 Found 478 hits with Last Name = 'roberts' and Initial = 'k'
Found 478 hits with Last Name = 'roberts' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50067499

((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(CO)=CC[C@H]3C(C)(C)Oc2c1 |r,c:18| Show InChI InChI=1S/C25H38O3/c1-6-7-8-9-12-24(2,3)18-14-21(27)23-19-13-17(16-26)10-11-20(19)25(4,5)28-22(23)15-18/h10,14-15,19-20,26-27H,6-9,11-13,16H2,1-5H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant cannabinoid CB2 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 21: 5859-62 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.099
BindingDB Entry DOI: 10.7270/Q2K074P5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418901
(CHEMBL1808415)Show SMILES Cc1cccc(NC(=O)NC2C(=O)N(CCN3CCOCC3)c3ccccc3N(CC3CCCCC3)C2=O)c1 Show InChI InChI=1S/C30H39N5O4/c1-22-8-7-11-24(20-22)31-30(38)32-27-28(36)34(15-14-33-16-18-39-19-17-33)25-12-5-6-13-26(25)35(29(27)37)21-23-9-3-2-4-10-23/h5-8,11-13,20,23,27H,2-4,9-10,14-19,21H2,1H3,(H2,31,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
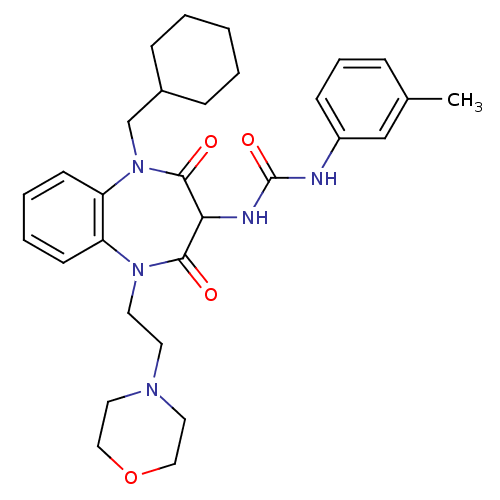
Cholecystokinin receptor type A
(RAT) | BDBM50418893
(CHEMBL1808400)Show SMILES CC(C)CCN1c2ccccc2N(CCN2CCOCC2)C(=O)C(NC(=O)Nc2cccc(C)c2)C1=O Show InChI InChI=1S/C28H37N5O4/c1-20(2)11-12-32-23-9-4-5-10-24(23)33(14-13-31-15-17-37-18-16-31)27(35)25(26(32)34)30-28(36)29-22-8-6-7-21(3)19-22/h4-10,19-20,25H,11-18H2,1-3H3,(H2,29,30,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
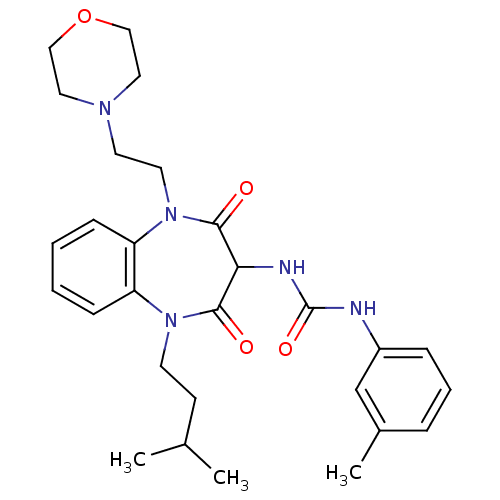
Cholecystokinin receptor type A
(RAT) | BDBM50418884
(CHEMBL1808414)Show SMILES O=C(NC1C(=O)N(CCN2CCOCC2)c2ccccc2N(CC2CCCCC2)C1=O)Nc1ccccc1 Show InChI InChI=1S/C29H37N5O4/c35-27-26(31-29(37)30-23-11-5-2-6-12-23)28(36)34(21-22-9-3-1-4-10-22)25-14-8-7-13-24(25)33(27)16-15-32-17-19-38-20-18-32/h2,5-8,11-14,22,26H,1,3-4,9-10,15-21H2,(H2,30,31,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
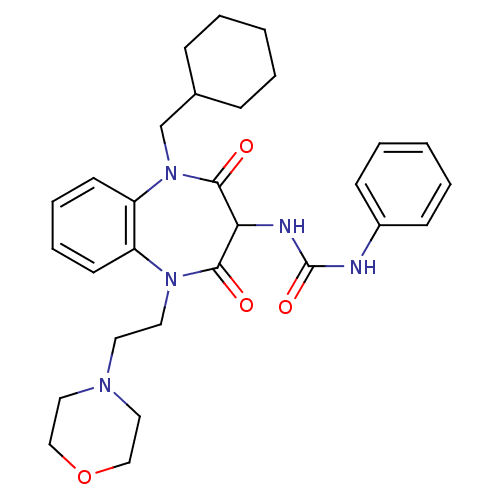
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50354474
(CHEMBL1836868)Show SMILES CCCCCN1C(=O)C(=NNC(=O)CN(C)c2ccc([N+]([O-])=O)c3nonc23)c2ccc(OC)cc12 |w:9.9| Show InChI InChI=1S/C23H25N7O6/c1-4-5-6-11-29-18-12-14(35-3)7-8-15(18)20(23(29)32)25-24-19(31)13-28(2)16-9-10-17(30(33)34)22-21(16)26-36-27-22/h7-10,12H,4-6,11,13H2,1-3H3,(H,24,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 387 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant cannabinoid CB2 receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 21: 5859-62 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.099
BindingDB Entry DOI: 10.7270/Q2K074P5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418896
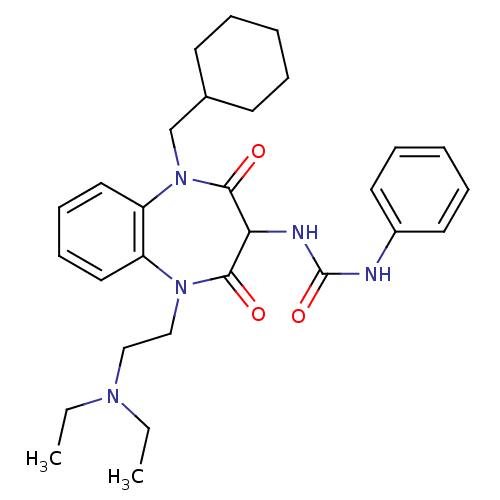
(CHEMBL1807152)Show SMILES CCN(CC)CCN1c2ccccc2N(CC2CCCCC2)C(=O)C(NC(=O)Nc2ccccc2)C1=O Show InChI InChI=1S/C29H39N5O3/c1-3-32(4-2)19-20-33-24-17-11-12-18-25(24)34(21-22-13-7-5-8-14-22)28(36)26(27(33)35)31-29(37)30-23-15-9-6-10-16-23/h6,9-12,15-18,22,26H,3-5,7-8,13-14,19-21H2,1-2H3,(H2,30,31,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 467 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418896

