMBL3359847)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CCN(CCCC(O)=O)CCc2c1 Show InChI InChI=1S/C26H28N4O4/c1-17(2)33-23-8-7-21(15-22(23)16-27)26-28-25(29-34-26)20-6-5-18-9-12-30(11-3-4-24(31)32)13-10-19(18)14-20/h5-8,14-15,17H,3-4,9-13H2,1-2H3,(H,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50034556

(CHEMBL3359844)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nc(no1)-c1cccc2CN(CCCC(O)=O)CCOc12 Show InChI InChI=1S/C24H26ClN3O5/c1-15(2)32-20-9-8-16(13-19(20)25)24-26-23(27-33-24)18-6-3-5-17-14-28(10-4-7-21(29)30)11-12-31-22(17)18/h3,5-6,8-9,13,15H,4,7,10-12,14H2,1-2H3,(H,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50034557

(CHEMBL3360360)Show SMILES [Na+].CC(C)Oc1ccc(cc1Cl)-c1nc(no1)-c1cccc2n(CCC([O-])=O)ncc12 Show InChI InChI=1S/C21H19ClN4O4.Na/c1-12(2)29-18-7-6-13(10-16(18)22)21-24-20(25-30-21)14-4-3-5-17-15(14)11-23-26(17)9-8-19(27)28;/h3-7,10-12H,8-9H2,1-2H3,(H,27,28);/q;+1/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50034554

(CHEMBL3359854)Show SMILES CC(C)Oc1ncc(cc1Cl)-c1nc(no1)-c1cccc2C(CCCC(O)=O)NCCOc12 Show InChI InChI=1S/C23H25ClN4O5/c1-13(2)32-23-17(24)11-14(12-26-23)22-27-21(28-33-22)16-6-3-5-15-18(7-4-8-19(29)30)25-9-10-31-20(15)16/h3,5-6,11-13,18,25H,4,7-10H2,1-2H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50034554

(CHEMBL3359854)Show SMILES CC(C)Oc1ncc(cc1Cl)-c1nc(no1)-c1cccc2C(CCCC(O)=O)NCCOc12 Show InChI InChI=1S/C23H25ClN4O5/c1-13(2)32-23-17(24)11-14(12-26-23)22-27-21(28-33-22)16-6-3-5-15-18(7-4-8-19(29)30)25-9-10-31-20(15)16/h3,5-6,11-13,18,25H,4,7-10H2,1-2H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50034555

(CHEMBL3359847)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CCN(CCCC(O)=O)CCc2c1 Show InChI InChI=1S/C26H28N4O4/c1-17(2)33-23-8-7-21(15-22(23)16-27)26-28-25(29-34-26)20-6-5-18-9-12-30(11-3-4-24(31)32)13-10-19(18)14-20/h5-8,14-15,17H,3-4,9-13H2,1-2H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50034556

(CHEMBL3359844)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nc(no1)-c1cccc2CN(CCCC(O)=O)CCOc12 Show InChI InChI=1S/C24H26ClN3O5/c1-15(2)32-20-9-8-16(13-19(20)25)24-26-23(27-33-24)18-6-3-5-17-14-28(10-4-7-21(29)30)11-12-31-22(17)18/h3,5-6,8-9,13,15H,4,7,10-12,14H2,1-2H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50034557

(CHEMBL3360360)Show SMILES [Na+].CC(C)Oc1ccc(cc1Cl)-c1nc(no1)-c1cccc2n(CCC([O-])=O)ncc12 Show InChI InChI=1S/C21H19ClN4O4.Na/c1-12(2)29-18-7-6-13(10-16(18)22)21-24-20(25-30-21)14-4-3-5-17-15(14)11-23-26(17)9-8-19(27)28;/h3-7,10-12H,8-9H2,1-2H3,(H,27,28);/q;+1/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50034554

(CHEMBL3359854)Show SMILES CC(C)Oc1ncc(cc1Cl)-c1nc(no1)-c1cccc2C(CCCC(O)=O)NCCOc12 Show InChI InChI=1S/C23H25ClN4O5/c1-13(2)32-23-17(24)11-14(12-26-23)22-27-21(28-33-22)16-6-3-5-15-18(7-4-8-19(29)30)25-9-10-31-20(15)16/h3,5-6,11-13,18,25H,4,7-10H2,1-2H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50034554

(CHEMBL3359854)Show SMILES CC(C)Oc1ncc(cc1Cl)-c1nc(no1)-c1cccc2C(CCCC(O)=O)NCCOc12 Show InChI InChI=1S/C23H25ClN4O5/c1-13(2)32-23-17(24)11-14(12-26-23)22-27-21(28-33-22)16-6-3-5-15-18(7-4-8-19(29)30)25-9-10-31-20(15)16/h3,5-6,11-13,18,25H,4,7-10H2,1-2H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50034555

(CHEMBL3359847)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CCN(CCCC(O)=O)CCc2c1 Show InChI InChI=1S/C26H28N4O4/c1-17(2)33-23-8-7-21(15-22(23)16-27)26-28-25(29-34-26)20-6-5-18-9-12-30(11-3-4-24(31)32)13-10-19(18)14-20/h5-8,14-15,17H,3-4,9-13H2,1-2H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50034554

(CHEMBL3359854)Show SMILES CC(C)Oc1ncc(cc1Cl)-c1nc(no1)-c1cccc2C(CCCC(O)=O)NCCOc12 Show InChI InChI=1S/C23H25ClN4O5/c1-13(2)32-23-17(24)11-14(12-26-23)22-27-21(28-33-22)16-6-3-5-15-18(7-4-8-19(29)30)25-9-10-31-20(15)16/h3,5-6,11-13,18,25H,4,7-10H2,1-2H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50034554

(CHEMBL3359854)Show SMILES CC(C)Oc1ncc(cc1Cl)-c1nc(no1)-c1cccc2C(CCCC(O)=O)NCCOc12 Show InChI InChI=1S/C23H25ClN4O5/c1-13(2)32-23-17(24)11-14(12-26-23)22-27-21(28-33-22)16-6-3-5-15-18(7-4-8-19(29)30)25-9-10-31-20(15)16/h3,5-6,11-13,18,25H,4,7-10H2,1-2H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50034555

(CHEMBL3359847)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CCN(CCCC(O)=O)CCc2c1 Show InChI InChI=1S/C26H28N4O4/c1-17(2)33-23-8-7-21(15-22(23)16-27)26-28-25(29-34-26)20-6-5-18-9-12-30(11-3-4-24(31)32)13-10-19(18)14-20/h5-8,14-15,17H,3-4,9-13H2,1-2H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50034556

(CHEMBL3359844)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nc(no1)-c1cccc2CN(CCCC(O)=O)CCOc12 Show InChI InChI=1S/C24H26ClN3O5/c1-15(2)32-20-9-8-16(13-19(20)25)24-26-23(27-33-24)18-6-3-5-17-14-28(10-4-7-21(29)30)11-12-31-22(17)18/h3,5-6,8-9,13,15H,4,7,10-12,14H2,1-2H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50034557

(CHEMBL3360360)Show SMILES [Na+].CC(C)Oc1ccc(cc1Cl)-c1nc(no1)-c1cccc2n(CCC([O-])=O)ncc12 Show InChI InChI=1S/C21H19ClN4O4.Na/c1-12(2)29-18-7-6-13(10-16(18)22)21-24-20(25-30-21)14-4-3-5-17-15(14)11-23-26(17)9-8-19(27)28;/h3-7,10-12H,8-9H2,1-2H3,(H,27,28);/q;+1/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263616

(1-(3-chlorophenylsulfonyl)-7-methyl-1,5,6,7,8,9-he...)Show SMILES CN1CCc2cc3ccn(c3cc2CC1)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H19ClN2O2S/c1-21-8-5-14-11-16-7-10-22(19(16)12-15(14)6-9-21)25(23,24)18-4-2-3-17(20)13-18/h2-4,7,10-13H,5-6,8-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using PPR substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50263565

(1-(pyridin-2-ylsulfonyl)-1,5,6,7,8,9-hexahydroazep...)Show InChI InChI=1S/C17H17N3O2S/c21-23(22,17-3-1-2-7-19-17)20-10-6-15-11-13-4-8-18-9-5-14(13)12-16(15)20/h1-3,6-7,10-12,18H,4-5,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by microtiter plate assays using PPR substrate |
Bioorg Med Chem Lett 18: 5698-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.010
BindingDB Entry DOI: 10.7270/Q28W3D5D |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50034617
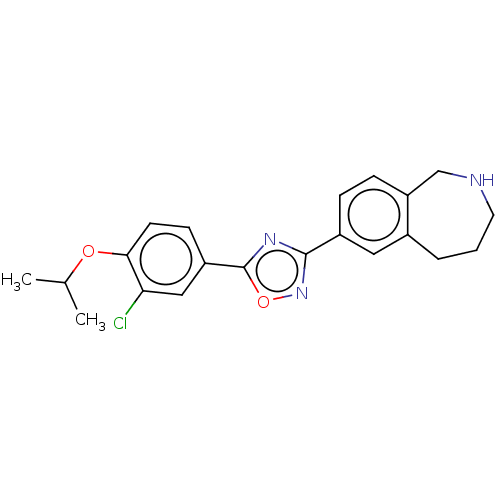
(CHEMBL3360373)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nc(no1)-c1ccc2CNCCCc2c1 Show InChI InChI=1S/C21H22ClN3O2/c1-13(2)26-19-8-7-16(11-18(19)22)21-24-20(25-27-21)15-5-6-17-12-23-9-3-4-14(17)10-15/h5-8,10-11,13,23H,3-4,9,12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 398 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1R expressed in RH7777 membranes assessed as [35S]GTPgammaS binding after 30 mins |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50034622