(CHEMBL1807152)Show SMILES CCN(CC)CCN1c2ccccc2N(CC2CCCCC2)C(=O)C(NC(=O)Nc2ccccc2)C1=O Show InChI InChI=1S/C29H39N5O3/c1-3-32(4-2)19-20-33-24-17-11-12-18-25(24)34(21-22-13-7-5-8-14-22)28(36)26(27(33)35)31-29(37)30-23-15-9-6-10-16-23/h6,9-12,15-18,22,26H,3-5,7-8,13-14,19-21H2,1-2H3,(H2,30,31,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 468 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418891
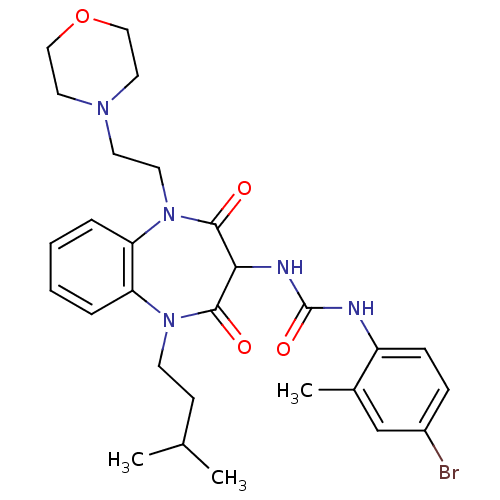
(CHEMBL1808405)Show SMILES CC(C)CCN1c2ccccc2N(CCN2CCOCC2)C(=O)C(NC(=O)Nc2ccc(Br)cc2C)C1=O Show InChI InChI=1S/C28H36BrN5O4/c1-19(2)10-11-33-23-6-4-5-7-24(23)34(13-12-32-14-16-38-17-15-32)27(36)25(26(33)35)31-28(37)30-22-9-8-21(29)18-20(22)3/h4-9,18-19,25H,10-17H2,1-3H3,(H2,30,31,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418898
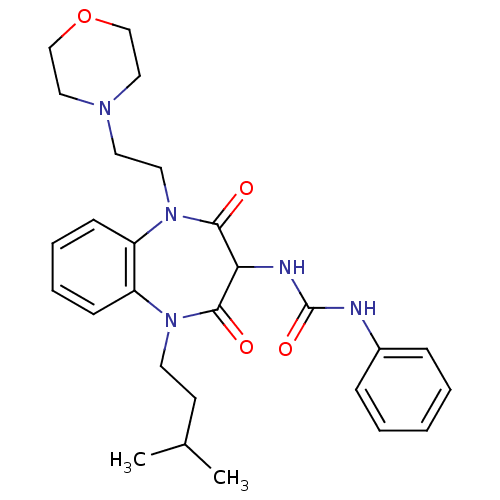
(CHEMBL1808398)Show SMILES CC(C)CCN1c2ccccc2N(CCN2CCOCC2)C(=O)C(NC(=O)Nc2ccccc2)C1=O Show InChI InChI=1S/C27H35N5O4/c1-20(2)12-13-31-22-10-6-7-11-23(22)32(15-14-30-16-18-36-19-17-30)26(34)24(25(31)33)29-27(35)28-21-8-4-3-5-9-21/h3-11,20,24H,12-19H2,1-2H3,(H2,28,29,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418878
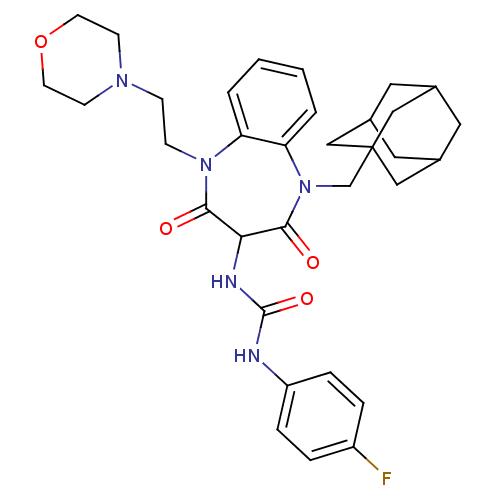
(CHEMBL1808418)Show SMILES Fc1ccc(NC(=O)NC2C(=O)N(CCN3CCOCC3)c3ccccc3N(CC34CC5CC(CC(C5)C3)C4)C2=O)cc1 |TLB:38:29:36:33.32.34,28:29:32:36.35.34,THB:38:33:36:29.37.30,37:35:32:38.29.30,37:29:32:36.35.34| Show InChI InChI=1S/C33H40FN5O4/c34-25-5-7-26(8-6-25)35-32(42)36-29-30(40)38(10-9-37-11-13-43-14-12-37)27-3-1-2-4-28(27)39(31(29)41)21-33-18-22-15-23(19-33)17-24(16-22)20-33/h1-8,22-24,29H,9-21H2,(H2,35,36,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418894
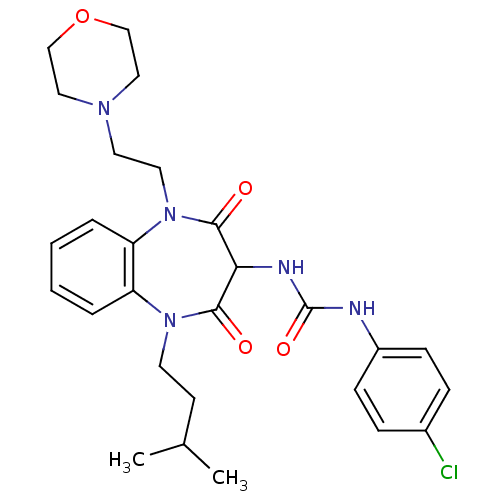
(CHEMBL1808399)Show SMILES CC(C)CCN1c2ccccc2N(CCN2CCOCC2)C(=O)C(NC(=O)Nc2ccc(Cl)cc2)C1=O Show InChI InChI=1S/C27H34ClN5O4/c1-19(2)11-12-32-22-5-3-4-6-23(22)33(14-13-31-15-17-37-18-16-31)26(35)24(25(32)34)30-27(36)29-21-9-7-20(28)8-10-21/h3-10,19,24H,11-18H2,1-2H3,(H2,29,30,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418897
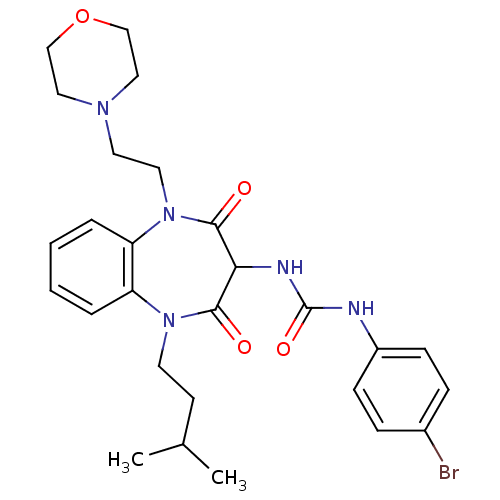
(CHEMBL1808402)Show SMILES CC(C)CCN1c2ccccc2N(CCN2CCOCC2)C(=O)C(NC(=O)Nc2ccc(Br)cc2)C1=O Show InChI InChI=1S/C27H34BrN5O4/c1-19(2)11-12-32-22-5-3-4-6-23(22)33(14-13-31-15-17-37-18-16-31)26(35)24(25(32)34)30-27(36)29-21-9-7-20(28)8-10-21/h3-10,19,24H,11-18H2,1-2H3,(H2,29,30,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418892
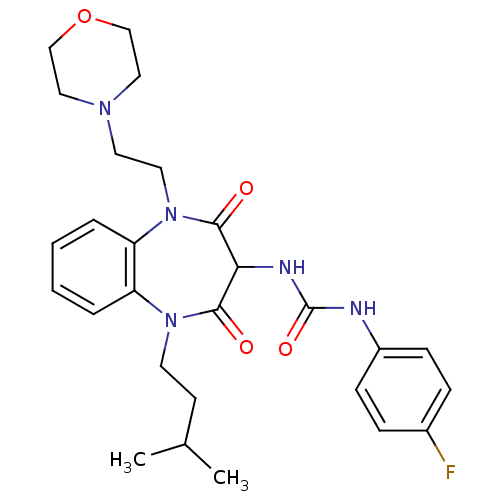
(CHEMBL1808403)Show SMILES CC(C)CCN1c2ccccc2N(CCN2CCOCC2)C(=O)C(NC(=O)Nc2ccc(F)cc2)C1=O Show InChI InChI=1S/C27H34FN5O4/c1-19(2)11-12-32-22-5-3-4-6-23(22)33(14-13-31-15-17-37-18-16-31)26(35)24(25(32)34)30-27(36)29-21-9-7-20(28)8-10-21/h3-10,19,24H,11-18H2,1-2H3,(H2,29,30,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418904
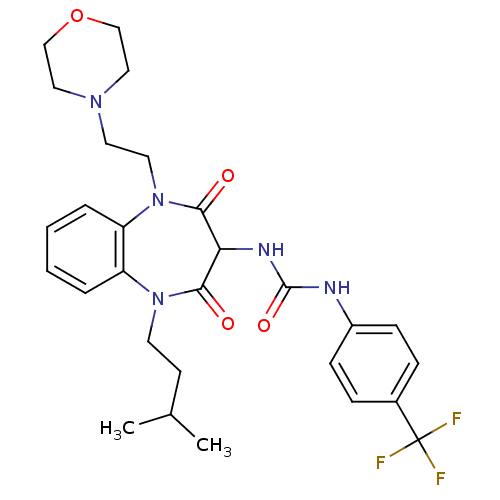
(CHEMBL1808401)Show SMILES CC(C)CCN1c2ccccc2N(CCN2CCOCC2)C(=O)C(NC(=O)Nc2ccc(cc2)C(F)(F)F)C1=O Show InChI InChI=1S/C28H34F3N5O4/c1-19(2)11-12-35-22-5-3-4-6-23(22)36(14-13-34-15-17-40-18-16-34)26(38)24(25(35)37)33-27(39)32-21-9-7-20(8-10-21)28(29,30)31/h3-10,19,24H,11-18H2,1-2H3,(H2,32,33,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418880
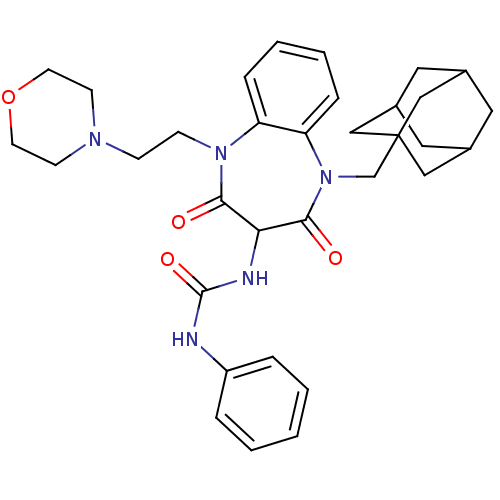
(CHEMBL1808416)Show SMILES O=C(NC1C(=O)N(CCN2CCOCC2)c2ccccc2N(CC23CC4CC(CC(C4)C2)C3)C1=O)Nc1ccccc1 |TLB:32:23:30:27.26.28,22:23:26:30.29.28,THB:32:27:30:23.31.24,31:29:26:32.23.24,31:23:26:30.29.28| Show InChI InChI=1S/C33H41N5O4/c39-30-29(35-32(41)34-26-6-2-1-3-7-26)31(40)38(22-33-19-23-16-24(20-33)18-25(17-23)21-33)28-9-5-4-8-27(28)37(30)11-10-36-12-14-42-15-13-36/h1-9,23-25,29H,10-22H2,(H2,34,35,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418880

(CHEMBL1808416)Show SMILES O=C(NC1C(=O)N(CCN2CCOCC2)c2ccccc2N(CC23CC4CC(CC(C4)C2)C3)C1=O)Nc1ccccc1 |TLB:32:23:30:27.26.28,22:23:26:30.29.28,THB:32:27:30:23.31.24,31:29:26:32.23.24,31:23:26:30.29.28| Show InChI InChI=1S/C33H41N5O4/c39-30-29(35-32(41)34-26-6-2-1-3-7-26)31(40)38(22-33-19-23-16-24(20-33)18-25(17-23)21-33)28-9-5-4-8-27(28)37(30)11-10-36-12-14-42-15-13-36/h1-9,23-25,29H,10-22H2,(H2,34,35,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418880

(CHEMBL1808416)Show SMILES O=C(NC1C(=O)N(CCN2CCOCC2)c2ccccc2N(CC23CC4CC(CC(C4)C2)C3)C1=O)Nc1ccccc1 |TLB:32:23:30:27.26.28,22:23:26:30.29.28,THB:32:27:30:23.31.24,31:29:26:32.23.24,31:23:26:30.29.28| Show InChI InChI=1S/C33H41N5O4/c39-30-29(35-32(41)34-26-6-2-1-3-7-26)31(40)38(22-33-19-23-16-24(20-33)18-25(17-23)21-33)28-9-5-4-8-27(28)37(30)11-10-36-12-14-42-15-13-36/h1-9,23-25,29H,10-22H2,(H2,34,35,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418881
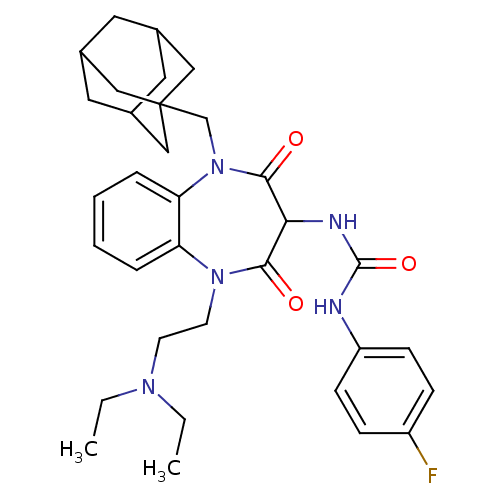
(CHEMBL1808422)Show SMILES CCN(CC)CCN1c2ccccc2N(CC23CC4CC(CC(C4)C2)C3)C(=O)C(NC(=O)Nc2ccc(F)cc2)C1=O |TLB:25:16:23:20.19.21,15:16:19:23.22.21,THB:25:20:23:16.24.17,24:22:19:25.16.17,24:16:19:23.22.21| Show InChI InChI=1S/C33H42FN5O3/c1-3-37(4-2)13-14-38-27-7-5-6-8-28(27)39(21-33-18-22-15-23(19-33)17-24(16-22)20-33)31(41)29(30(38)40)36-32(42)35-26-11-9-25(34)10-12-26/h5-12,22-24,29H,3-4,13-21H2,1-2H3,(H2,35,36,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418887
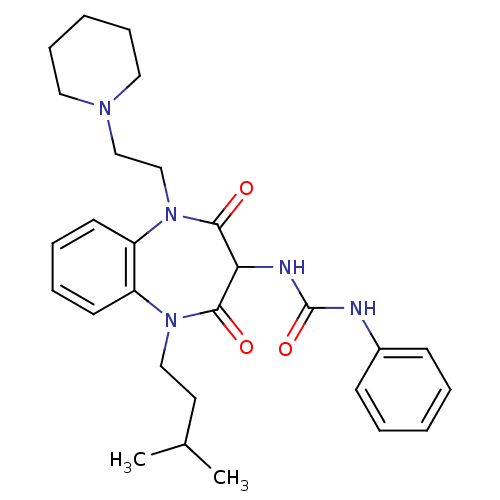
(CHEMBL1808410)Show SMILES CC(C)CCN1c2ccccc2N(CCN2CCCCC2)C(=O)C(NC(=O)Nc2ccccc2)C1=O Show InChI InChI=1S/C28H37N5O3/c1-21(2)15-18-32-23-13-7-8-14-24(23)33(20-19-31-16-9-4-10-17-31)27(35)25(26(32)34)30-28(36)29-22-11-5-3-6-12-22/h3,5-8,11-14,21,25H,4,9-10,15-20H2,1-2H3,(H2,29,30,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418879
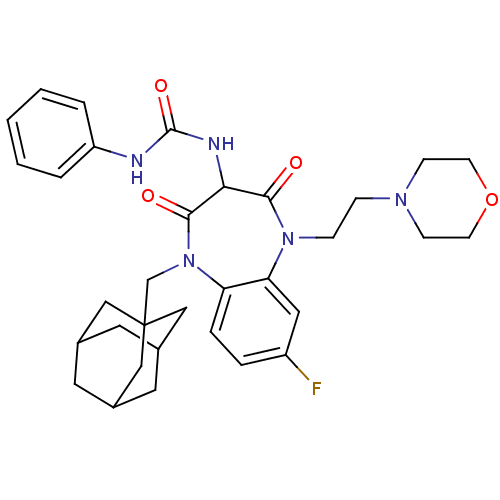
(CHEMBL1808417)Show SMILES Fc1ccc2N(CC34CC5CC(CC(C5)C3)C4)C(=O)C(NC(=O)Nc3ccccc3)C(=O)N(CCN3CCOCC3)c2c1 |TLB:16:7:14:11.10.12,6:7:10:14.13.12,THB:16:11:14:7.15.8,15:13:10:16.7.8,15:7:10:14.13.12| Show InChI InChI=1S/C33H40FN5O4/c34-25-6-7-27-28(17-25)38(9-8-37-10-12-43-13-11-37)30(40)29(36-32(42)35-26-4-2-1-3-5-26)31(41)39(27)21-33-18-22-14-23(19-33)16-24(15-22)20-33/h1-7,17,22-24,29H,8-16,18-21H2,(H2,35,36,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418879

(CHEMBL1808417)Show SMILES Fc1ccc2N(CC34CC5CC(CC(C5)C3)C4)C(=O)C(NC(=O)Nc3ccccc3)C(=O)N(CCN3CCOCC3)c2c1 |TLB:16:7:14:11.10.12,6:7:10:14.13.12,THB:16:11:14:7.15.8,15:13:10:16.7.8,15:7:10:14.13.12| Show InChI InChI=1S/C33H40FN5O4/c34-25-6-7-27-28(17-25)38(9-8-37-10-12-43-13-11-37)30(40)29(36-32(42)35-26-4-2-1-3-5-26)31(41)39(27)21-33-18-22-14-23(19-33)16-24(15-22)20-33/h1-7,17,22-24,29H,8-16,18-21H2,(H2,35,36,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418878

(CHEMBL1808418)Show SMILES Fc1ccc(NC(=O)NC2C(=O)N(CCN3CCOCC3)c3ccccc3N(CC34CC5CC(CC(C5)C3)C4)C2=O)cc1 |TLB:38:29:36:33.32.34,28:29:32:36.35.34,THB:38:33:36:29.37.30,37:35:32:38.29.30,37:29:32:36.35.34| Show InChI InChI=1S/C33H40FN5O4/c34-25-5-7-26(8-6-25)35-32(42)36-29-30(40)38(10-9-37-11-13-43-14-12-37)27-3-1-2-4-28(27)39(31(29)41)21-33-18-22-15-23(19-33)17-24(16-22)20-33/h1-8,22-24,29H,9-21H2,(H2,35,36,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418878

(CHEMBL1808418)Show SMILES Fc1ccc(NC(=O)NC2C(=O)N(CCN3CCOCC3)c3ccccc3N(CC34CC5CC(CC(C5)C3)C4)C2=O)cc1 |TLB:38:29:36:33.32.34,28:29:32:36.35.34,THB:38:33:36:29.37.30,37:35:32:38.29.30,37:29:32:36.35.34| Show InChI InChI=1S/C33H40FN5O4/c34-25-5-7-26(8-6-25)35-32(42)36-29-30(40)38(10-9-37-11-13-43-14-12-37)27-3-1-2-4-28(27)39(31(29)41)21-33-18-22-15-23(19-33)17-24(16-22)20-33/h1-8,22-24,29H,9-21H2,(H2,35,36,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418896

(CHEMBL1807152)Show SMILES CCN(CC)CCN1c2ccccc2N(CC2CCCCC2)C(=O)C(NC(=O)Nc2ccccc2)C1=O Show InChI InChI=1S/C29H39N5O3/c1-3-32(4-2)19-20-33-24-17-11-12-18-25(24)34(21-22-13-7-5-8-14-22)28(36)26(27(33)35)31-29(37)30-23-15-9-6-10-16-23/h6,9-12,15-18,22,26H,3-5,7-8,13-14,19-21H2,1-2H3,(H2,30,31,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418879