(CHEMBL3360372)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CNCCOc2c1 Show InChI InChI=1S/C21H20N4O3/c1-13(2)27-18-6-5-15(9-17(18)11-22)21-24-20(25-28-21)14-3-4-16-12-23-7-8-26-19(16)10-14/h3-6,9-10,13,23H,7-8,12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 79 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1R expressed in RH7777 membranes assessed as [35S]GTPgammaS binding after 30 mins |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50034624
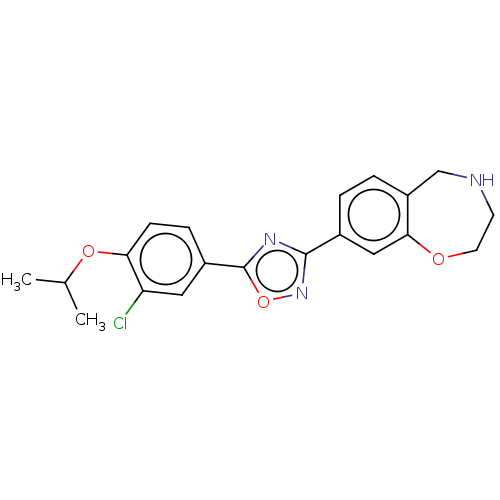
(CHEMBL3360371)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nc(no1)-c1ccc2CNCCOc2c1 Show InChI InChI=1S/C20H20ClN3O3/c1-12(2)26-17-6-5-14(9-16(17)21)20-23-19(24-27-20)13-3-4-15-11-22-7-8-25-18(15)10-13/h3-6,9-10,12,22H,7-8,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 316 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1R expressed in RH7777 membranes assessed as [35S]GTPgammaS binding after 30 mins |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50034626
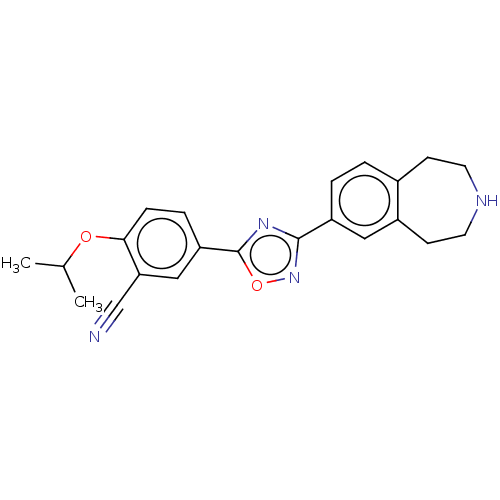
(CHEMBL3360370)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CCNCCc2c1 Show InChI InChI=1S/C22H22N4O2/c1-14(2)27-20-6-5-18(12-19(20)13-23)22-25-21(26-28-22)17-4-3-15-7-9-24-10-8-16(15)11-17/h3-6,11-12,14,24H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1R expressed in RH7777 membranes assessed as [35S]GTPgammaS binding after 30 mins |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50034627
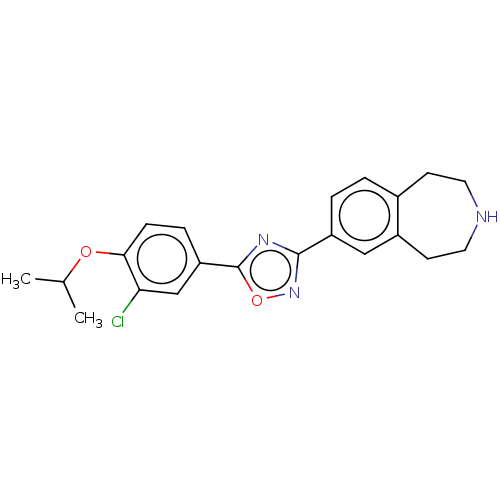
(CHEMBL3360369)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nc(no1)-c1ccc2CCNCCc2c1 Show InChI InChI=1S/C21H22ClN3O2/c1-13(2)26-19-6-5-17(12-18(19)22)21-24-20(25-27-21)16-4-3-14-7-9-23-10-8-15(14)11-16/h3-6,11-13,23H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 158 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1R expressed in RH7777 membranes assessed as [35S]GTPgammaS binding after 30 mins |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50034628
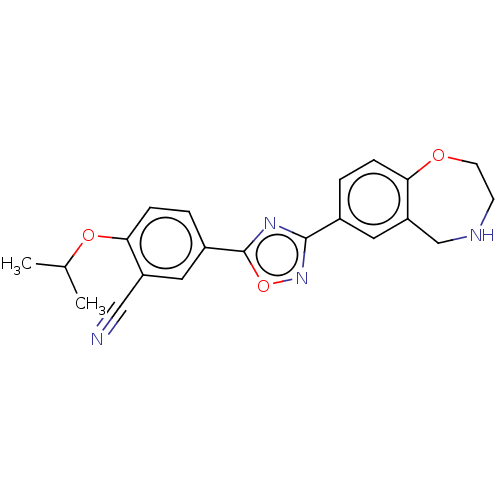