(CHEMBL1808417)Show SMILES Fc1ccc2N(CC34CC5CC(CC(C5)C3)C4)C(=O)C(NC(=O)Nc3ccccc3)C(=O)N(CCN3CCOCC3)c2c1 |TLB:16:7:14:11.10.12,6:7:10:14.13.12,THB:16:11:14:7.15.8,15:13:10:16.7.8,15:7:10:14.13.12| Show InChI InChI=1S/C33H40FN5O4/c34-25-6-7-27-28(17-25)38(9-8-37-10-12-43-13-11-37)30(40)29(36-32(42)35-26-4-2-1-3-5-26)31(41)39(27)21-33-18-22-14-23(19-33)16-24(15-22)20-33/h1-7,17,22-24,29H,8-16,18-21H2,(H2,35,36,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418883
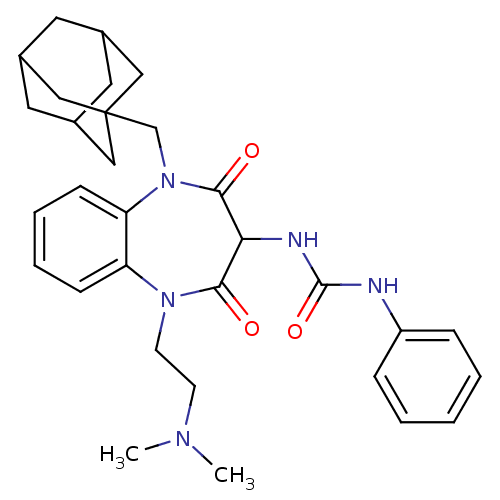
(CHEMBL1808420)Show SMILES CN(C)CCN1c2ccccc2N(CC23CC4CC(CC(C4)C2)C3)C(=O)C(NC(=O)Nc2ccccc2)C1=O |TLB:23:14:21:18.17.19,13:14:17:21.20.19,THB:23:18:21:14.22.15,22:20:17:23.14.15,22:14:17:21.20.19| Show InChI InChI=1S/C31H39N5O3/c1-34(2)12-13-35-25-10-6-7-11-26(25)36(20-31-17-21-14-22(18-31)16-23(15-21)19-31)29(38)27(28(35)37)33-30(39)32-24-8-4-3-5-9-24/h3-11,21-23,27H,12-20H2,1-2H3,(H2,32,33,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418886
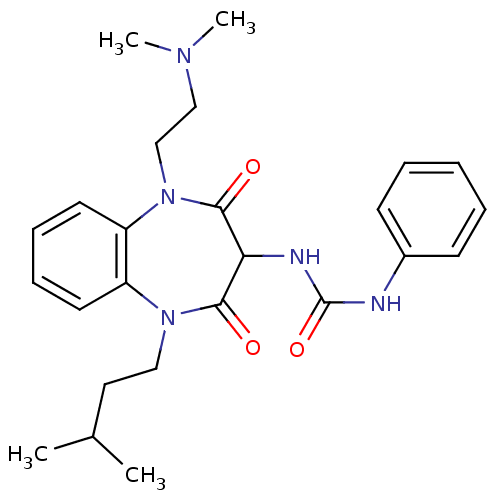
(CHEMBL1808411)Show SMILES CC(C)CCN1c2ccccc2N(CCN(C)C)C(=O)C(NC(=O)Nc2ccccc2)C1=O Show InChI InChI=1S/C25H33N5O3/c1-18(2)14-15-29-20-12-8-9-13-21(20)30(17-16-28(3)4)24(32)22(23(29)31)27-25(33)26-19-10-6-5-7-11-19/h5-13,18,22H,14-17H2,1-4H3,(H2,26,27,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis subsp. tularensis (strain S...) | BDBM50436715
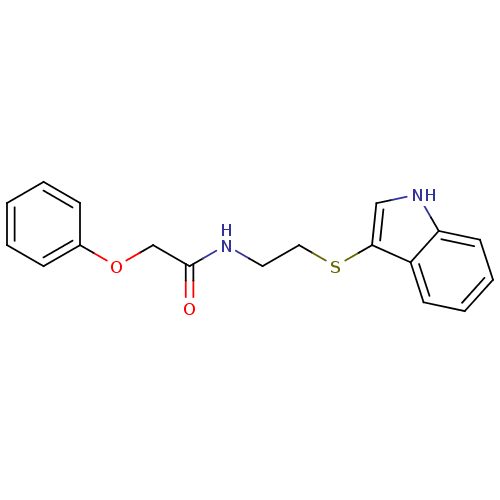
(CHEMBL2398661)Show InChI InChI=1S/C18H18N2O2S/c21-18(13-22-14-6-2-1-3-7-14)19-10-11-23-17-12-20-16-9-5-4-8-15(16)17/h1-9,12,20H,10-11,13H2,(H,19,21) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Naval Research Laboratories
Curated by ChEMBL
| Assay Description
Competitive inhibition of C-terminal His6-tagged Francisella tularensis SCHU S4 FabI expressed in Escherichia coli BL21 (DE3) using CrCoA as substrat... |
J Med Chem 56: 5275-87 (2014)
Article DOI: 10.1021/jm4001242
BindingDB Entry DOI: 10.7270/Q2D79CTG |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418899
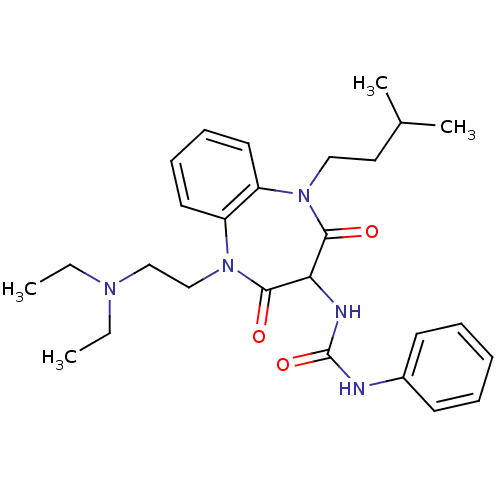
(CHEMBL1808413)Show SMILES CCN(CC)CCN1c2ccccc2N(CCC(C)C)C(=O)C(NC(=O)Nc2ccccc2)C1=O Show InChI InChI=1S/C27H37N5O3/c1-5-30(6-2)18-19-32-23-15-11-10-14-22(23)31(17-16-20(3)4)25(33)24(26(32)34)29-27(35)28-21-12-8-7-9-13-21/h7-15,20,24H,5-6,16-19H2,1-4H3,(H2,28,29,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418889
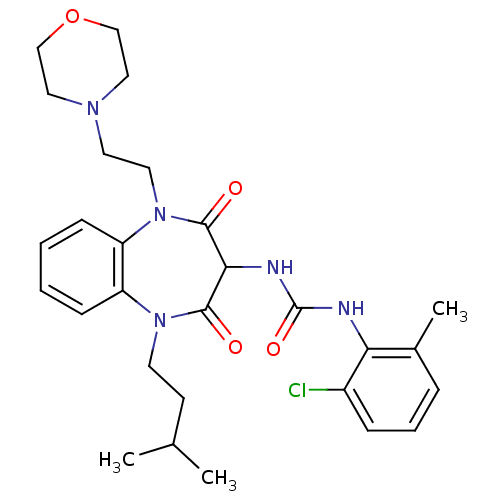
(CHEMBL1808407)Show SMILES CC(C)CCN1c2ccccc2N(CCN2CCOCC2)C(=O)C(NC(=O)Nc2c(C)cccc2Cl)C1=O Show InChI InChI=1S/C28H36ClN5O4/c1-19(2)11-12-33-22-9-4-5-10-23(22)34(14-13-32-15-17-38-18-16-32)27(36)25(26(33)35)31-28(37)30-24-20(3)7-6-8-21(24)29/h4-10,19,25H,11-18H2,1-3H3,(H2,30,31,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418888
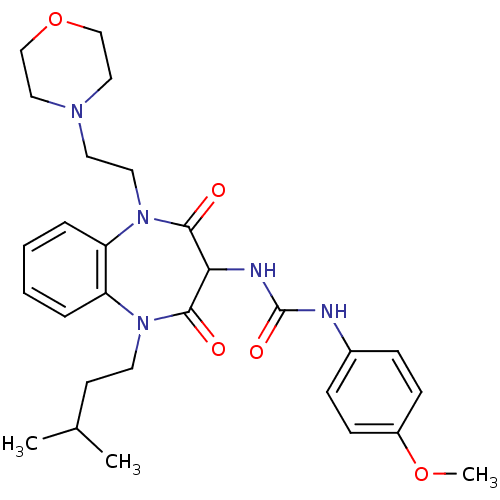
(CHEMBL1808408)Show SMILES COc1ccc(NC(=O)NC2C(=O)N(CCC(C)C)c3ccccc3N(CCN3CCOCC3)C2=O)cc1 Show InChI InChI=1S/C28H37N5O5/c1-20(2)12-13-32-23-6-4-5-7-24(23)33(15-14-31-16-18-38-19-17-31)27(35)25(26(32)34)30-28(36)29-21-8-10-22(37-3)11-9-21/h4-11,20,25H,12-19H2,1-3H3,(H2,29,30,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418890
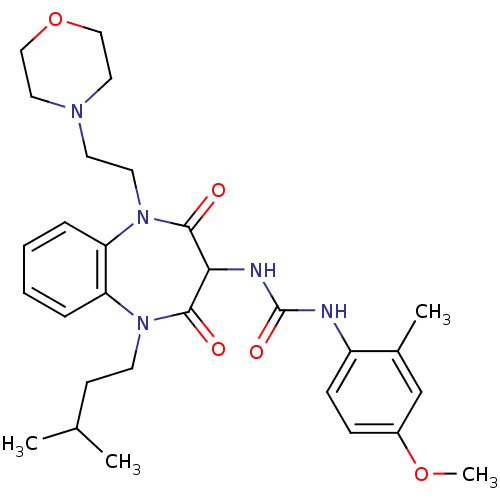
(CHEMBL1806517)Show SMILES COc1ccc(NC(=O)NC2C(=O)N(CCC(C)C)c3ccccc3N(CCN3CCOCC3)C2=O)c(C)c1 Show InChI InChI=1S/C29H39N5O5/c1-20(2)11-12-33-24-7-5-6-8-25(24)34(14-13-32-15-17-39-18-16-32)28(36)26(27(33)35)31-29(37)30-23-10-9-22(38-4)19-21(23)3/h5-10,19-20,26H,11-18H2,1-4H3,(H2,30,31,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis subsp. tularensis (strain S...) | BDBM50436718
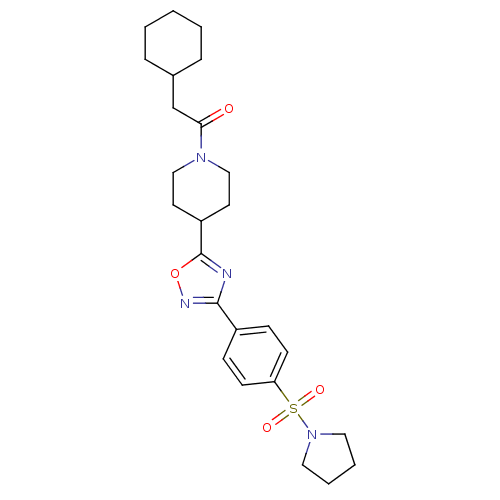
(CHEMBL1341978)Show SMILES O=C(CC1CCCCC1)N1CCC(CC1)c1nc(no1)-c1ccc(cc1)S(=O)(=O)N1CCCC1 Show InChI InChI=1S/C25H34N4O4S/c30-23(18-19-6-2-1-3-7-19)28-16-12-21(13-17-28)25-26-24(27-33-25)20-8-10-22(11-9-20)34(31,32)29-14-4-5-15-29/h8-11,19,21H,1-7,12-18H2 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Naval Research Laboratories
Curated by ChEMBL
| Assay Description
Competitive inhibition of C-terminal His6-tagged Francisella tularensis SCHU S4 FabI expressed in Escherichia coli BL21 (DE3) using CrCoA as substrat... |
J Med Chem 56: 5275-87 (2014)
Article DOI: 10.1021/jm4001242
BindingDB Entry DOI: 10.7270/Q2D79CTG |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418902
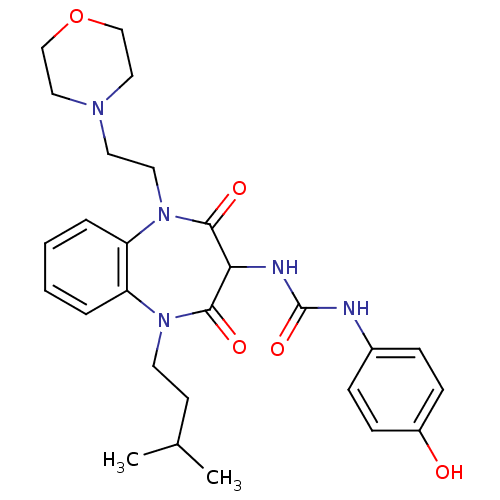
(CHEMBL1808409)Show SMILES CC(C)CCN1c2ccccc2N(CCN2CCOCC2)C(=O)C(NC(=O)Nc2ccc(O)cc2)C1=O Show InChI InChI=1S/C27H35N5O5/c1-19(2)11-12-31-22-5-3-4-6-23(22)32(14-13-30-15-17-37-18-16-30)26(35)24(25(31)34)29-27(36)28-20-7-9-21(33)10-8-20/h3-10,19,24,33H,11-18H2,1-2H3,(H2,28,29,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418900
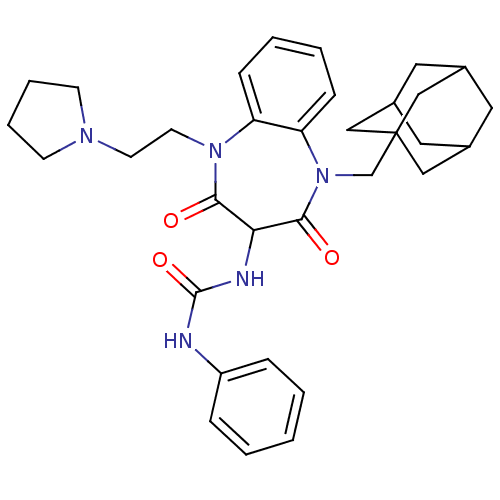