(CHEMBL3360368)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2OCCNCc2c1 Show InChI InChI=1S/C21H20N4O3/c1-13(2)27-19-6-4-15(10-16(19)11-22)21-24-20(25-28-21)14-3-5-18-17(9-14)12-23-7-8-26-18/h3-6,9-10,13,23H,7-8,12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1R expressed in RH7777 membranes assessed as [35S]GTPgammaS binding after 30 mins |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50034629
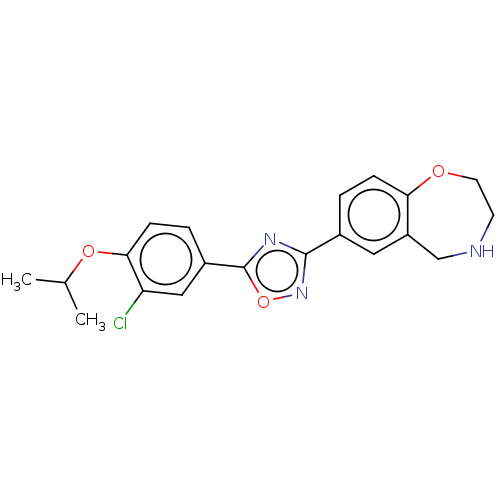
(CHEMBL3360367)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nc(no1)-c1ccc2OCCNCc2c1 Show InChI InChI=1S/C20H20ClN3O3/c1-12(2)26-18-6-4-14(10-16(18)21)20-23-19(24-27-20)13-3-5-17-15(9-13)11-22-7-8-25-17/h3-6,9-10,12,22H,7-8,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 126 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1R expressed in RH7777 membranes assessed as [35S]GTPgammaS binding after 30 mins |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50034630
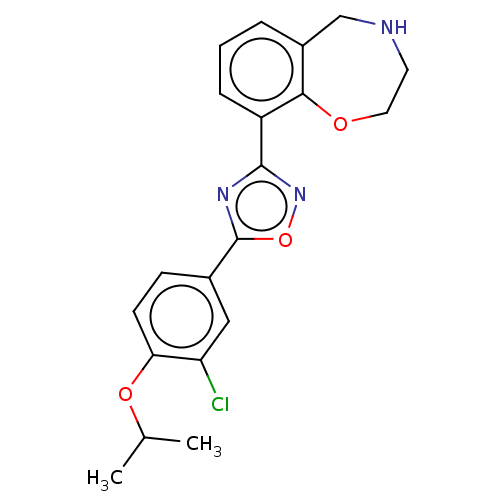
(CHEMBL3360366)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nc(no1)-c1cccc2CNCCOc12 Show InChI InChI=1S/C20H20ClN3O3/c1-12(2)26-17-7-6-13(10-16(17)21)20-23-19(24-27-20)15-5-3-4-14-11-22-8-9-25-18(14)15/h3-7,10,12,22H,8-9,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 79 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1R expressed in RH7777 membranes assessed as [35S]GTPgammaS binding after 30 mins |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50034631
(CHEMBL3360365)Show SMILES CC(C)Oc1ccc(cc1Cl)-c1nc(no1)-c1cccc2CNCCc12 Show InChI InChI=1S/C20H20ClN3O2/c1-12(2)25-18-7-6-13(10-17(18)21)20-23-19(24-26-20)16-5-3-4-14-11-22-9-8-15(14)16/h3-7,10,12,22H,8-9,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1R expressed in RH7777 membranes assessed as [35S]GTPgammaS binding after 30 mins |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50034632

(CHEMBL3360364)Show SMILES CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CNCCc2c1 Show InChI InChI=1S/C21H20N4O2/c1-13(2)26-19-6-5-16(10-18(19)11-22)21-24-20(25-27-21)15-3-4-17-12-23-8-7-14(17)9-15/h3-6,9-10,13,23H,7-8,12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1R expressed in RH7777 membranes assessed as [35S]GTPgammaS binding after 30 mins |
J Med Chem 57: 10424-42 (2014)
Article DOI: 10.1021/jm5010336
BindingDB Entry DOI: 10.7270/Q2DF6SSX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data