(CHEMBL1808419)Show SMILES O=C(NC1C(=O)N(CCN2CCCC2)c2ccccc2N(CC23CC4CC(CC(C4)C2)C3)C1=O)Nc1ccccc1 |TLB:31:22:29:26.25.27,21:22:25:29.28.27,THB:31:26:29:22.30.23,30:28:25:31.22.23,30:22:25:29.28.27| Show InChI InChI=1S/C33H41N5O3/c39-30-29(35-32(41)34-26-8-2-1-3-9-26)31(40)38(22-33-19-23-16-24(20-33)18-25(17-23)21-33)28-11-5-4-10-27(28)37(30)15-14-36-12-6-7-13-36/h1-5,8-11,23-25,29H,6-7,12-22H2,(H2,34,35,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418903
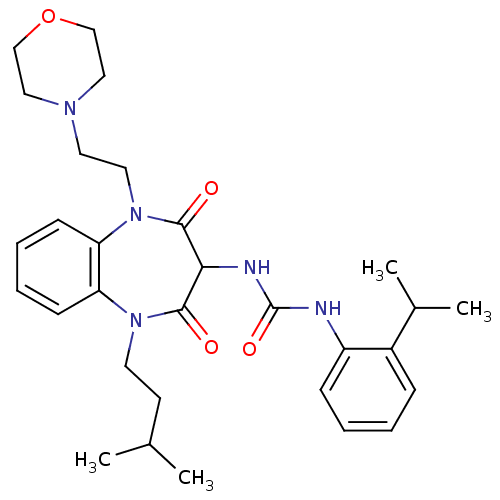
(CHEMBL1808406)Show SMILES CC(C)CCN1c2ccccc2N(CCN2CCOCC2)C(=O)C(NC(=O)Nc2ccccc2C(C)C)C1=O Show InChI InChI=1S/C30H41N5O4/c1-21(2)13-14-34-25-11-7-8-12-26(25)35(16-15-33-17-19-39-20-18-33)29(37)27(28(34)36)32-30(38)31-24-10-6-5-9-23(24)22(3)4/h5-12,21-22,27H,13-20H2,1-4H3,(H2,31,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418895
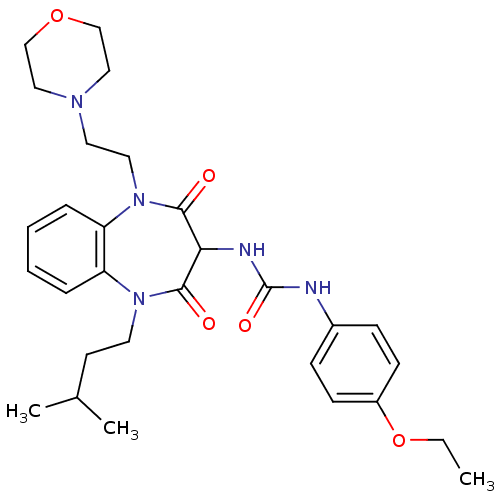
(CHEMBL1808404)Show SMILES CCOc1ccc(NC(=O)NC2C(=O)N(CCC(C)C)c3ccccc3N(CCN3CCOCC3)C2=O)cc1 Show InChI InChI=1S/C29H39N5O5/c1-4-39-23-11-9-22(10-12-23)30-29(37)31-26-27(35)33(14-13-21(2)3)24-7-5-6-8-25(24)34(28(26)36)16-15-32-17-19-38-20-18-32/h5-12,21,26H,4,13-20H2,1-3H3,(H2,30,31,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418885
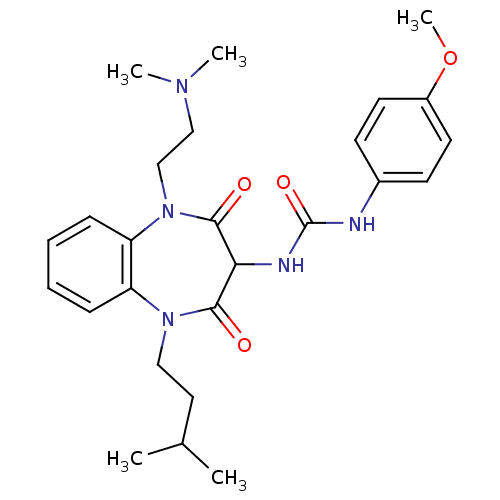
(CHEMBL1808412)Show SMILES COc1ccc(NC(=O)NC2C(=O)N(CCC(C)C)c3ccccc3N(CCN(C)C)C2=O)cc1 Show InChI InChI=1S/C26H35N5O4/c1-18(2)14-15-30-21-8-6-7-9-22(21)31(17-16-29(3)4)25(33)23(24(30)32)28-26(34)27-19-10-12-20(35-5)13-11-19/h6-13,18,23H,14-17H2,1-5H3,(H2,27,28,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50418882
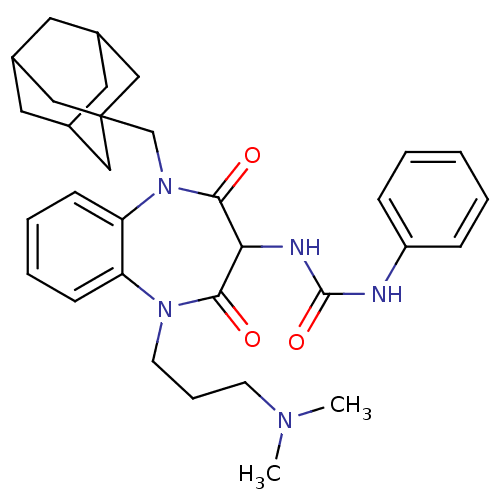
(CHEMBL1808421)Show SMILES CN(C)CCCN1c2ccccc2N(CC23CC4CC(CC(C4)C2)C3)C(=O)C(NC(=O)Nc2ccccc2)C1=O |TLB:24:15:22:19.18.20,14:15:18:22.21.20,THB:24:19:22:15.23.16,23:21:18:24.15.16,23:15:18:22.21.20| Show InChI InChI=1S/C32H41N5O3/c1-35(2)13-8-14-36-26-11-6-7-12-27(26)37(21-32-18-22-15-23(19-32)17-24(16-22)20-32)30(39)28(29(36)38)34-31(40)33-25-9-4-3-5-10-25/h3-7,9-12,22-24,28H,8,13-21H2,1-2H3,(H2,33,34,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCK8 from rat pancreas CCK1 receptor at by liquid scintillation counting |
Bioorg Med Chem 19: 4257-73 (2011)
Article DOI: 10.1016/j.bmc.2011.05.057
BindingDB Entry DOI: 10.7270/Q2416Z93 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505991

(CHEMBL4470113)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cc2C(=O)N(Cc3cccc(Cl)c3)CCn2c1 Show InChI InChI=1S/C23H22ClN7O/c1-15-12-25-23(27-20-6-7-26-29(20)2)28-21(15)17-11-19-22(32)31(9-8-30(19)14-17)13-16-4-3-5-18(24)10-16/h3-7,10-12,14H,8-9,13H2,1-2H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265958
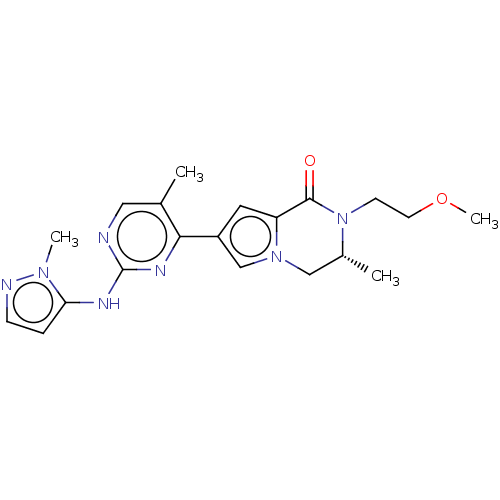
(CHEMBL4086507)Show SMILES COCCN1[C@H](C)Cn2cc(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-13-10-21-20(23-17-5-6-22-25(17)3)24-18(13)15-9-16-19(28)27(7-8-29-4)14(2)11-26(16)12-15/h5-6,9-10,12,14H,7-8,11H2,1-4H3,(H,21,23,24)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505989

(CHEMBL4551714)Show SMILES COC[C@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C25H28N8O2/c1-16-11-26-25(29-22-8-9-27-31(22)3)30-23(16)18-10-21-24(34)33(13-19-7-5-6-17(2)28-19)20(15-35-4)14-32(21)12-18/h5-12,20H,13-15H2,1-4H3,(H,26,29,30)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50094464

(CHEMBL3590107 | US10525036, Example SCH772984 | US...)Show SMILES O=C(CN1CC[C@H](C1)C(=O)Nc1ccc2[nH]nc(-c3ccncc3)c2c1)N1CCN(CC1)c1ccc(cc1)-c1ncccn1 |r| Show InChI InChI=1S/C22H27NO3/c1-2-15-23-16-13-20(14-17-23)26-21(24)22(25,18-9-5-3-6-10-18)19-11-7-4-8-12-19/h3-12,20,25H,2,13-17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using Erktide as substrate preincubated with enzyme for 20 mins prior to substrate addition by... |
J Med Chem 58: 4790-801 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00466
BindingDB Entry DOI: 10.7270/Q2V989SJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505994

(CHEMBL4440109)Show SMILES C[C@H]1Cn2cc(nc2C(=O)N1Cc1ccc(F)c(F)c1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C23H22F2N8O/c1-13-9-26-23(29-19-6-7-27-31(19)3)30-20(13)18-12-32-10-14(2)33(22(34)21(32)28-18)11-15-4-5-16(24)17(25)8-15/h4-9,12,14H,10-11H2,1-3H3,(H,26,29,30)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50094465
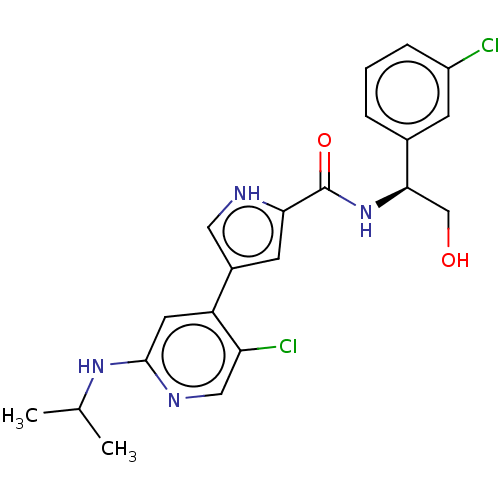
(CHEMBL3590106 | US10525036, Example BVD-523 | US10...)Show SMILES CC(C)Nc1cc(-c2c[nH]c(c2)C(=O)N[C@H](CO)c2cccc(Cl)c2)c(Cl)cn1 |r| Show InChI InChI=1S/C19H21NO3/c21-18(23-17-11-13-20-14-12-17)19(22,15-7-3-1-4-8-15)16-9-5-2-6-10-16/h1-10,17,20,22H,11-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using Erktide as substrate preincubated with enzyme for 20 mins prior to substrate addition by... |
J Med Chem 58: 4790-801 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00466
BindingDB Entry DOI: 10.7270/Q2V989SJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505988
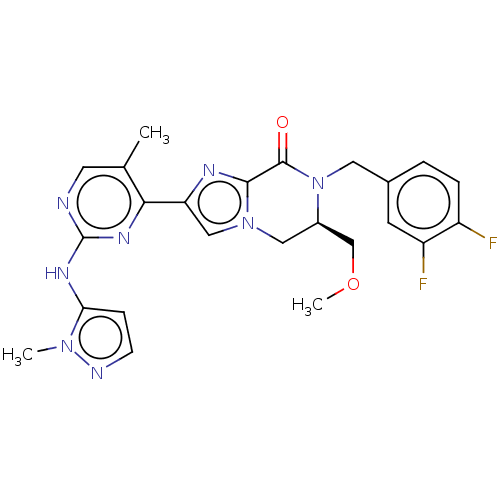
(CHEMBL4482864)Show SMILES COC[C@H]1Cn2cc(nc2C(=O)N1Cc1ccc(F)c(F)c1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H24F2N8O2/c1-14-9-27-24(30-20-6-7-28-32(20)2)31-21(14)19-12-33-11-16(13-36-3)34(23(35)22(33)29-19)10-15-4-5-17(25)18(26)8-15/h4-9,12,16H,10-11,13H2,1-3H3,(H,27,30,31)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50094464

(CHEMBL3590107 | US10525036, Example SCH772984 | US...)Show SMILES O=C(CN1CC[C@H](C1)C(=O)Nc1ccc2[nH]nc(-c3ccncc3)c2c1)N1CCN(CC1)c1ccc(cc1)-c1ncccn1 |r| Show InChI InChI=1S/C22H27NO3/c1-2-15-23-16-13-20(14-17-23)26-21(24)22(25,18-9-5-3-6-10-18)19-11-7-4-8-12-19/h3-12,20,25H,2,13-17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using Erktide as substrate preincubated with enzyme for 20 mins prior to substrate addition by... |
J Med Chem 58: 4790-801 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00466
BindingDB Entry DOI: 10.7270/Q2V989SJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265960

(CHEMBL4098608)Show SMILES COCCN1[C@@H](C)Cn2cc(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-13-10-21-20(23-17-5-6-22-25(17)3)24-18(13)15-9-16-19(28)27(7-8-29-4)14(2)11-26(16)12-15/h5-6,9-10,12,14H,7-8,11H2,1-4H3,(H,21,23,24)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505993

(CHEMBL4587118)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cc2C(=O)N(Cc3ccc(F)c(F)c3)CCn2c1 Show InChI InChI=1S/C23H21F2N7O/c1-14-11-26-23(28-20-5-6-27-30(20)2)29-21(14)16-10-19-22(33)32(8-7-31(19)13-16)12-15-3-4-17(24)18(25)9-15/h3-6,9-11,13H,7-8,12H2,1-2H3,(H,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265961

(CHEMBL4090886)Show InChI InChI=1S/C19H23N7O2/c1-13-11-20-19(22-16-4-5-21-24(16)2)23-17(13)14-10-15-18(27)25(8-9-28-3)6-7-26(15)12-14/h4-5,10-12H,6-9H2,1-3H3,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data